Abstract
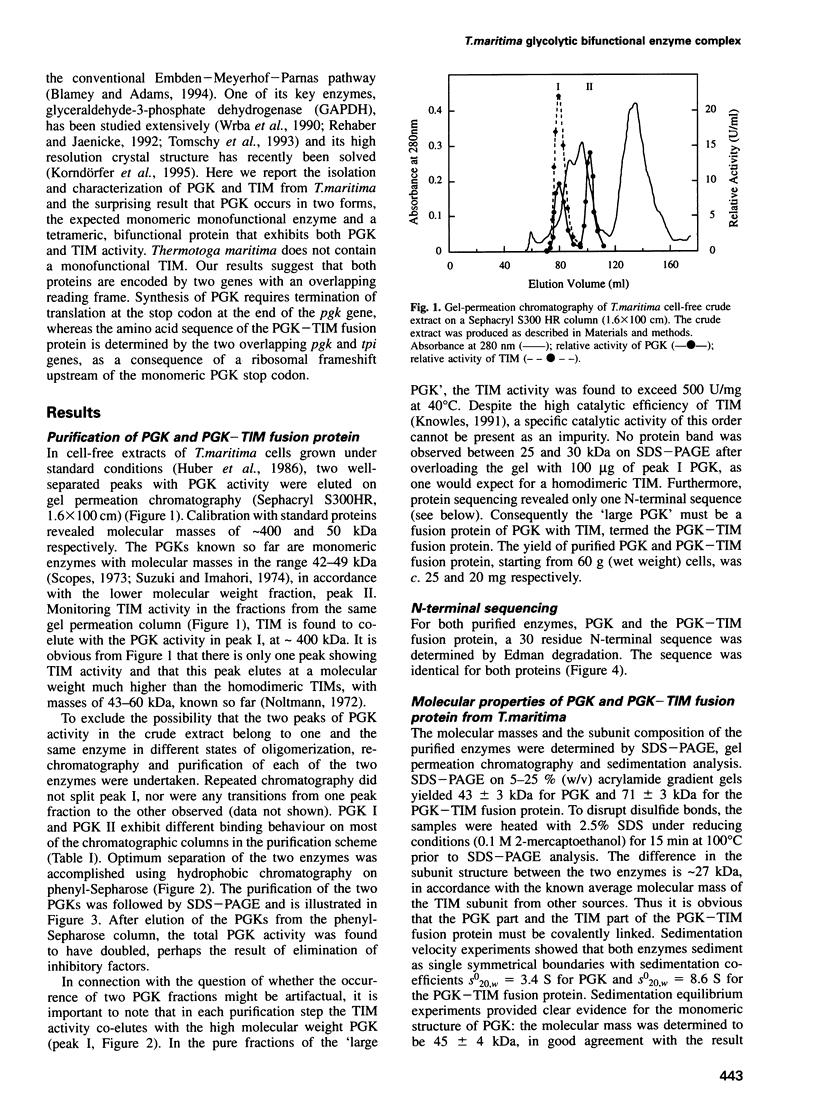
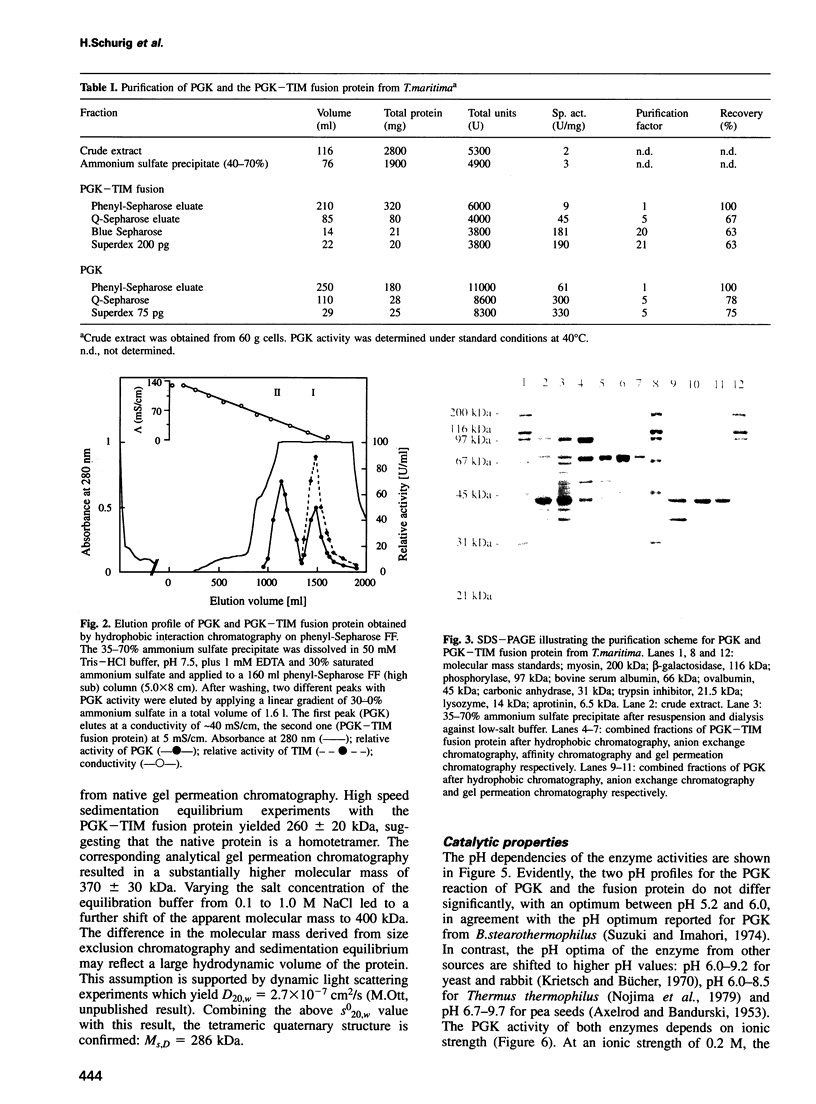
Phosphoglycerate kinase (PGK) from the hyperthermophilic bacterium Thermotoga maritima has been purified to homogeneity. A second larger enzyme with PGK activity and identical N-terminal sequence was also found. Surprisingly, this enzyme displayed triosephosphate isomerase (TIM) activity. No other TIM is detectable in T. maritima crude extracts. As shown by ultracentrifugal analysis, PGK is a 43 kDa monomer, whereas the bifunctional PGK-TIM fusion protein is a homotetramer of 240-285 kDa. SDS-PAGE indicated a subunit size of 70 kDa for the fusion protein. Both enzymes show high thermostability. Measurements of the catalytic properties revealed no extraordinary results. pH optima, Km values and activation energies were found to be in the range observed for other PGKs and TIMs investigated so far. The corresponding pgk and tpi genes are part of the apparent gap operon of T. maritima. This gene segment contains two overlapping reading frames, where the 43 kDa PGK is encoded by the upstream open reading frame, the pgk gene. On the other hand, the 70 kDa PGK-TIM fusion protein is encoded jointly by the pgk gene and the overlapping downstream open reading frame of the tpi gene. A programmed frameshift may be responsible for this fusion. A comparison of the amino acid sequence of both the PGK and the TIM parts of the fusion protein with those of known PGKs and TIMs reveals high similarity to the corresponding enzymes from different procaryotic and eucaryotic organisms.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., BANDURSKI R. S. Phosphoglyceryl kinase in higher plants. J Biol Chem. 1953 Oct;204(2):939–948. [PubMed] [Google Scholar]
- Adamski F. M., Donly B. C., Tate W. P. Competition between frameshifting, termination and suppression at the frameshift site in the Escherichia coli release factor-2 mRNA. Nucleic Acids Res. 1993 Nov 11;21(22):5074–5078. doi: 10.1093/nar/21.22.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J. F., Weiss R. B., Gesteland R. F. Ribosome gymnastics--degree of difficulty 9.5, style 10.0. Cell. 1990 Aug 10;62(3):413–423. doi: 10.1016/0092-8674(90)90007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J. F., Weiss R. B., Thompson S., Gesteland R. F. Towards a genetic dissection of the basis of triplet decoding, and its natural subversion: programmed reading frame shifts and hops. Annu Rev Genet. 1991;25:201–228. doi: 10.1146/annurev.ge.25.120191.001221. [DOI] [PubMed] [Google Scholar]
- Banks R. D., Blake C. C., Evans P. R., Haser R., Rice D. W., Hardy G. W., Merrett M., Phillips A. W. Sequence, structure and activity of phosphoglycerate kinase: a possible hinge-bending enzyme. Nature. 1979 Jun 28;279(5716):773–777. doi: 10.1038/279773a0. [DOI] [PubMed] [Google Scholar]
