Abstract
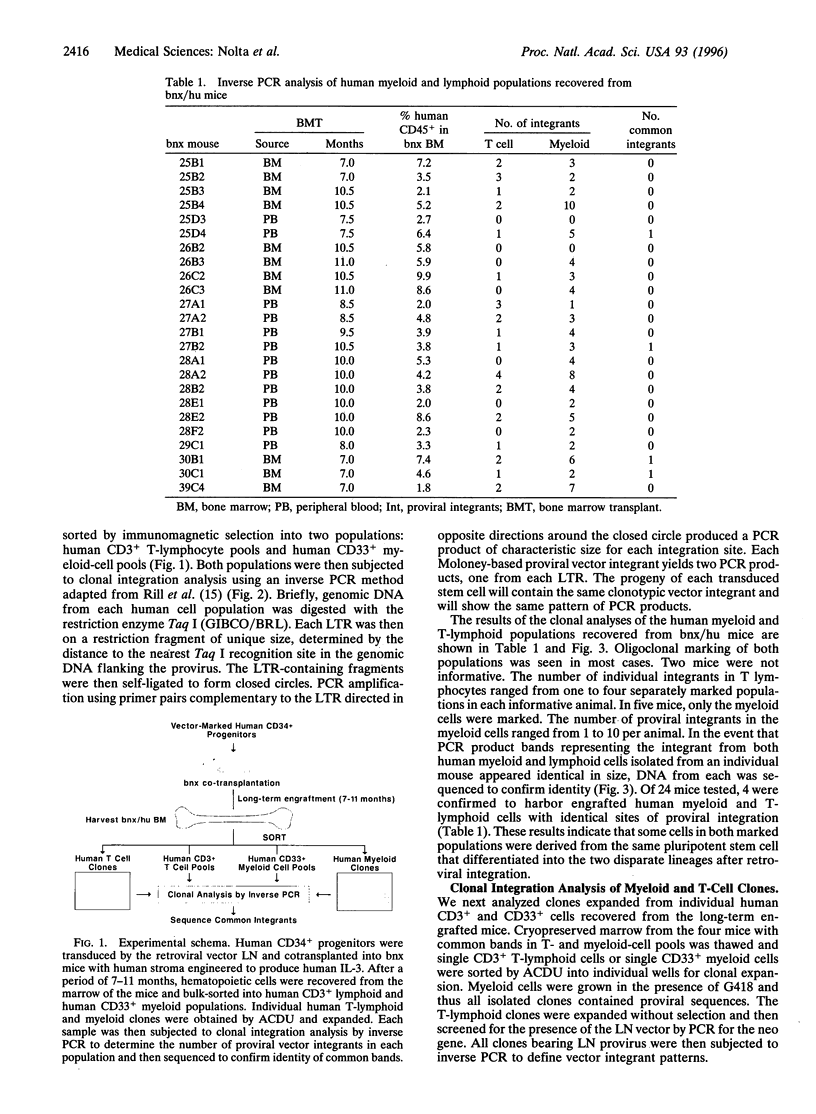
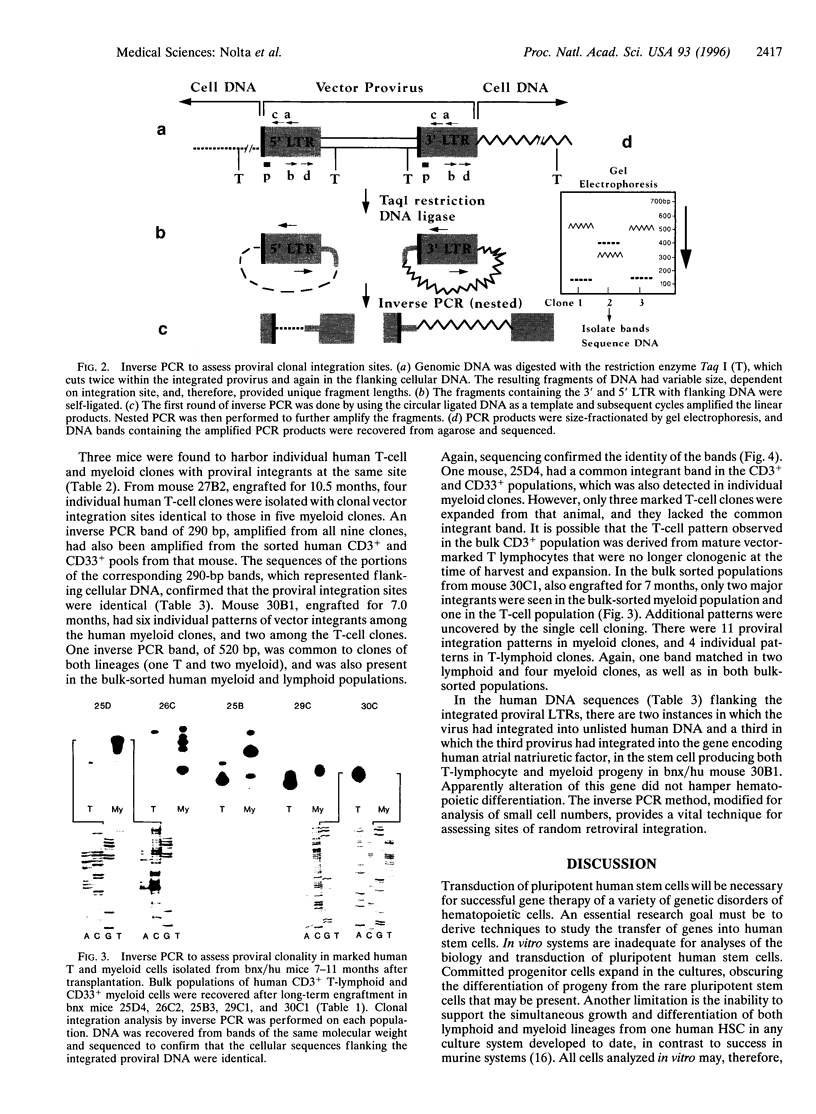
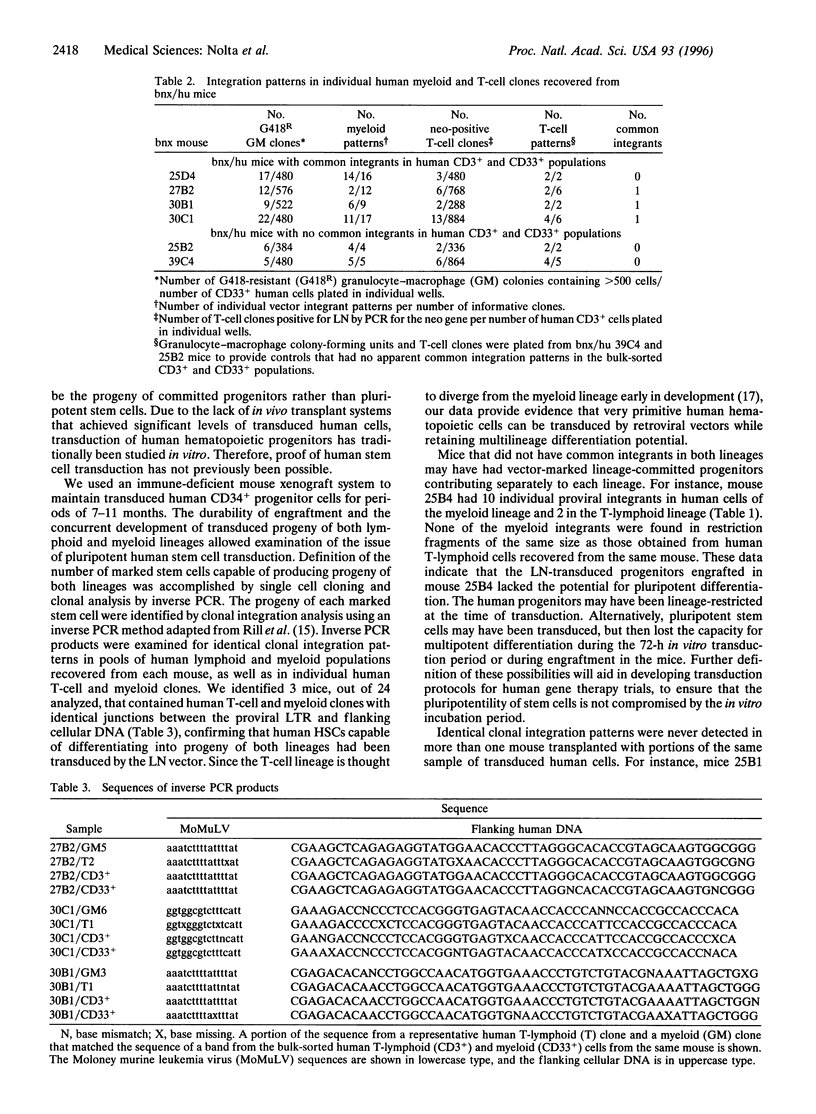
Gene transduction of pluripotent human hematopoietic stem cells (HSCs) is necessary for successful gene therapy of genetic disorders involving hematolymphoid cells. Evidence for transduction of pluripotent HSCs can be deduced from the demonstration of a retroviral vector integrated into the same cellular chromosomal DNA site in myeloid and lymphoid cells descended from a common HSC precursor. CD34+ progenitors from human bone marrow and mobilized peripheral blood were transduced by retroviral vectors and used for long-term engraftment in immune-deficient (beige/nude/XIS) mice. Human lymphoid and myeloid populations were recovered from the marrow of the mice after 7-11 months, and individual human granulocyte-macrophage and T-cell clones were isolated and expanded ex vivo. Inverse PCR from the retroviral long terminal repeat into the flanking genomic DNA was performed on