Abstract
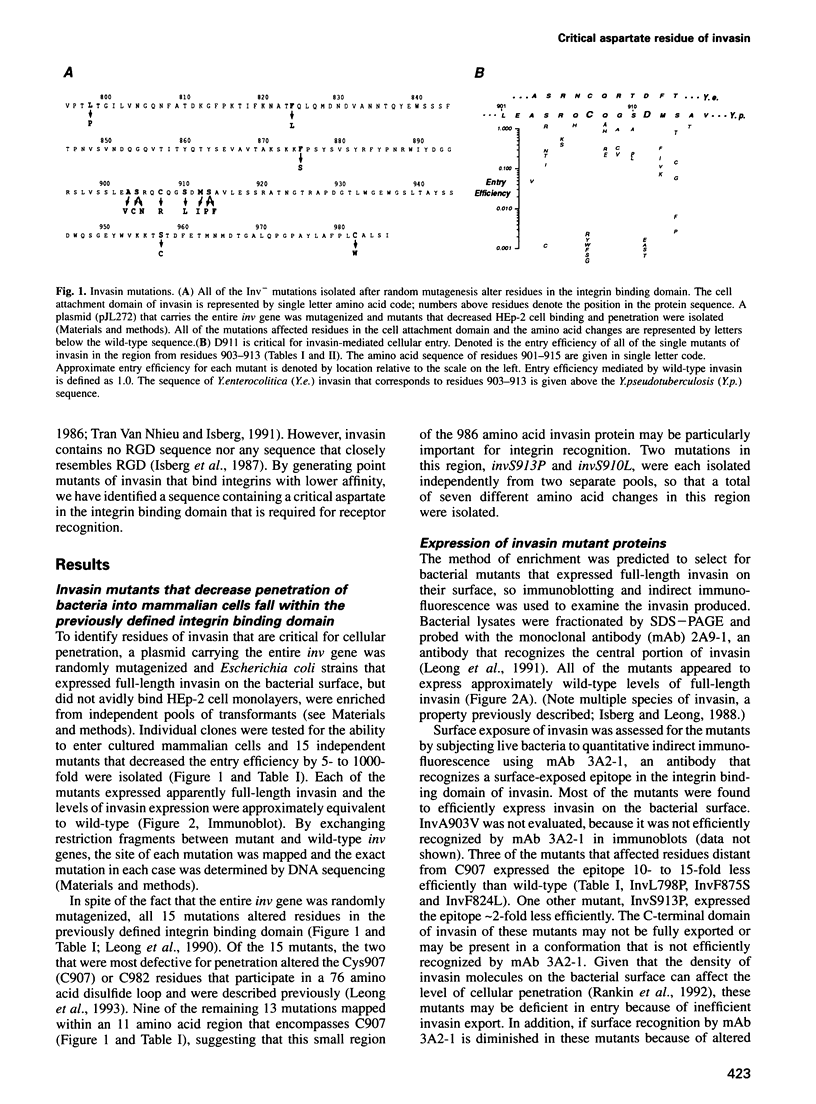
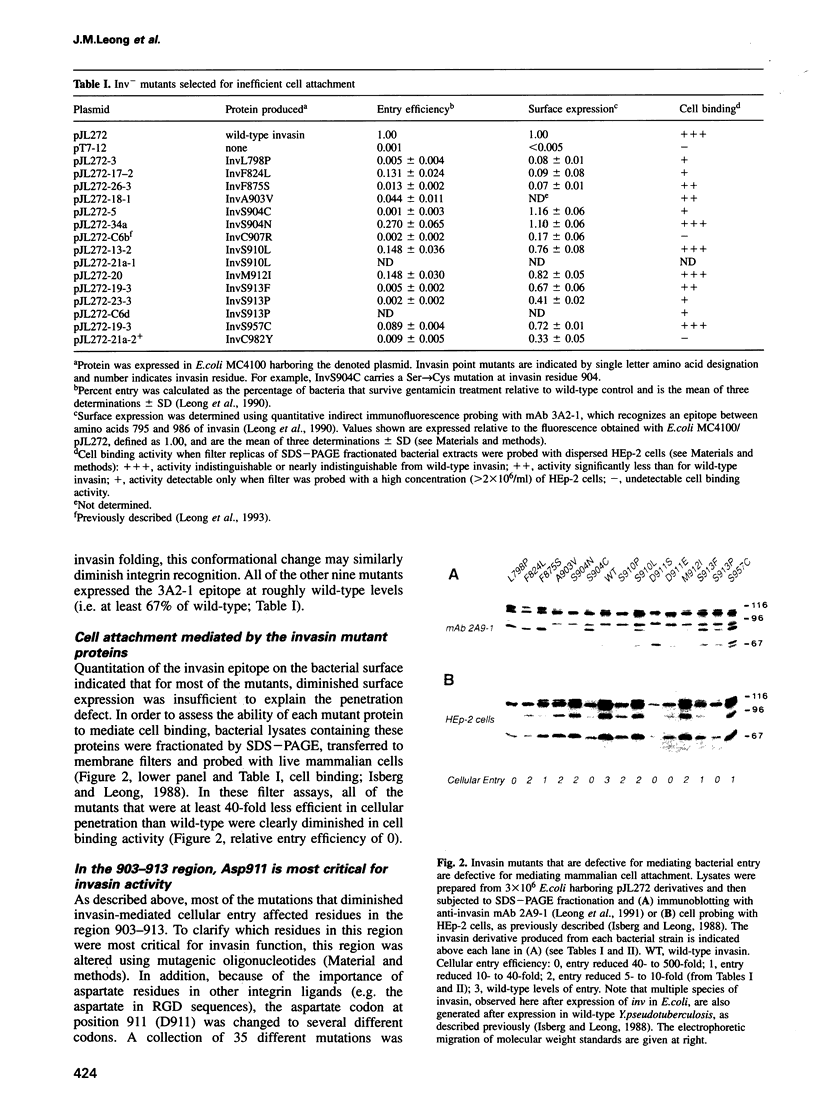
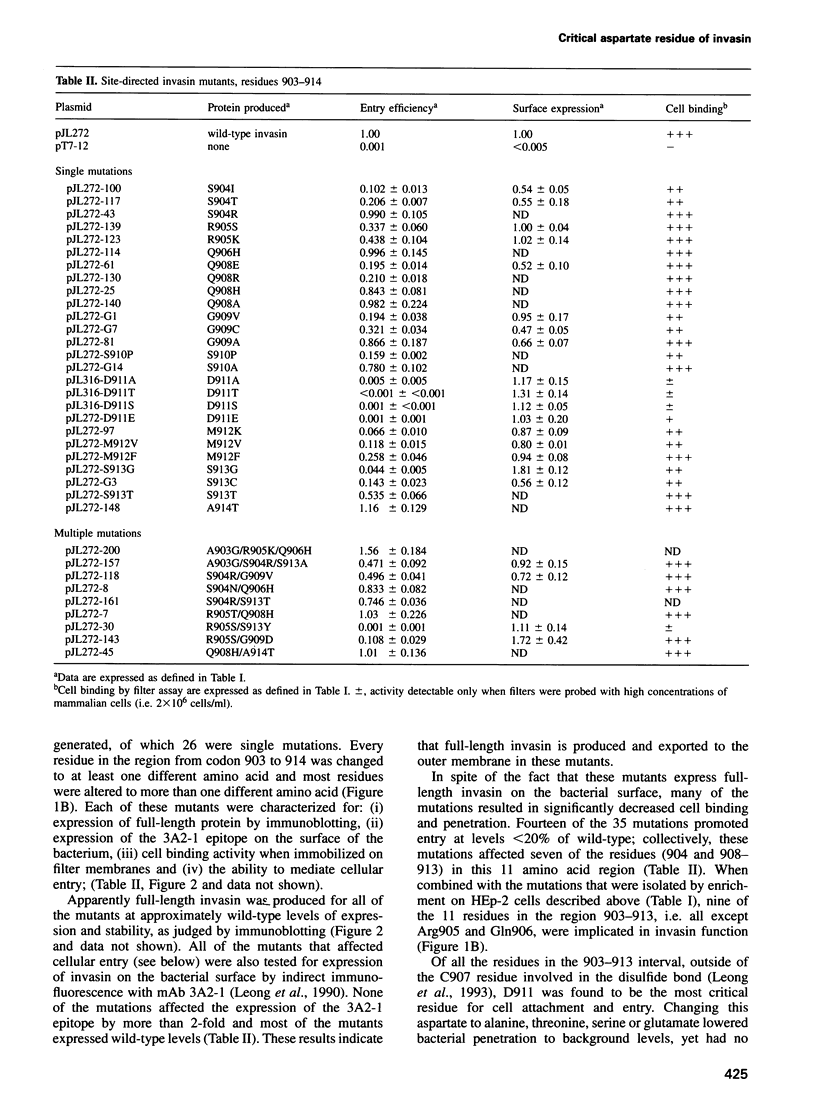
The Yersinia pseudotuberculosis invasin protein mediates bacterial entry into mammalian cells by binding multiple beta 1-chain integrins. Invasin binding to purified alpha 5 beta 1 integrin is inhibited by Arg-Gly-Asp (RGD)-containing peptides, although invasin contains no RGD sequence. Fifteen mutations that diminished binding and bacterial entry were isolated after mutagenesis of the entire inv gene. All of the mutations altered residues within the C-terminal 192 amino acids of invasin, previously delineated as the integrin binding domain, and 10 of the mutations fell within an 11 residue region. This small region was subjected to site-directed mutagenesis and almost half of the 35 mutations generated decreased invasin-mediated entry. D911 within this region was the most critical residue, as even a conservative glutamate substitution abolished bacterial penetration. Purified invasin derivatives altered at this residue were defective in promoting cell attachment and this defect was reflected in a 10-fold or greater increase in IC50 for integrin binding. D911 may have a function similar to that of the aspartate residue in RGD-containing sequences.
Full text
PDF
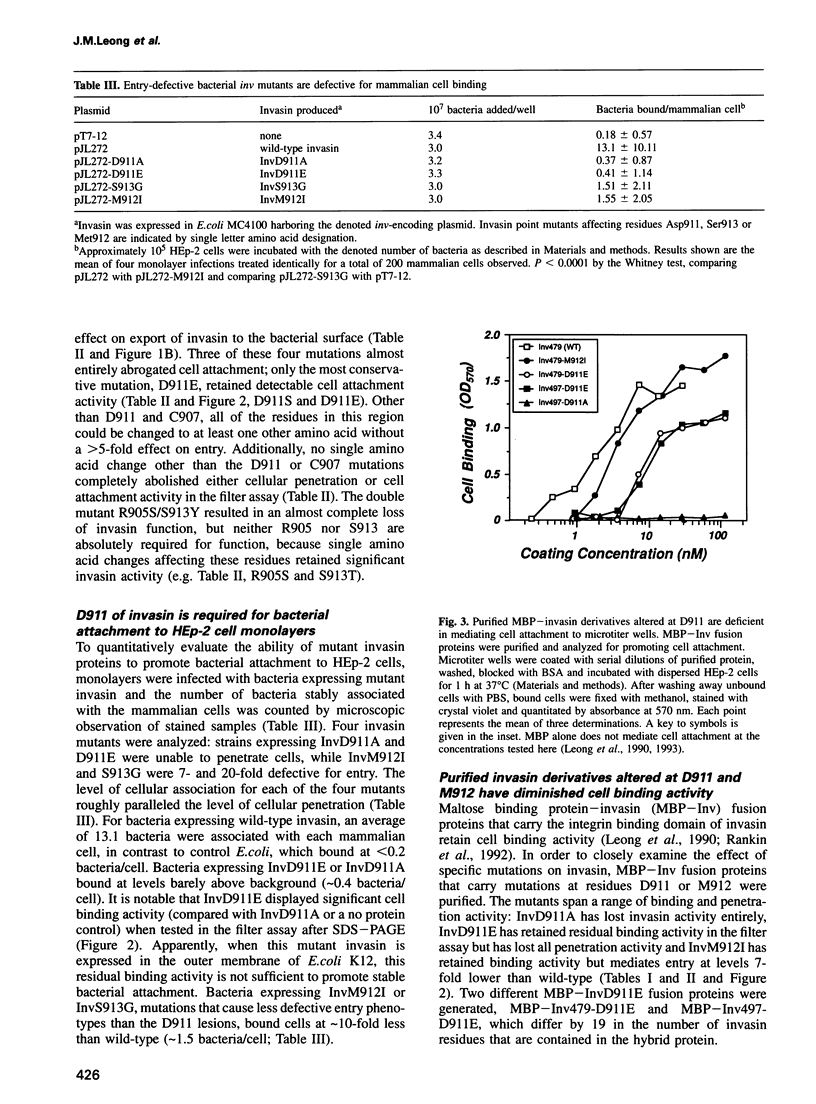
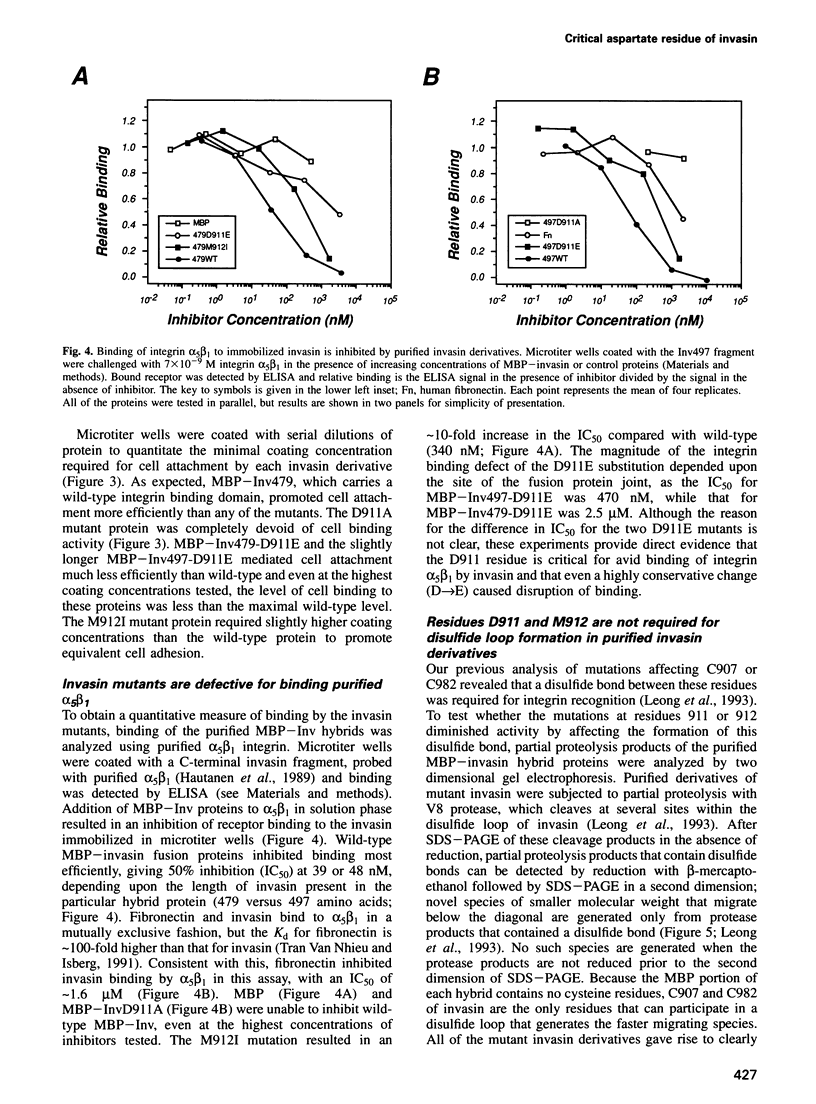
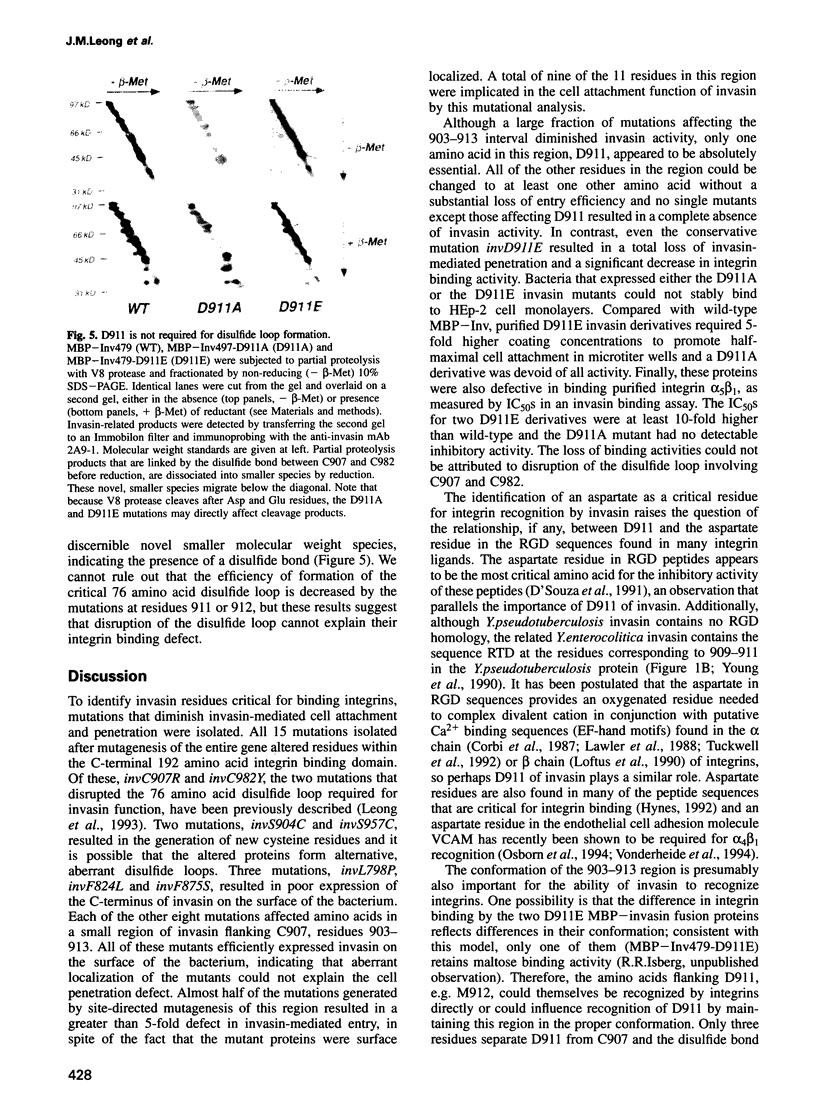



