Abstract
Induction of apoptosis in lymphocytes, which may account for the therapeutic effects of glucocorticoids in various diseases including leukemia, depends on the glucocorticoid receptor. However, the events leading from the activated receptor to cell lysis are not understood. A prevailing hypothesis postulates induction of so-called 'lysis genes' by the activated receptor. In this study, we show that an activation-deficient glucocorticoid receptor mutant is as effective as the wild-type receptor in repression of AP-1 activity, inhibition of interleukin-2 production, inhibition of c-myc expression and induction of apoptosis. Furthermore, we show that retinoic acid can also induce apoptosis in these cells through the retinoic acid receptor, whose repressive functions but not target site specificity, are similar to those of the glucocorticoid receptor. Therefore, the primary effect of the receptor in glucocorticoid-mediated apoptosis correlates with transcriptional repression rather than activation and could be mediated by interference with other transcription factors required for cell survival.
Full text
PDF
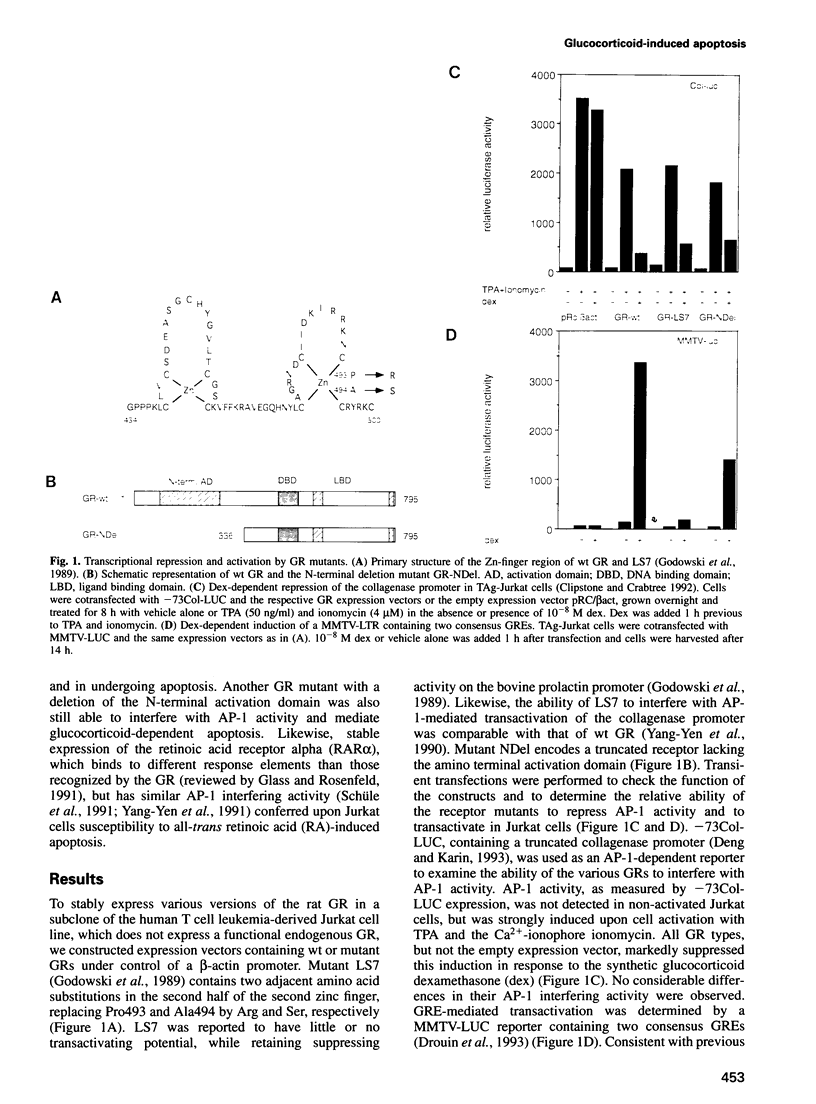



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alles A. J., Sulik K. K. Retinoic acid-induced spina bifida: evidence for a pathogenetic mechanism. Development. 1990 Jan;108(1):73–81. doi: 10.1242/dev.108.Supplement.73. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Wong-Staal F., Gallo R. C. Dexamethasone-mediated inhibition of human T cell growth factor and gamma-interferon messenger RNA. J Immunol. 1984 Jul;133(1):273–276. [PubMed] [Google Scholar]