- Belasco J. G., Herlihy J. M., Knowles J. R. Critical ionization states in the reaction catalyzed by triosephosphate isomerase. Biochemistry. 1978 Jul 25;17(15):2971–2978. doi: 10.1021/bi00608a005. [DOI] [PubMed] [Google Scholar]
- Blacklow S. C., Knowles J. R. How can a catalytic lesion be offset? The energetics of two pseudorevertant triosephosphate isomerases. Biochemistry. 1990 May 1;29(17):4099–4108. doi: 10.1021/bi00469a012. [DOI] [PubMed] [Google Scholar]
- Blacklow S. C., Raines R. T., Lim W. A., Zamore P. D., Knowles J. R. Triosephosphate isomerase catalysis is diffusion controlled. Appendix: Analysis of triose phosphate equilibria in aqueous solution by 31P NMR. Biochemistry. 1988 Feb 23;27(4):1158–1167. doi: 10.1021/bi00404a013. [DOI] [PubMed] [Google Scholar]
- Blamey J. M., Adams M. W. Characterization of an ancestral type of pyruvate ferredoxin oxidoreductase from the hyperthermophilic bacterium, Thermotoga maritima. Biochemistry. 1994 Feb 1;33(4):1000–1007. doi: 10.1021/bi00170a019. [DOI] [PubMed] [Google Scholar]
- Burton P. M., Waley S. G. Kinetics of triose phosphate isomerase. Biochim Biophys Acta. 1968 Mar 25;151(3):714–715. doi: 10.1016/0005-2744(68)90028-4. [DOI] [PubMed] [Google Scholar]
- Böhm G., Jaenicke R. Relevance of sequence statistics for the properties of extremophilic proteins. Int J Pept Protein Res. 1994 Jan;43(1):97–106. doi: 10.1111/j.1399-3011.1994.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Davies G. J., Gamblin S. J., Littlechild J. A., Watson H. C. The structure of a thermally stable 3-phosphoglycerate kinase and a comparison with its mesophilic equivalent. Proteins. 1993 Mar;15(3):283–289. doi: 10.1002/prot.340150306. [DOI] [PubMed] [Google Scholar]
- Davies G. J., Littlechild J. A., Watson H. C., Hall L. Sequence and expression of the gene encoding 3-phosphoglycerate kinase from Bacillus stearothermophilus. Gene. 1991 Dec 20;109(1):39–45. doi: 10.1016/0378-1119(91)90586-z. [DOI] [PubMed] [Google Scholar]
- Eikmanns B. J. Identification, sequence analysis, and expression of a Corynebacterium glutamicum gene cluster encoding the three glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase, 3-phosphoglycerate kinase, and triosephosphate isomerase. J Bacteriol. 1992 Oct;174(19):6076–6086. doi: 10.1128/jb.174.19.6076-6086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry S., Hensel R. Purification and characterization of D-glyceraldehyde-3-phosphate dehydrogenase from the thermophilic archaebacterium Methanothermus fervidus. Eur J Biochem. 1987 May 15;165(1):147–155. doi: 10.1111/j.1432-1033.1987.tb11205.x. [DOI] [PubMed] [Google Scholar]
