Abstract
Background:
In the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) trial, tolvaptan significantly reduced expansion of kidney volume and loss of kidney function.
Objective:
To determine how benefits observed in the TEMPO trial might relate to longer-term health outcomes such as progression to end-stage renal disease (ESRD) and mortality in addition to its cost-effectiveness.
Design:
A decision-analytic model.
Data Sources:
Published literature.
Target Population:
Persons with early Autosomal Dominant Polycystic Kidney Disease (ADPKD).
Time Horizon:
Lifetime.
Perspective:
Societal.
Interventions:
We compared a strategy where patients receive tolvaptan therapy until death, development of ESRD, or liver complications to one where they do not receive tolvaptan.
Outcome Measures:
Median age at ESRD onset, life expectancy, discounted quality-adjusted life years (QALYs) and lifetime costs (in 2010 USD), and incremental cost-effectiveness ratios.
Results of Base Case Analysis:
Tolvaptan prolonged the median age at ESRD onset by 6.5 years and increased life expectancy by 2.6 years. At a drug cost of $5,760 per month, tolvaptan cost $744,100 per QALY gained compared to standard care.
Results of Sensitivity Analysis:
For patients with ADPKD progressing more slowly, tolvaptan’s cost per QALY gained was even higher.
Limitations:
Although the TEMPO trial followed patients for 3 years, our main analysis assumed that the clinical benefits of tolvaptan persisted over patients’ lifetimes.
Conclusions and Relevance:
Assuming that tolvaptan’s benefits persist longer term, the drug may slow progression to ESRD and reduce mortality. However, barring an approximately 95% reduction in the price of tolvaptan, its cost-effectiveness does not compare favorably with many other commonly accepted medical interventions.
Keywords: autosomal dominant polycystic kidney disease, tolvaptan, cost-effectiveness
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) occurs in approximately 1 in every 1000 individuals and comprises 5% of the end-stage renal disease (ESRD) population in the United States.(1, 2) Patients with ADPKD suffer from progressive enlargement of intra-renal cysts and decline in glomerular filtration rate (GFR), generally leading to ESRD in the sixth or seventh decade.(3, 4) Unfortunately, therapeutic strategies that slow progression to ESRD in patients with diabetes-related and other forms of glomerular disease have proved unsuccessful at slowing progression among patients with ADPKD.(5, 6)
Recently, the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) trial compared an anti-diuretic hormone antagonist – tolvaptan – to placebo for reduction of total kidney volume growth and preservation of kidney function in patients with ADPKD.(7) In this randomized controlled trial, tolvaptan administered twice daily significantly reduced growth in kidney volume and reduced the rate of estimated glomerular filtration rate (eGFR) decline over a 3-year period. It is unknown how these effects might translate into longer-term health benefits, whether treating patients with tolvaptan might improve health at an acceptable cost, and how clinical benefits might vary if tolvaptan were prescribed outside of a carefully selected clinical trial population.
We used a decision-analytic model to determine the expected benefit from tolvaptan over the lifetime of patients with ADPKD. We focused on tolvaptan therapy’s effect on patient-oriented outcomes such as progression to ESRD and mortality. Additionally, we assessed the cost-effectiveness of tolvaptan in different populations with ADPKD and considered a variety of alternative assumptions when extrapolating short-term trial results to a chronic condition.
Methods
Decision-analytic model
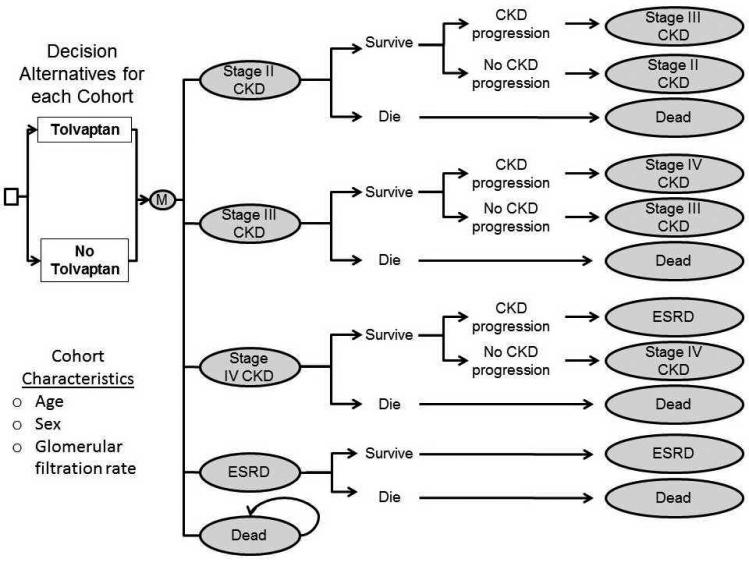
We developed a Markov model of CKD to evaluate tolvaptan therapy for slowing the rate of loss of kidney function in the following cohorts:
1) The base case – 40 year-old men and women with early ADPKD, defined by an eGFR of 80ml/min/1.73m2.