- Baxter J. D., Harris A. W., Tomkins G. M., Cohn M. Glucocorticoid receptors in lymphoma cells in culture: relationship to glucocorticoid killing activity. Science. 1971 Jan 15;171(3967):189–191. doi: 10.1126/science.171.3967.189. [DOI] [PubMed] [Google Scholar]
- Bazar L. S., Deeg H. J. Ultraviolet B-induced DNA fragmentation (apoptosis) in activated T-lymphocytes and Jurkat cells is augmented by inhibition of RNA and protein synthesis. Exp Hematol. 1992 Jan;20(1):80–86. [PubMed] [Google Scholar]
- Bourgeois S., Newby R. F. Diploid and haploid states of the glucocorticoid receptor gene of mouse lymphoid cell lines. Cell. 1977 Jun;11(2):423–430. doi: 10.1016/0092-8674(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Caron-Leslie L. M., Schwartzman R. A., Gaido M. L., Compton M. M., Cidlowski J. A. Identification and characterization of glucocorticoid-regulated nuclease(s) in lymphoid cells undergoing apoptosis. J Steroid Biochem Mol Biol. 1991;40(4-6):661–671. doi: 10.1016/0960-0760(91)90288-g. [DOI] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Deng T., Karin M. JunB differs from c-Jun in its DNA-binding and dimerization domains, and represses c-Jun by formation of inactive heterodimers. Genes Dev. 1993 Mar;7(3):479–490. doi: 10.1101/gad.7.3.479. [DOI] [PubMed] [Google Scholar]
- Dieken E. S., Miesfeld R. L. Transcriptional transactivation functions localized to the glucocorticoid receptor N terminus are necessary for steroid induction of lymphocyte apoptosis. Mol Cell Biol. 1992 Feb;12(2):589–597. doi: 10.1128/mcb.12.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd D. R., Miesfeld R. L. Evidence that glucocorticoid- and cyclic AMP-induced apoptotic pathways in lymphocytes share distal events. Mol Cell Biol. 1992 Aug;12(8):3600–3608. doi: 10.1128/mcb.12.8.