- Fabry S., Heppner P., Dietmaier W., Hensel R. Cloning and sequencing the gene encoding 3-phosphoglycerate kinase from mesophilic Methanobacterium bryantii and thermophilic Methanothermus fervidus. Gene. 1990 Jul 2;91(1):19–25. doi: 10.1016/0378-1119(90)90157-m. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Zhao H., Vimaladithan A. A novel programed frameshift expresses the POL3 gene of retrotransposon Ty3 of yeast: frameshifting without tRNA slippage. Cell. 1993 Jul 16;74(1):93–103. doi: 10.1016/0092-8674(93)90297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber G. K., Petsko G. A. The evolution of alpha/beta barrel enzymes. Trends Biochem Sci. 1990 Jun;15(6):228–234. doi: 10.1016/0968-0004(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Weiss R. B., Atkins J. F. Recoding: reprogrammed genetic decoding. Science. 1992 Sep 18;257(5077):1640–1641. doi: 10.1126/science.1529352. [DOI] [PubMed] [Google Scholar]
- Hecht K., Wrba A., Jaenicke R. Catalytic properties of thermophilic lactate dehydrogenase and halophilic malate dehydrogenase at high temperature and low water activity. Eur J Biochem. 1989 Jul 15;183(1):69–74. doi: 10.1111/j.1432-1033.1989.tb14897.x. [DOI] [PubMed] [Google Scholar]
- Hensel R., Laumann S., Lang J., Heumann H., Lottspeich F. Characterization of two D-glyceraldehyde-3-phosphate dehydrogenases from the extremely thermophilic archaebacterium Thermoproteus tenax. Eur J Biochem. 1987 Dec 30;170(1-2):325–333. doi: 10.1111/j.1432-1033.1987.tb13703.x. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Protein stability and molecular adaptation to extreme conditions. Eur J Biochem. 1991 Dec 18;202(3):715–728. doi: 10.1111/j.1432-1033.1991.tb16426.x. [DOI] [PubMed] [Google Scholar]
- João H. C., Williams R. J. The anatomy of a kinase and the control of phosphate transfer. Eur J Biochem. 1993 Aug 15;216(1):1–18. doi: 10.1111/j.1432-1033.1993.tb18110.x. [DOI] [PubMed] [Google Scholar]
- Juszczak A., Aono S., Adams M. W. The extremely thermophilic eubacterium, Thermotoga maritima, contains a novel iron-hydrogenase whose cellular activity is dependent upon tungsten. J Biol Chem. 1991 Jul 25;266(21):13834–13841. [PubMed] [Google Scholar]
- Kim C. W., Markiewicz P., Lee J. J., Schierle C. F., Miller J. H. Studies of the hyperthermophile Thermotoga maritima by random sequencing of cDNA and genomic libraries. Identification and sequencing of the trpEG (D) operon. J Mol Biol. 1993 Jun 20;231(4):960–981. doi: 10.1006/jmbi.1993.1345. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Enzyme catalysis: not different, just better. Nature. 1991 Mar 14;350(6314):121–124. doi: 10.1038/350121a0. [DOI] [PubMed] [Google Scholar]
- Krietsch W. K., Bücher T. 3-phosphoglycerate kinase from rabbit sceletal muscle and yeast. Eur J Biochem. 1970 Dec;17(3):568–580. doi: 10.1111/j.1432-1033.1970.tb01202.x. [DOI] [PubMed] [Google Scholar]