2) Additional cohorts of men and women who might be prescribed tolvaptan in clinical practice defined by: a) age and b) eGFR at initiation of tolvaptan; and c) rate of eGFR decline without tolvaptan.
The starting age and eGFR for the base case were selected to approximate the TEMPO trial’s study population and were similar to a large observation trial used for model validation.(4) The model simulated patients over their lifetimes in 3-month intervals. Individuals progress through CKD stages 2 (eGFR: 60-89 ml/min/1.73m2), 3a (eGFR: 45-59 ml/min/1.73m2), 3b (eGFR: 30-44 ml/min/1.73m2), 4 (eGFR: 15-29 ml/min/1.73m2) and 5 (eGFR: <15 ml/min/1.73m2). (Figure 1) Costs, health-related quality-of-life, and mortality rates varied by CKD stage. Mortality rates at different CKD stages for patients with ADPKD were derived from studies of the general CKD population. Once patients progress to stage 5 CKD, we assumed they experience costs equal to the averages of similarly-aged U.S. patients with ESRD. We assumed mortality rates in stage 5 CKD were equal to those of similarly-aged U.S. patients with ESRD adjusted to account for lower mortality in ESRD among patients with ADPKD.(8) Modeled outcomes included median age at ESRD onset, life years, quality-adjusted life years (QALYs), direct healthcare costs in 2010 US dollars and incremental cost-effectiveness ratios (ICERs) discounted at 3% annually.(9, 10) We report costs and ICERs rounded to the nearest $100. The analysis adopted a societal perspective.
Figure 1. Decision Analytic Model Design.
Schematic of the Markov model of kidney disease. Stage 3 chronic kidney disease (CKD) was subdivided into stages 3a and 3b (not shown in the schematic). We used SAS 9.2 (SAS Institute Inc.) in our microsimulation to convert eGFR progression to CKD stage progression in autosomal dominant polycystic kidney disease (ADPKD) and then used TreeAge Pro 2009 (TreeAge Software Inc.) to perform cost-effectiveness analyses. “ESRD” is end-stage renal disease.
Model inputs, including those from the TEMPO trial, were derived from published literature. (Table 1) The TEMPO trial included a subset of the ADPKD population with total kidney volume exceeding 750 ml (assessed by MRI) and a creatinine clearance > 60 ml/min/1.73m2 (excluding 25% of patients assessed for eligibility). In the base case, the rate of eGFR decline without tolvaptan was that observed in the placebo arm of the TEMPO trial.(7) Variability in eGFR decline was derived from a large observational cohort of patients with ADPKD.(4) We undertook a separate microsimulation to convert annual rates of progression into probabilities of transitioning among CKD stages. (Supplement; Modeling CKD Progression) Mortality rates for patients in each CKD stage were calculated by multiplying CKD stage-specific mortality hazards by age- and sex-specific U.S. life table mortality rates.(11, 12) We compared the age of ESRD predicted from our model to two observational cohorts of patients with ADPKD to assess its face validity. (Supplement; Model Validation)
Table 1.