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J., Sun Y. L., Chamberland M., Gauthier Y., De Léan A., Nemer M., Schmidt T. J. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO J. 1993 Jan;12(1):145–156. doi: 10.1002/j.1460-2075.1993.tb05640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Galili U. Glucocorticoid induced cytolysis of human normal and malignant lymphocytes. J Steroid Biochem. 1983 Jul;19(1B):483–490. doi: 10.1016/0022-4731(83)90207-8. [DOI] [PubMed] [Google Scholar]
- Gasson J. C., Bourgeois S. A new determinant of glucocorticoid sensitivity in lymphoid cell lines. J Cell Biol. 1983 Feb;96(2):409–415. doi: 10.1083/jcb.96.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol. 1979 Oct;123(4):1624–1631. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Goya L., Maiyar A. C., Ge Y., Firestone G. L. Glucocorticoids induce a G1/G0 cell cycle arrest of Con8 rat mammary tumor cells that is synchronously reversed by steroid withdrawal or addition of transforming growth factor-alpha. Mol Endocrinol. 1993 Sep;7(9):1121–1132. doi: 10.1210/mend.7.9.8247014. [DOI] [PubMed] [Google Scholar]
- Harbour D. V., Chambon P., Thompson E. B. Steroid mediated lysis of lymphoblasts requires the DNA binding region of the steroid hormone receptor. J Steroid Biochem. 1990 Jan;35(1):1–9. doi: 10.1016/0022-4731(90)90137-h. [DOI] [PubMed] [Google Scholar]
- Harmon J. M., Norman M. R., Fowlkes B. J., Thompson E. B. Dexamethasone induces irreversible G1 arrest and death of a human lymphoid cell line. J Cell Physiol. 1979 Feb;98(2):267–278. doi: 10.1002/jcp.1040980203. [DOI] [PubMed] [Google Scholar]