- Lambeir A. M., Opperdoes F. R., Wierenga R. K. Kinetic properties of triose-phosphate isomerase from Trypanosoma brucei brucei. A comparison with the rabbit muscle and yeast enzymes. Eur J Biochem. 1987 Oct 1;168(1):69–74. doi: 10.1111/j.1432-1033.1987.tb13388.x. [DOI] [PubMed] [Google Scholar]
- Levin M. E., Hendrix R. W., Casjens S. R. A programmed translational frameshift is required for the synthesis of a bacteriophage lambda tail assembly protein. J Mol Biol. 1993 Nov 5;234(1):124–139. doi: 10.1006/jmbi.1993.1568. [DOI] [PubMed] [Google Scholar]
- Lodi P. J., Knowles J. R. Neutral imidazole is the electrophile in the reaction catalyzed by triosephosphate isomerase: structural origins and catalytic implications. Biochemistry. 1991 Jul 16;30(28):6948–6956. doi: 10.1021/bi00242a020. [DOI] [PubMed] [Google Scholar]
- Lolis E., Alber T., Davenport R. C., Rose D., Hartman F. C., Petsko G. A. Structure of yeast triosephosphate isomerase at 1.9-A resolution. Biochemistry. 1990 Jul 17;29(28):6609–6618. doi: 10.1021/bi00480a009. [DOI] [PubMed] [Google Scholar]
- Mori N., Singer-Sam J., Riggs A. D. Evolutionary conservation of the substrate-binding cleft of phosphoglycerate kinases. FEBS Lett. 1986 Aug 18;204(2):313–317. doi: 10.1016/0014-5793(86)80835-3. [DOI] [PubMed] [Google Scholar]
- Nojima H., Ikai A., Oshima T., Noda H. Reversible thermal unfolding of thermostable phosphoglycerate kinase. Thermostability associated with mean zero enthalpy change. J Mol Biol. 1977 Nov 5;116(3):429–442. doi: 10.1016/0022-2836(77)90078-x. [DOI] [PubMed] [Google Scholar]
- Nojima H., Noda H. Kietics of thermal unfolding and refolding of thermostable phosphoglycerate kinase. J Biochem. 1979 Oct;86(4):1055–1065. doi: 10.1093/oxfordjournals.jbchem.a132600. [DOI] [PubMed] [Google Scholar]
- Nojima H., Oshima T., Noda H. Purification and properties of phosphoglycerate kinase from Thermus thermophilus strain HB8. J Biochem. 1979 Jun;85(6):1509–1517. doi: 10.1093/oxfordjournals.jbchem.a132480. [DOI] [PubMed] [Google Scholar]
- Ostendorp R., Liebl W., Schurig H., Jaenicke R. The L-lactate dehydrogenase gene of the hyperthermophilic bacterium Thermotoga maritima cloned by complementation in Escherichia coli. Eur J Biochem. 1993 Sep 15;216(3):709–715. doi: 10.1111/j.1432-1033.1993.tb18190.x. [DOI] [PubMed] [Google Scholar]
- Plaut B., Knowles J. R. pH-dependence of the triose phosphate isomerase reaction. Biochem J. 1972 Sep;129(2):311–320. doi: 10.1042/bj1290311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehaber V., Jaenicke R. Stability and reconstitution of D-glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic eubacterium Thermotoga maritima. J Biol Chem. 1992 Jun 5;267(16):10999–11006. [PubMed] [Google Scholar]