Model Inputs
| Intervention Parameters: | (Value) | (Range) | |
| Percent reduction in rate of CKD progression from tolvaptan(7)† | 26.0 | 16.0 | 77.0 |
| Change in quality of life due to tolvaptan - QALY(7) | 1.00 | 0.96 | 1.04 |
| Cost of tolvaptan per month*(18) | 5760 | 0.00 | 18,240 |
| Proportion of patients who discontinue tolvaptan(7) | 0.15 | 0.10 | 0.20 |
| Annual cost of laboratory tests and monitoring*(19,20) | 62.8 | (+−20%) | |
| Natural History Parameters: | (Value) | (Range) | |
| Age-based probability of death in healthy individuals(12) | (U.S. Life tables) | (+−10%) | |
| Increased risk of death (CKD stage 3a) - hazard ratio(l1)† | 1.20 | 1.10 | 1.30 |
| Increased risk of death (CKD stage 3b) - hazard ratio(l1)† | 1.80 | 1.70 | 1.90 |
| Increased risk of death (CKD stage 4) - hazard ratio(l1)† | 3.20 | 3.00 | 3.40 |
| Probability of death from ESRD(1, 8) | (see Supplement) | (+−20%) | |
| Baseline rate of CKD progression (ml/min/1.73m2yr)(7) | 3.70 | 2.00 | 4.40 |
| CKD-Related Quality of life assumptions | (QALY) | (Range) | |
| QALY - CKD stage 3a(15, 16) | 0.88 | 0.78 | 0.98 |
| QALY - CKD stage 3b(15, 16) | 0.86 | 0.76 | 0.96 |
| QALY - CKD Stage 4(15,16) | 0.85 | 0.75 | 0.95 |
| QALY - ESRD(15, 16) | 0.77 | 0.67 | 0.87 |
| CKD-Related Cost assumptions* | ($) | (Range) | |
| Annual added cost of Stage 3a CKD(17) | 1,833 | (50%-200%) | |
| Annual added cost of Stage 3b CKD(17) | 4507 | (50%-200%) | |
| Annual added cost of Stage 4 CKD(17) | 5,844 | (50%-200%) | |
| Cost of ESRD*(l) | (see Supplement) | (50%-200%) | |
range in sensitivity analysis obtained from 95% confidence intervals reported in literature.
costs are in 2010 USD.
Note: Quality-of-life assumed to decrease as patients age and differs by sex as observed in the Beaver Dam Study (see appendix).(13) Decreases in health-related quality-of-life were applied multiplicatively for patients with chronic kidney disease (CKD) stages 3a, 3b, 4, and end-stage renal disease (ESRD) according to published time tradeoff estimates that adjusted for age, gender, and diabetes.(16) Patients with stage 2 CKD are assumed to have similar costs and quality of life as the general population. Costs included background medical costs. (see Supplement) Costs for CKD stages 3a, 3b and 4 were added to stage 2 costs. ESRD costs and mortality stratified by age and ESRD duration are derived from national averages published by USRDS (27) “QALY” is quality-adjusted life year.
Benefits and Adverse Effects from Tolvaptan
In TEMPO, tolvaptan significantly reduced the rate of eGFR decline. We used the observed difference in the rate of eGFR decline from the TEMPO trial (–2.72 versus –3.70 ml/min/1.73m2 in patients randomized to tolvaptan and placebo, respectively, corresponding to a 26% relative reduction).(7) Since the observed difference derives from an intention-to-treat analysis, it represents the average decline in eGFR of patients who took tolvaptan for the entire 3 years and of those who discontinued therapy. Though TEMPO included 3-year follow-up, we assumed the attenuation in eGFR decline persisted until patients developed ESRD and that patients taking tolvaptan after 3 years continued doing so until the development of ESRD.
In TEMPO, patients receiving tolvaptan experienced increased incidence of side effects including thirst, polyuria, and elevated hepatic enzymes. Some also experienced reduced kidney pain. It is unknown whether these effects produced a net increase or decrease in the average health-related quality-of-life. In the base case, we assumed that side effects and pain-reducing treatment benefits offset each other such that tolvaptan only modified health-related quality-of-life through attenuating eGFR decline.
Costs and Quality-of-Life
Costs and health-related quality-of-life varied according to age based on non-CKD populations.(13, 14) (Supplement; Selected Model Assumptions) Patients with stage 2 CKD and ADPKD were assumed to have the same health-related quality-of-life as healthy individuals, consistent with patient surveys.(15) After progression to CKD stage 3a, we added separate cost increases and quality-of-life decrements for each CKD stage derived from the general CKD population.(16, 17)
Tolvaptan costs included medication, laboratory, and clinical follow-up.(18-20) Due to the observed elevation in liver enzymes in patients receiving tolvaptan during TEMPO, we assumed in the base case that liver enzymes are monitored twice yearly in patients taking tolvaptan. We used a factor of 0.64 to convert average wholesale prices to lowest prices, consistent with Congressional Budget Office estimates.(21) Because tolvaptan is not yet sold for ADPKD treatment, we assumed in the base case that 95mg of tolvaptan (the average TEMPO trial dose) would be offered at the same daily price as the 30mg dose currently approved for hyponatremia treatment. This represents a 68% discount in cost per milligram, a conservative assumption biasing the analysis in favor of tolvaptan. Additionally, costs in our model accounted for the assumption that 15.4% of patients originally placed on tolvaptan would discontinue therapy, consistent with the TEMPO trial.