- Hay N., Takimoto M., Bishop J. M. A FOS protein is present in a complex that binds a negative regulator of MYC. Genes Dev. 1989 Mar;3(3):293–303. doi: 10.1101/gad.3.3.293. [DOI] [PubMed] [Google Scholar]
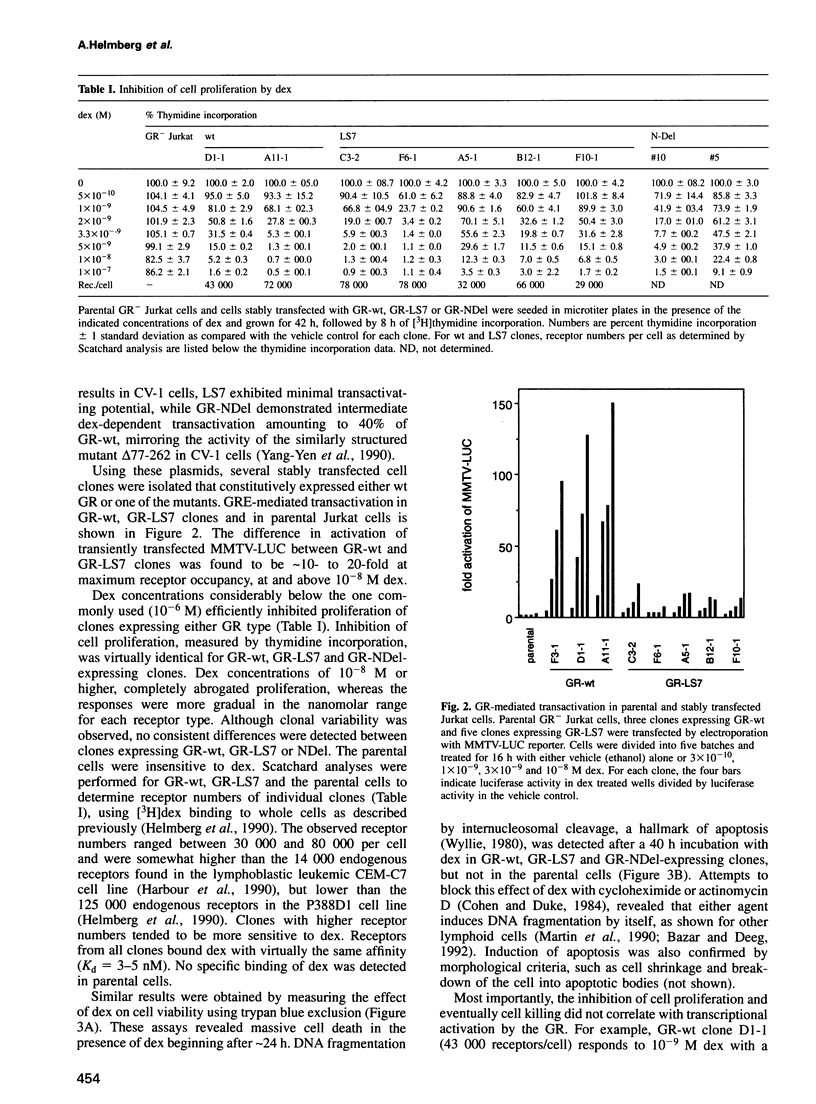
- Helmberg A., Fässler R., Geley S., Jöhrer K., Kroemer G., Böck G., Kofler R. Glucocorticoid-regulated gene expression in the immune system. Analysis of glucocorticoid-regulated transcripts from the mouse macrophage-like cell line P388D1. J Immunol. 1990 Dec 15;145(12):4332–4337. [PubMed] [Google Scholar]
- Hollenberg S. M., Evans R. M. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988 Dec 2;55(5):899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Jain J., Valge-Archer V. E., Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992 Feb 15;148(4):1240–1250. [PubMed] [Google Scholar]
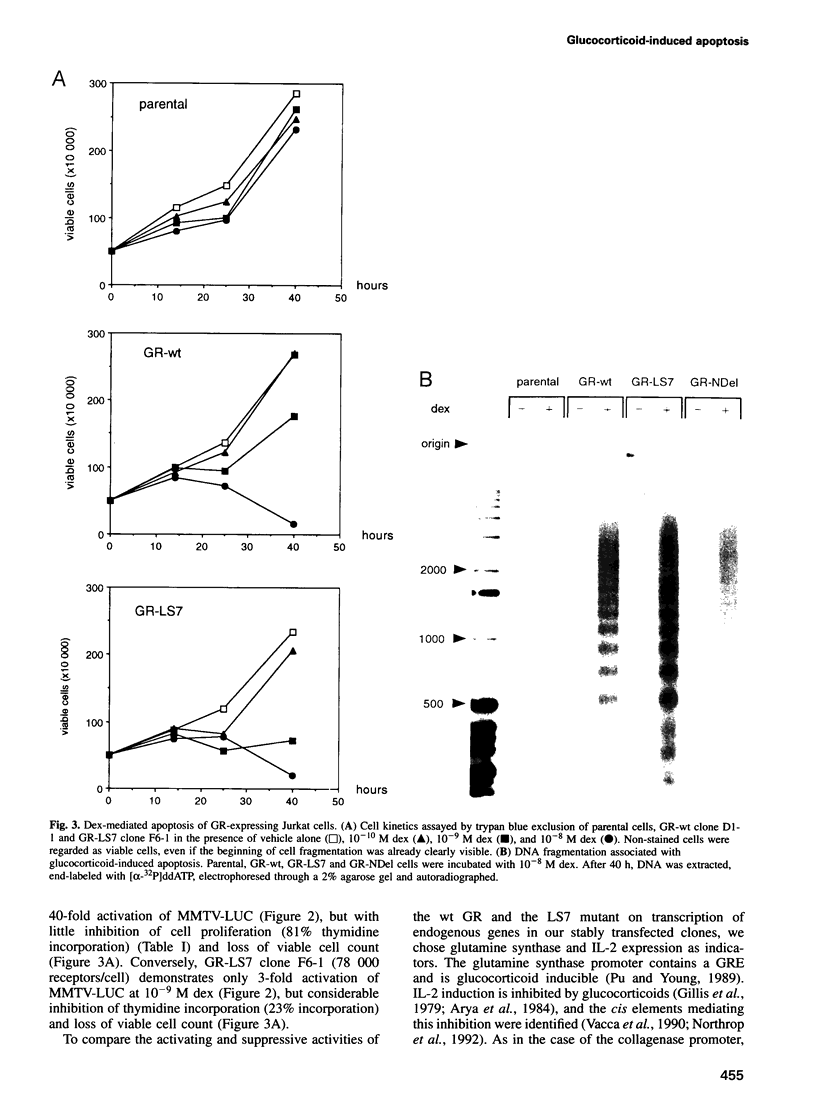
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kizaki M., Ikeda Y., Tanosaki R., Nakajima H., Morikawa M., Sakashita A., Koeffler H. P. Effects of novel retinoic acid compound, 9-cis-retinoic acid, on proliferation, differentiation, and expression of retinoic acid receptor-alpha and retinoid X receptor-alpha RNA by HL-60 cells. Blood. 1993 Dec 15;82(12):3592–3599. [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
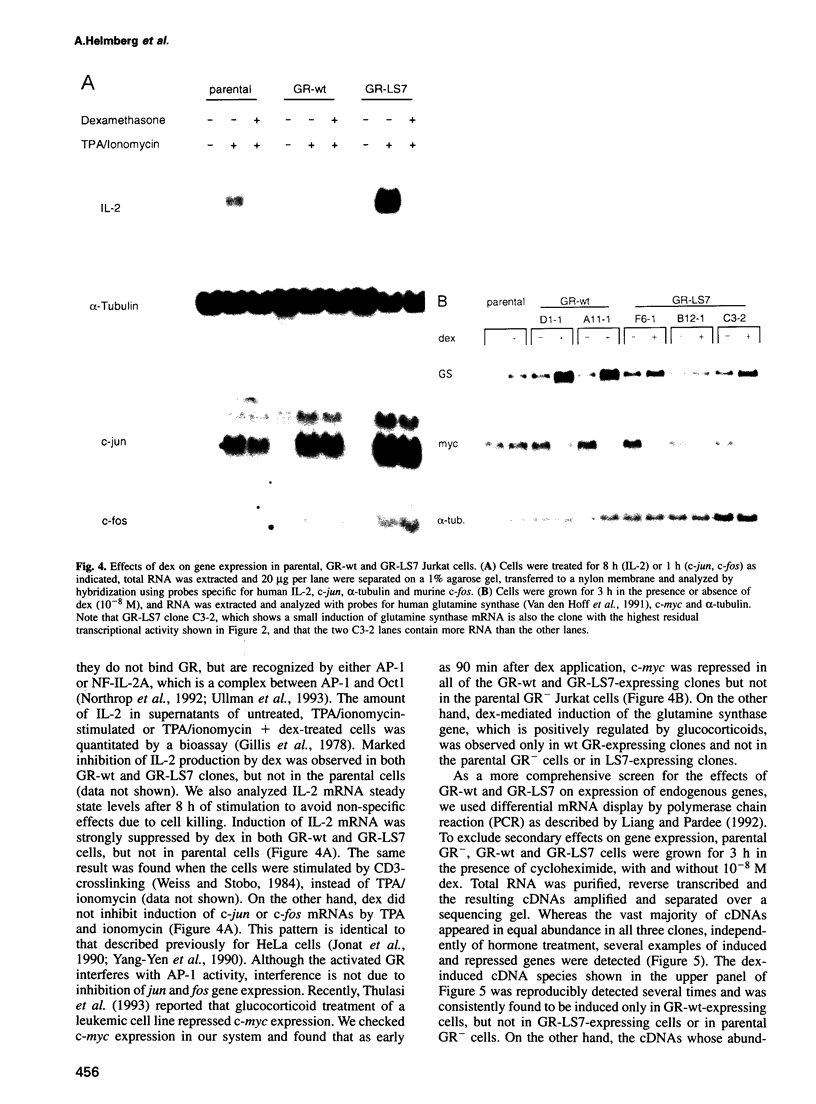
- Martin S. J., Lennon S. V., Bonham A. M., Cotter T. G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990 Sep 15;145(6):1859–1867. [PubMed] [Google Scholar]