- Rentier-Delrue F., Mande S. C., Moyens S., Terpstra P., Mainfroid V., Goraj K., Lion M., Hol W. G., Martial J. A. Cloning and overexpression of the triosephosphate isomerase genes from psychrophilic and thermophilic bacteria. Structural comparison of the predicted protein sequences. J Mol Biol. 1993 Jan 5;229(1):85–93. doi: 10.1006/jmbi.1993.1010. [DOI] [PubMed] [Google Scholar]
- Schläpfer B. S., Zuber H. Cloning and sequencing of the genes encoding glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase and triosephosphate isomerase (gap operon) from mesophilic Bacillus megaterium: comparison with corresponding sequences from thermophilic Bacillus stearothermophilus. Gene. 1992 Dec 1;122(1):53–62. doi: 10.1016/0378-1119(92)90031-j. [DOI] [PubMed] [Google Scholar]
- Skerra A., Pfitzinger I., Plückthun A. The functional expression of antibody Fv fragments in Escherichia coli: improved vectors and a generally applicable purification technique. Biotechnology (N Y) 1991 Mar;9(3):273–278. doi: 10.1038/nbt0391-273. [DOI] [PubMed] [Google Scholar]
- Stoll V. S., Blanchard J. S. Buffers: principles and practice. Methods Enzymol. 1990;182:24–38. doi: 10.1016/0076-6879(90)82006-n. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Imahori K. Phosphoglycerate kinase of Bacillus stearothermophilus. J Biochem. 1974 Oct;76(4):771–782. [PubMed] [Google Scholar]
- Tomschy A., Glockshuber R., Jaenicke R. Functional expression of D-glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic eubacterium Thermotoga maritima in Escherichia coli. Authenticity and kinetic properties of the recombinant enzyme. Eur J Biochem. 1993 May 15;214(1):43–50. doi: 10.1111/j.1432-1033.1993.tb17894.x. [DOI] [PubMed] [Google Scholar]
- Wagner L. A., Weiss R. B., Driscoll R., Dunn D. S., Gesteland R. F. Transcriptional slippage occurs during elongation at runs of adenine or thymine in Escherichia coli. Nucleic Acids Res. 1990 Jun 25;18(12):3529–3535. doi: 10.1093/nar/18.12.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. C., Littlechild J. A. Isoenzymes of phosphoglycerate kinase: evolutionary conservation of the structure of this glycolytic enzyme. Biochem Soc Trans. 1990 Apr;18(2):187–190. doi: 10.1042/bst0180187. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Noble M. E., Davenport R. C. Comparison of the refined crystal structures of liganded and unliganded chicken, yeast and trypanosomal triosephosphate isomerase. J Mol Biol. 1992 Apr 20;224(4):1115–1126. doi: 10.1016/0022-2836(92)90473-w. [DOI] [PubMed] [Google Scholar]
- Wrba A., Schweiger A., Schultes V., Jaenicke R., Závodszky P. Extremely thermostable D-glyceraldehyde-3-phosphate dehydrogenase from the eubacterium Thermotoga maritima. Biochemistry. 1990 Aug 21;29(33):7584–7592. doi: 10.1021/bi00485a007. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]