Sensitivity Analyses
One-way sensitivity analyses varied all model inputs. Probabilistic sensitivity analyses evaluated how the simultaneous uncertainties about model parameters might influence outcomes. We also examined several alternative treatment scenarios. First, we explored cost-effectiveness at varying tolvaptan price discounts focusing on commonly used willingness to pay thresholds of $50,000 and $100,000 per QALY gained. Then, we explored the cost-effectiveness under scenarios where 1) patients only take tolvaptan for 3 years; 2) patients continue to take tolvaptan beyond 3 years with a discontinuation rate observed in the TEMPO trial; (Supplement; Modeling Tolvaptan Discontinuation) 3) efficacy of tolvaptan wanes at a rate of 5% every year; 4) heterogeneity in treatment effect. (Supplement; Additional Sensitivity Analyses)
The National Institutes of Health and Agency for Healthcare Research and Quality were not involved in the design, conduct, and analysis of this study or in the decision to submit the manuscript for publication.
Reproducible Research Statement: Study Protocol: Available from Dr. Erickson (kevine1@stanford.edu). Statistical Code: Not available. Data Set: Available from Dr. Erickson.
Results
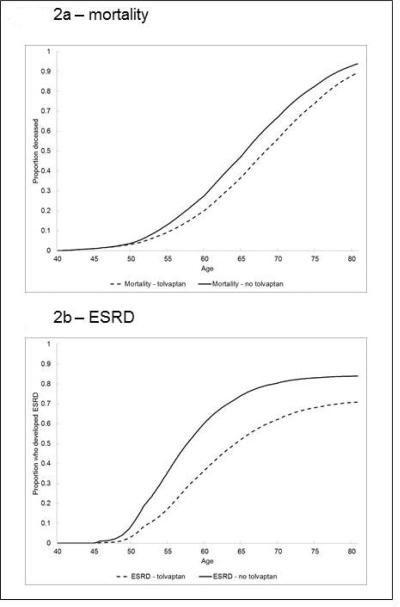
Initiating therapy with tolvaptan in 40 year-olds with ADPKD and eGFR of 80 ml/min/1.73m2 delayed the median age of developing ESRD by 6.3 years in women and 6.8 years in men. Tolvaptan therapy yielded an average increase in life expectancy of 2.8 years in women and 2.3 years in men. (Table 2; Figure 2) Therapy with tolvaptan yielded an increase of 1.2 discounted QALYs in women and 1.1 discounted QALYs in men. Total lifetime medical costs were substantially higher in patients receiving tolvaptan ($858,300 higher in women and $830,100 higher in men). Combining health benefits and costs, for this patient group, tolvaptan therapy cost $720,600 per QALY gained in women and $769,500 in men. In a balanced cohort of women and men, tolvaptan increased median time to ESRD by 6.5 years, increased life expectancy by 2.6 years, and cost $744,100 per QALY gained. (Table 2)
Table 2.
Results of Base Case Analysis
| Cost ($) | QALYs (discounted) |
Life Years (undiscounted) |
Mean Age at Death |
Median Age of Developing ESRD |
ICER ($/QALY) |
|
|---|---|---|---|---|---|---|
| Men: | ||||||
| Tolvaptan | 1,207,500 | 15.3 | 27.7 | 67.7 | 64.8 | |
| No Tolvaptan | 377,400 | 14.2 | 25.4 | 65.4 | 58.0 | |
| Difference | 830,100 | 1.1 | 2.3 | 6.8 | 769,500 | |
| Women: | ||||||
| Tolvaptan | 1,255,400 | 15.3 | 29.1 | 69.1 | 63.8 | |
| No Tolvaptan | 397,100 | 14.1 | 26.3 | 66.3 | 57.5 | |
| Difference | 858,300 | 1.2 | 2.8 | 6.3 | 720,600 | |
| Pooled: | ||||||
| Tolvaptan | 1,231,400 | 15.3 | 28.4 | 68.4 | 64.3 | |
| No Tolvaptan | 387,200 | 14.2 | 25.9 | 65.9 | 57.8 | |
| Difference | 844,200 | 1.1 | 2.6 | 6.5 | 744,100 |
Note: “Pooled” is a cohort consisting of 50% men and 50% women. Costs are rounded to nearest $100. Because of rounding, incremental cost-effectiveness ratios (ICERs) and pooled life years may be different than is suggested by illustrated costs, quality-adjusted life years (QALYs) and life years. “ESRD” is end-stage renal disease.