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aota S., Nagai T., Yamada K. M. Characterization of regions of fibronectin besides the arginine-glycine-aspartic acid sequence required for adhesive function of the cell-binding domain using site-directed mutagenesis. J Biol Chem. 1991 Aug 25;266(24):15938–15943. [PubMed] [Google Scholar]
- Argraves W. S., Pytela R., Suzuki S., Millán J. L., Pierschbacher M. D., Ruoslahti E. cDNA sequences from the alpha subunit of the fibronectin receptor predict a transmembrane domain and a short cytoplasmic peptide. J Biol Chem. 1986 Oct 5;261(28):12922–12924. [PubMed] [Google Scholar]
- Barker P. L., Bullens S., Bunting S., Burdick D. J., Chan K. S., Deisher T., Eigenbrot C., Gadek T. R., Gantzos R., Lipari M. T. Cyclic RGD peptide analogues as antiplatelet antithrombotics. J Med Chem. 1992 May 29;35(11):2040–2048. doi: 10.1021/jm00089a014. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bliska J. B., Copass M. C., Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect Immun. 1993 Sep;61(9):3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Galán J. E., Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993 Jun 4;73(5):903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- Bowditch R. D., Hariharan M., Tominna E. F., Smith J. W., Yamada K. M., Getzoff E. D., Ginsberg M. H. Identification of a novel integrin binding site in fibronectin. Differential utilization by beta 3 integrins. J Biol Chem. 1994 Apr 8;269(14):10856–10863. [PubMed] [Google Scholar]