- Miyazaki J., Takaki S., Araki K., Tashiro F., Tominaga A., Takatsu K., Yamamura K. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989 Jul 15;79(2):269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- Nazareth L. V., Harbour D. V., Thompson E. B. Mapping the human glucocorticoid receptor for leukemic cell death. J Biol Chem. 1991 Jul 15;266(20):12976–12980. [PubMed] [Google Scholar]
- Norman M. R., Thompson E. B. Characterization of a glucocorticoid-sensitive human lymphoid cell line. Cancer Res. 1977 Oct;37(10):3785–3791. [PubMed] [Google Scholar]
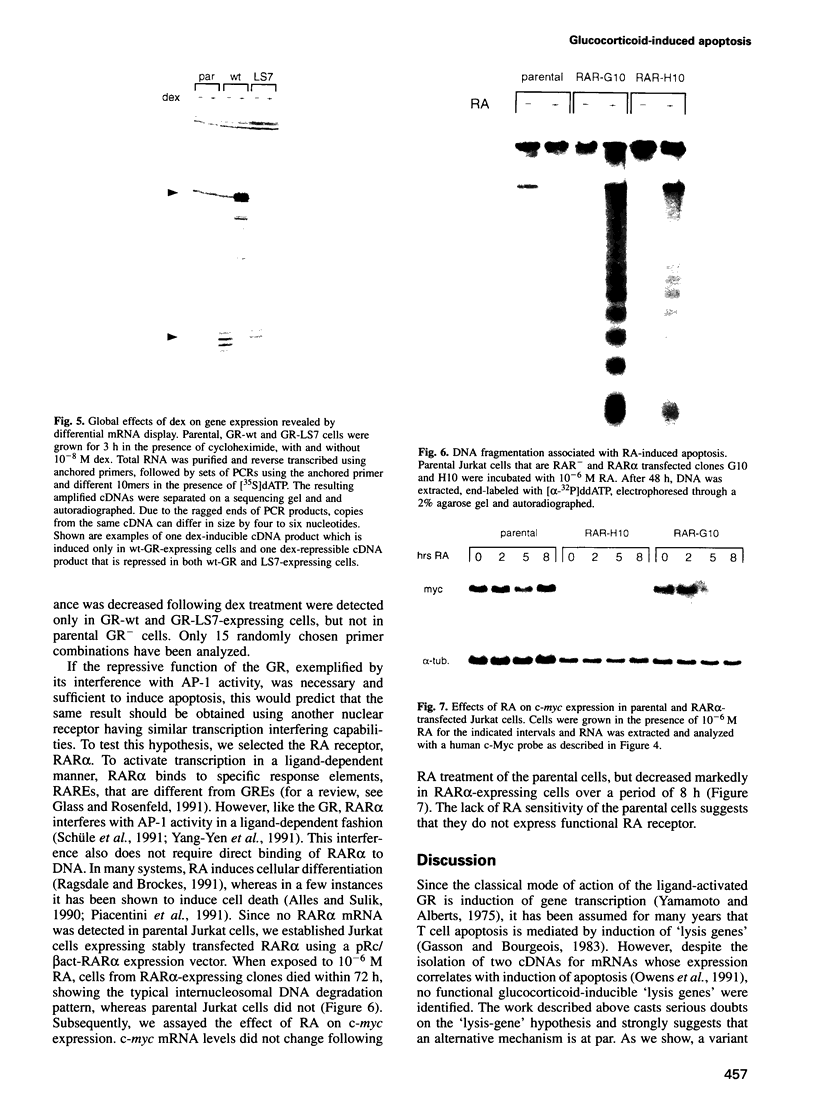
- Northrop J. P., Crabtree G. R., Mattila P. S. Negative regulation of interleukin 2 transcription by the glucocorticoid receptor. J Exp Med. 1992 May 1;175(5):1235–1245. doi: 10.1084/jem.175.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Banion M. K., Levenson R. M., Brinckmann U. G., Young D. A. Glucocorticoid modulation of transformed cell proliferation is oncogene specific and correlates with effects on c-myc levels. Mol Endocrinol. 1992 Sep;6(9):1371–1380. doi: 10.1210/mend.6.9.1331773. [DOI] [PubMed] [Google Scholar]
- Owens G. P., Hahn W. E., Cohen J. J. Identification of mRNAs associated with programmed cell death in immature thymocytes. Mol Cell Biol. 1991 Aug;11(8):4177–4188. doi: 10.1128/mcb.11.8.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrillo J. E., Fauci A. S. Mechanisms of glucocorticoid action on immune processes. Annu Rev Pharmacol Toxicol. 1979;19:179–201. doi: 10.1146/annurev.pa.19.040179.001143. [DOI] [PubMed] [Google Scholar]
- Piacentini M., Fesus L., Farrace M. G., Ghibelli L., Piredda L., Melino G. The expression of "tissue" transglutaminase in two human cancer cell lines is related with the programmed cell death (apoptosis). Eur J Cell Biol. 1991 Apr;54(2):246–254. [PubMed] [Google Scholar]