Figure 2. Simulated Mortality and Age of End-Stage Renal Disease (ESRD) Onset With and Without Tolvaptan.
Tolvaptan therapy prolongs median age to development of ESRD by 6.5 years and extends life by an average of 2.6 years.
Cost-effectiveness in Additional Patient Cohorts
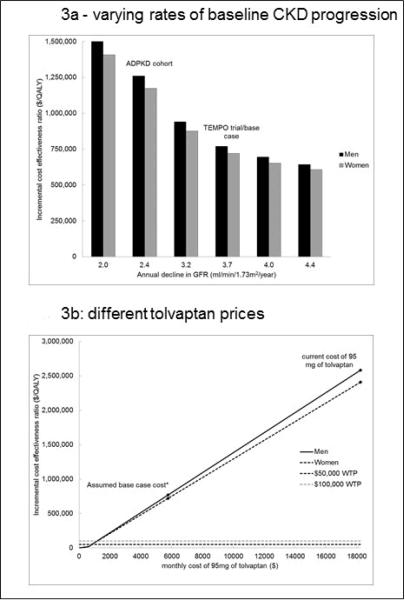
Tolvaptan therapy was less cost-effective when given to patients with slower rates of eGFR decline. (Figure 3a) When tolvaptan is given to patients with a rate of eGFR decline of 2.4 ml/min/1.73m2/year (the rate observed in a large cohort of ADPKD patients(3)) tolvaptan cost $1,215,200 per QALY gained. Because, for a given eGFR when starting tolvaptan, older patients are more likely to die from other causes before experiencing CKD progression, tolvaptan was less cost-effective in older patients. For instance, tolvaptan cost 54% more per QALY gained in 65 year-olds ($1,147,800 per QALY gained) compared to 40 year-olds. (Supplement Figure 1). The cost per QALY gained also depended on the eGFR at which tolvaptan was begun. The cost per QALY gained in 40 year-olds was 16% lower when tolvaptan is started at an eGFR of 75 ml/min/1.73m2 ($626,938 per QALY) compared to the base case with a starting eGFR of 80 ml/min/1.73m2. (Supplement Figure 2)
Figure 3. Cost-Effectiveness under Different Model Assumptions.
Tolvaptan is less cost-effective with slower rates of baseline kidney disease progression. Tolvaptan cost less than $100,000 per quality-adjusted life year (QALY) gained if 95 mg per day is offered at or below $1,155 per month (approximately where the dotted $100,000 WTP line crosses the lines for men and women). The decline in estimated glomerular filtration rate (eGFR) from a cohort of patients with autosomal dominant polycystic kidney disease (ADPKD) was –2.4 ml/min/1.73m2/year.(3) Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) trial/base case decline in eGFR of –3.7 ml/min/1.73m2/year observed in placebo group of the TEMPO trial.(7) “WTP” is willingness to pay. The horizontal $50,000 and $100,000 WTP lines represent societal willingness to pay thresholds – the amount of money society would be willing to pay to increase quality-adjusted life expectancy by one year. Assumed base case cost of $5,760 per month for 95mg of tolvaptan equals the current cost of 30mg tablets. Cost of 95 mg of tolvaptan is $18,240 based on current cost of 30mg tablets.