- Corbi A. L., Miller L. J., O'Connor K., Larson R. S., Springer T. A. cDNA cloning and complete primary structure of the alpha subunit of a leukocyte adhesion glycoprotein, p150,95. EMBO J. 1987 Dec 20;6(13):4023–4028. doi: 10.1002/j.1460-2075.1987.tb02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney H. S., Simpson W. A., Beachey E. H. Binding of streptococcal lipoteichoic acid to fatty acid-binding sites on human plasma fibronectin. J Bacteriol. 1983 Feb;153(2):763–770. doi: 10.1128/jb.153.2.763-770.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Plow E. F. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991 Jul;16(7):246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Falkow S., Isberg R. R., Portnoy D. A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock J. I., Fröman G., Jönsson K., Guss B., Signäs C., Nilsson B., Raucci G., Hök M., Wadström T., Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987 Aug;6(8):2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler R. G., Degnen G. E., Cox E. C. Mutational specificity of a conditional Escherichia coli mutator, mutD5. Mol Gen Genet. 1974;133(3):179–191. doi: 10.1007/BF00267667. [DOI] [PubMed] [Google Scholar]
- Grützkau A., Hanski C., Hahn H., Riecken E. O. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990 Sep;31(9):1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Hanski C., Kutschka U., Schmoranzer H. P., Naumann M., Stallmach A., Hahn H., Menge H., Riecken E. O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989 Mar;57(3):673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautanen A., Gailit J., Mann D. M., Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J Biol Chem. 1989 Jan 25;264(3):1437–1442. [PubMed] [Google Scholar]