- Pu H. F., Young A. P. The structure of the chicken glutamine synthetase-encoding gene. Gene. 1989 Sep 1;81(1):169–175. doi: 10.1016/0378-1119(89)90348-x. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Yang N., Kliewer S., Ransone L. J., Bolado J., Verma I. M., Evans R. M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Glynn J. M., Guilbert L. J., Cotter T. G., Bissonnette R. P., Green D. R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992 Jul 10;257(5067):212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Ninomiya-Tsuji J., Matsumoto K., Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992 Jul 10;70(1):57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Isolation of lymphoma cell variants resistant to killing by glucocorticoids. Cell. 1974 Aug;2(4):213–220. doi: 10.1016/0092-8674(74)90013-0. [DOI] [PubMed] [Google Scholar]
- Su B., Jacinto E., Hibi M., Kallunki T., Karin M., Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994 Jun 3;77(5):727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Sánchez I., Goya L., Vallerga A. K., Firestone G. L. Glucocorticoids reversibly arrest rat hepatoma cell growth by inducing an early G1 block in cell cycle progression. Cell Growth Differ. 1993 Mar;4(3):215–225. [PubMed] [Google Scholar]
- Thulasi R., Harbour D. V., Thompson E. B. Suppression of c-myc is a critical step in glucocorticoid-induced human leukemic cell lysis. J Biol Chem. 1993 Aug 25;268(24):18306–18312. [PubMed] [Google Scholar]
- Tilly J. L., Hsueh A. J. Microscale autoradiographic method for the qualitative and quantitative analysis of apoptotic DNA fragmentation. J Cell Physiol. 1993 Mar;154(3):519–526. doi: 10.1002/jcp.1041540310. [DOI] [PubMed] [Google Scholar]