Cost of Tolvaptan
Tolvaptan therapy would be more cost-effective if it were offered at a lower price. At a willingness to pay threshold of $100,000 per QALY gained, therapy with tolvaptan would be cost-effective in both men and women if offered at or below $1,155 per month (80% below the base case price and 94% below the current price per milligram). At a willingness to pay threshold of $50,000 per QALY gained, therapy with tolvaptan would be cost-effective in both men and women if offered at or below $805 per month (86% below the base case price and 96% below the current price per milligram). (Figure 3b)
Sensitivity Analyses
While the cost per QALY of therapy with tolvaptan depended most on the reduction in the rate of eGFR decline and changes in health-related quality-of-life associated with treatment, it remained above $500,000 per QALY gained across all uncertainty ranges. With a 37% reduction in the rate of eGFR decline – the largest treatment effect within the TEMPO trial’s 95% confidence interval – tolvaptan therapy cost $517,400 per QALY gained. (Supplement Figure 3) If tolvaptan therapy resulted in a 4% increase in quality of life from reduced kidney pain, it cost $532,300 per QALY gained. (Supplement Figure 4) The cost per QALY gained was less sensitive to the other model parameters. (Supplement Figures 5-6)
Probabilistic sensitivity analyses considered the simultaneous uncertainty of all model parameters. In women, the cost per QALY gained exceeded $392,500 in 99% of 10,000 simulations, while in men the cost per QALY gained exceeded $398,300 in 99% of 10,000 simulations. (Supplement Figures 7-8) In a second set of probabilistic sensitivity analyses, we considered simultaneous model parameter uncertainty if tolvaptan were available at an average of $576 per month, which represents a 90% reduction from the base case price and a 97% reduction from the current price per milligram. At this price, tolvaptan therapy cost less than $50,000 per QALY gained in 78% and 79% of simulations in men and women, respectively, and cost less than $100,000 per QALY gained in 96% of simulations in men and 97% of simulations in women, holding efficacy assumptions constant. (Supplement Figures 9-10)
Results were relatively insensitive to alternative scenarios where we varied the duration of therapy and efficacy of tolvaptan over time. If tolvaptan were given for only 3 years to patients with an eGFR of 80 ml/min/1.73m2 – similar to the TEMPO trial – the ICER was $575,150 per QALY gained (77% of the base case). If patients continue taking tolvaptan after 3 years, but discontinue the medication at the same rate observed in the TEMPO trial, the ICER for tolvaptan therapy is $770,900 per QALY gained (104% of the base case). If tolvaptan loses 5% of its efficacy every year, it costs $985,600 per QALY gained (132% of the base case). An analysis accounting for potential treatment effect heterogeneity found that tolvaptan cost $831,000 per QALY gained (112% of the base case).
Conclusions
If tolvaptan’s effectiveness in ADPKD patients can be sustained, it could produce substantial health benefits, but likely at a very high cost. Initiating therapy with tolvaptan in 40 year-olds with ADPKD and an eGFR of 80 ml/min/1.73m2 would be expected to delay the average age of progression to ESRD by 6.5 years and enhance survival by 2.6 years. While a reduction in progression to ESRD of this magnitude could translate into significantly lower medical costs from ESRD, (1) the likely high cost of tolvaptan surpasses these averted costs. Despite including a 68% reduction in the per milligram price of tolvaptan in our base case, we found that tolvaptan therapy cost nearly three-quarters of a million dollars per QALY gained. Tolvaptan therapy would have to be offered at or below $1,155 per month (a 94% discount in its current price per milligram) to cost less than $100,000 per QALY gained in this population.
Our findings can aid clinical decision making by quantifying how tolvaptan might improve well-being for patients with ADPKD. The TEMPO trial’s primary outcome was reduction in kidney volume, and a secondary end-point was reduction in the rate of eGFR decline from tolvaptan therapy. While these are reasonable surrogate endpoints, neither has been definitively linked to outcomes that affect patients’ well-being. A recent editorial urged that drugs developed for use in CKD be broadly used only after demonstrating significant benefits on outcomes directly relevant to patients – in general, either living longer or feeling better.(22) It is noteworthy that the National Kidney Foundation, with the support of multiple industry partners, recently sponsored a symposium in conjunction with the US Food and Drug Administration (FDA), at which more liberal criteria for eGFR endpoints in clinical trials were debated.(23, 24) By simulating the observed effects of tolvaptan over a patient’s lifetime and extrapolating them to therapeutic benefits expressed in terms of expected gains in longevity, health-related quality-of-life and reduction in progression to ESRD, our analysis uses a variety of data sources to bridge surrogate trial end-points to outcomes that affect patients’ well-being. Our findings can also inform costs and efficacy thresholds necessary for future interventions in ADPKD.
Due to an observed increase in liver enzymes in approximately 1-2 percent of patients given tolvaptan in TEMPO along with post-marketing surveillance data, the FDA recently determined that tolvaptan should not be used for more than 30 days to treat patients with low serum sodium concentrations (hyponatremia).(25) To our knowledge, tolvaptan has not been reviewed by the FDA for use in ADPKD. Tolvaptan may be less hazardous in patients with ADPKD and normal baseline liver function relative to patients with heart and/or liver failure with hyponatremia. By assessing the magnitude of potential down-stream effects of its use, our findings can help inform regulatory agencies and individual clinicians as they weigh the benefits of tolvaptan against potential risks and costs for patients with ADPKD.