- Hill D. E., Hope I. A., Macke J. P., Struhl K. Saturation mutagenesis of the yeast his3 regulatory site: requirements for transcriptional induction and for binding by GCN4 activator protein. Science. 1986 Oct 24;234(4775):451–457. doi: 10.1126/science.3532321. [DOI] [PubMed] [Google Scholar]
- Hoepelman A. I., Tuomanen E. I. Consequences of microbial attachment: directing host cell functions with adhesins. Infect Immun. 1992 May;60(5):1729–1733. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Cultured mammalian cells attach to the invasin protein of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6682–6686. doi: 10.1073/pnas.85.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R. Pathways for the penetration of enteroinvasive Yersinia into mammalian cells. Mol Biol Med. 1990 Feb;7(1):73–82. [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J Bacteriol. 1983 Apr;154(1):253–260. doi: 10.1128/jb.154.1.253-260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. M., Fournier R. S., Isberg R. R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990 Jun;9(6):1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. M., Fournier R. S., Isberg R. R. Mapping and topographic localization of epitopes of the Yersinia pseudotuberculosis invasin protein. Infect Immun. 1991 Oct;59(10):3424–3433. doi: 10.1128/iai.59.10.3424-3433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. M., Moitoso de Vargas L., Isberg R. R. Binding of cultured mammalian cells to immobilized bacteria. Infect Immun. 1992 Feb;60(2):683–686. doi: 10.1128/iai.60.2.683-686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. M., Morrissey P. E., Isberg R. R. A 76-amino acid disulfide loop in the Yersinia pseudotuberculosis invasin protein is required for integrin receptor recognition. J Biol Chem. 1993 Sep 25;268(27):20524–20532. [PubMed] [Google Scholar]
- Loftus J. C., O'Toole T. E., Plow E. F., Glass A., Frelinger A. L., 3rd, Ginsberg M. H. A beta 3 integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science. 1990 Aug 24;249(4971):915–918. doi: 10.1126/science.2392682. [DOI] [PubMed] [Google Scholar]
- Maina C. V., Riggs P. D., Grandea A. G., 3rd, Slatko B. E., Moran L. S., Tagliamonte J. A., McReynolds L. A., Guan C. D. An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from maltose-binding protein. Gene. 1988 Dec 30;74(2):365–373. doi: 10.1016/0378-1119(88)90170-9. [DOI] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil K. T., Hoess R. H., Jackson S. A., Ramachandran N. S., Mousa S. A., DeGrado W. F. Identification of novel peptide antagonists for GPIIb/IIIa from a conformationally constrained phage peptide library. Proteins. 1992 Dec;14(4):509–515. doi: 10.1002/prot.340140411. [DOI] [PubMed] [Google Scholar]