- Ucker D. S. Cytotoxic T lymphocytes and glucocorticoids activate an endogenous suicide process in target cells. Nature. 1987 May 7;327(6117):62–64. doi: 10.1038/327062a0. [DOI] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Admon A., Crabtree G. R. Jun family members are controlled by a calcium-regulated, cyclosporin A-sensitive signaling pathway in activated T lymphocytes. Genes Dev. 1993 Feb;7(2):188–196. doi: 10.1101/gad.7.2.188. [DOI] [PubMed] [Google Scholar]
- Vacca A., Martinotti S., Screpanti I., Maroder M., Felli M. P., Farina A. R., Gismondi A., Santoni A., Frati L., Gulino A. Transcriptional regulation of the interleukin 2 gene by glucocorticoid hormones. Role of steroid receptor and antigen-responsive 5'-flanking sequences. J Biol Chem. 1990 May 15;265(14):8075–8080. [PubMed] [Google Scholar]
- Van den Hoff M. J., Geerts W. J., Das A. T., Moorman A. F., Lamers W. H. cDNA sequence of the long mRNA for human glutamine synthase. Biochim Biophys Acta. 1991 Oct 8;1090(2):249–251. doi: 10.1016/0167-4781(91)90111-x. [DOI] [PubMed] [Google Scholar]
- Weiss A., Stobo J. D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984 Nov 1;160(5):1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S., Döbbeling U., Rusconi S. Interference and synergism of glucocorticoid receptor and octamer factors. EMBO J. 1991 Sep;10(9):2513–2521. doi: 10.1002/j.1460-2075.1991.tb07791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Zhang X. K., Graupner G., Tzukerman M., Sakamoto B., Karin M., Pfahl M. Antagonism between retinoic acid receptors and AP-1: implications for tumor promotion and inflammation. New Biol. 1991 Dec;3(12):1206–1219. [PubMed] [Google Scholar]