In considering the generalizability of the TEMPO trial it is important to recognize that its population was not typical of patients with ADPKD. An important criterion for trial entry was a total kidney volume >750 ml. Patients with ADPKD and higher kidney volumes are at higher risk for more rapid progression to ESRD relative to patients with more modest kidney volumes.(3, 26) Assuming that tolvaptan reduces the rate of eGFR decline by attenuating cyst growth, the TEMPO trial may have overestimated the therapeutic benefit of tolvaptan to the ADPKD population at large. Indeed, in a cohort of patients with ADPKD of similar age and eGFR but with no kidney volume criterion for inclusion, the mean kidney volume was 562 ml and the average rate of eGFR decline was –2.4 ml/min/1.73m2, in contrast to 1,693 ml and –3.7 ml/min/1.73m2, respectively, in TEMPO. Among unselected patients with lower mean kidney volumes and slower rates of eGFR decline, the ICER would $1.2 million per QALY – a figure well above any accepted cost-effectiveness threshold.
This study has several limitations. First, the TEMPO trial only followed patients for 3 years. In our primary analysis we assumed that the effectiveness and clinical benefits of tolvaptan observed in the TEMPO trial persist over patients’ lifetimes in order to extrapolate clinical outcomes of survival and end-stage renal disease. We also assume that the relative risk reduction from tolvaptan is constant across different levels of eGFR and rates of eGFR decline. Yet, evidence from the TEMPO trial suggests that the treatment effect may vary by kidney volume, with a larger relative risk reduction in patients with larger kidney volume. The possibility that the treatment effect increases or diminishes with progression of CKD requires further examination in the future. Second, we are limited to examining quality-of-life changes from tolvaptan in a sensitivity analysis due to lack of information about the actual magnitude of benefit (in terms of reduced kidney pain) and side-effects such as increased thirst. The incremental cost-effectiveness results could vary based on the relative importance of pain and other adverse effects (in particular, liver injury, in view of recent FDA action). Pain was not assessed systematically in the TEMPO trial. Despite making a number of assumptions that likely favor the cost-effectiveness of tolvaptan (such as the reduced per milligram price and the assumption that changes in the cost and quality-of-life from kidney disease progression is similar to the general CKD population), tolvaptan therapy remained much more expensive than many commonly accepted therapies. Notably, even if the price of tolvaptan were reduced substantially, then assumptions on effectiveness for time periods >3 years would need to be carefully considered (and perhaps formally tested) before drawing conclusions.
While tolvaptan therapy in patients with ADPKD could delay the development of ESRD by several years and in turn enhance survival, current medication prices erode its cost-effectiveness. Tolvaptan therapy would compare favorably with other commonly accepted therapies if its price per milligram were reduced by 93%-95% and if it were reserved for use in patients with larger kidney volumes and more rapid progression to ESRD without therapy.
Supplementary Material
Acknowledgement
Funding:
F32 HS019178 from AHRQ (Dr. Erickson); DK085446 (Dr. Chertow); AG037593 (Dr. Goldhaber-Fiebert).