- Osborn L., Vassallo C., Browning B. G., Tizard R., Haskard D. O., Benjamin C. D., Dougas I., Kirchhausen T. Arrangement of domains, and amino acid residues required for binding of vascular cell adhesion molecule-1 to its counter-receptor VLA-4 (alpha 4 beta 1). J Cell Biol. 1994 Feb;124(4):601–608. doi: 10.1083/jcb.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe J. C., Miller V. L. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6473–6477. doi: 10.1073/pnas.90.14.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J Biol Chem. 1987 Dec 25;262(36):17294–17298. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Argraves S., Suzuki S., Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Samanen J., Ali F., Romoff T., Calvo R., Sorenson E., Vasko J., Storer B., Berry D., Bennett D., Strohsacker M. Development of a small RGD peptide fibrinogen receptor antagonist with potent antiaggregatory activity in vitro. J Med Chem. 1991 Oct;34(10):3114–3125. doi: 10.1021/jm00114a022. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Lechene C., Ingber D. E. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin alpha 5 beta 1, independent of cell shape. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M., Richard S., Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990 Mar;58(3):841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Isberg R. R. Isolation and identification of eukaryotic receptors promoting bacterial internalization. Methods Enzymol. 1994;236:307–318. doi: 10.1016/0076-6879(94)36023-5. [DOI] [PubMed] [Google Scholar]
- Tuckwell D. S., Brass A., Humphries M. J. Homology modelling of integrin EF-hands. Evidence for widespread use of a conserved cation-binding site. Biochem J. 1992 Jul 1;285(Pt 1):325–331. doi: 10.1042/bj2850325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nhieu G. T., Isberg R. R. The Yersinia pseudotuberculosis invasin protein and human fibronectin bind to mutually exclusive sites on the alpha 5 beta 1 integrin receptor. J Biol Chem. 1991 Dec 25;266(36):24367–24375. [PubMed] [Google Scholar]
- Van de Water L., Destree A. T., Hynes R. O. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983 Apr 8;220(4593):201–204. doi: 10.1126/science.6338594. [DOI] [PubMed] [Google Scholar]
- Vonderheide R. H., Tedder T. F., Springer T. A., Staunton D. E. Residues within a conserved amino acid motif of domains 1 and 4 of VCAM-1 are required for binding to VLA-4. J Cell Biol. 1994 Apr;125(1):215–222. doi: 10.1083/jcb.125.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Isberg R. R. Cellular internalization in the absence of invasin expression is promoted by the Yersinia pseudotuberculosis yadA product. Infect Immun. 1993 Sep;61(9):3907–3913. doi: 10.1128/iai.61.9.3907-3913.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. B., Miller V. L., Falkow S., Schoolnik G. K. Sequence, localization and function of the invasin protein of Yersinia enterocolitica. Mol Microbiol. 1990 Jul;4(7):1119–1128. doi: 10.1111/j.1365-2958.1990.tb00686.x. [DOI] [PubMed] [Google Scholar]