Primary Funding Source: AHRQ F32:HS019178; Dr. Erickson
Footnotes
Author Mailing Addresses:
Kevin F. Erickson, MD, MS Stanford University School of Medicine 117 Encina Commons Stanford, CA 94305-6019
Jeremy D. Goldhaber-Fiebert PhD Stanford University School of Medicine 117 Encina Commons Stanford, CA 94305-6019
Glenn M. Chertow, MD, MPH Stanford University School of Medicine Division of Nephrology 777 Welch Road, Suite DE Palo Alto, CA 94304
References
- 1.USRDS . United States Renal Data System. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 2.Levy M, Feingold J. Estimating prevalence in single-gene kidney diseases progressing to renal failure. Kidney International. 2000;58(3):925–43. doi: 10.1046/j.1523-1755.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 3.Fick-Brosnahan GM, Belz MM, McFann KK, Johnson AM, Schrier RW. Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: a longitudinal study. American Journal of Kidney Diseases. 2002;39(6):1127–34. doi: 10.1053/ajkd.2002.33379. [DOI] [PubMed] [Google Scholar]
- 4.Schrier RW, McFann KK, Johnson AM. Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney International. 2003;63(2):678–85. doi: 10.1046/j.1523-1755.2003.00776.x. [DOI] [PubMed] [Google Scholar]
- 5.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. New England Journal of Medicine. 1996;334(15):939–45. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 6.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. New England Journal of Medicine. 1994;330(13):877–84. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 7.Torres V, Chapman A, Devuyst O, Gansevoort R, Grantham J, Higashihara E, et al. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2012;367(25):2407–18. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrone RD, Ruthazer R, Terrin NC. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. American Journal of Kidney Diseases. 2001;38(4):777–84. doi: 10.1053/ajkd.2001.27720. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–8. [PubMed] [Google Scholar]
- 10.United States Department of Labor: Bureau of Labor Statistics . Consumer Price Index. Washington, DC: 2011. [Google Scholar]
- 11.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New England Journal of Medicine. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [Erratum appears in N Engl J Med. 2008;18(4):4] [DOI] [PubMed] [Google Scholar]
- 12.Elizabeth Arias PD. United States Life Tables, 2006. National Vital Statistics Reports. 2010;58(21) [PubMed] [Google Scholar]
- 13.Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13(2):89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 14.Meara E, White C, Cutler DM. Trends in medical spending by age, 1963-2000. Health Aff (Millwood) 2004;23(4):176–83. doi: 10.1377/hlthaff.23.4.176. [DOI] [PubMed] [Google Scholar]
- 15.Rizk D, Jurkovitz C, Veledar E, Bagby S, Baumgarten DA, Rahbari-Oskoui F, et al. Quality of life in autosomal dominant polycystic kidney disease patients not yet on dialysis. Clinical Journal of The American Society of Nephrology: CJASN. 2009;4(3):560–6. doi: 10.2215/CJN.02410508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68(6):2801–8. doi: 10.1111/j.1523-1755.2005.00752.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith GAN DH, Gullion CM, Johnson ES, Keith D. Predicting Costs Of Care In Chronic Kidney Disease: The Role Of Comorbid Conditions. The Internet Journal of Nephrology. 2007;4(1) [Google Scholar]
- 18.Red Book, Pharmacy's Fundamental Reference. Physician's Desk Reference Inc; Montvale, NJ: 2010. [Google Scholar]
- 19.Department of Health and Human Services: Center for Medicare and Medicaid Services . Physician Fee Schedule. Washington, DC: Jun, 2011. Accessed online at: http://www.cms.gov/apps/physician-fee-schedule/overview.aspx. [Google Scholar]
- 20.Department of Health and Human Services: Center for Medicare and Medicaid Services . Clinical Laboratory Fee Schedule. Washington, DC: Apr, 2010. Accessed online at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html. [Google Scholar]
- 21.Holtz-Eakin D. Prices for Brand-Name Drugs Under Selected Federal Programs. Congressional Budget Office; Washington, DC: 2005. [Google Scholar]
- 22.Perkovic V, Neal B. Trials in Kidney Disease -- Time to EVOLVE. N Engl J Med. 2012;367:2541–2. doi: 10.1056/NEJMe1212368. [DOI] [PubMed] [Google Scholar]
- 23.NKF . Nephrology News & Issues Magazine. Grand View Media Group; Birmingham, AL: 2012. FDA to examine new definitions of GFR decline as endpoints in kidney disease trials. [Google Scholar]
- 24.Nephrology News & Issues Magazine. Grand View Media Group; Birmingham, AL: 2012. Earlier endpoints proposed for chronic kidney disease trials. [Google Scholar]
- 25.United States Food and Drug Administration . FDA Drug Safety Podcast: FDA limits duration and usage of Samsca (tolvaptan) due to possible liver injury leading to organ transplant or death. Washington, DC: 2013. [Google Scholar]
- 26.Tokiwa S, Muto S, China T, Horie S. The relationship between renal volume and renal function in autosomal dominant polycystic kidney disease. Clinical & Experimental Nephrology. 2011;15(4):539–45. doi: 10.1007/s10157-011-0428-y. [DOI] [PubMed] [Google Scholar]
- 27.Leibson CL, Hu T, Brown RD, Hass SL, OFallon WM, Whisnant JP. Utilization of acute care services in the year before and after first stroke: A population-based study. Neurology. 1996;46(3):861–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.