each sorted cell population. The recovered cellular DNA segments that flanked proviral integrants were sequenced to confirm identity. Three mice were found (of 24 informative mice) to contain human lymphoid and myeloid populations with identical proviral integration sites, confirming that pluripotent human HSCs had been transduced.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaese R. M., Culver K. W., Chang L., Anderson W. F., Mullen C., Nienhuis A., Carter C., Dunbar C., Leitman S., Berger M. Treatment of severe combined immunodeficiency disease (SCID) due to adenosine deaminase deficiency with CD34+ selected autologous peripheral blood cells transduced with a human ADA gene. Amendment to clinical research project, Project 90-C-195, January 10, 1992. Hum Gene Ther. 1993 Aug;4(4):521–527. doi: 10.1089/hum.1993.4.4-521. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., Moritz T., Donahue R. E., Luskey B. D., Kessler S. W., Martin D. I., Orkin S. H., Nienhuis A. W., Williams D. A. Long-term in vivo expression of a murine adenosine deaminase gene in rhesus monkey hematopoietic cells of multiple lineages after retroviral mediated gene transfer into CD34+ bone marrow cells. Blood. 1993 Oct 1;82(7):1975–1980. [PubMed] [Google Scholar]
- Brenner M. K., Rill D. R., Holladay M. S., Heslop H. E., Moen R. C., Buschle M., Krance R. A., Santana V. M., Anderson W. F., Ihle J. N. Gene marking to determine whether autologous marrow infusion restores long-term haemopoiesis in cancer patients. Lancet. 1993 Nov 6;342(8880):1134–1137. doi: 10.1016/0140-6736(93)92122-a. [DOI] [PubMed] [Google Scholar]
- Capel B., Hawley R. G., Mintz B. Long- and short-lived murine hematopoietic stem cell clones individually identified with retroviral integration markers. Blood. 1990 Jun 15;75(12):2267–2270. [PubMed] [Google Scholar]
- Carter R. F., Abrams-Ogg A. C., Dick J. E., Kruth S. A., Valli V. E., Kamel-Reid S., Dubé I. D. Autologous transplantation of canine long-term marrow culture cells genetically marked by retroviral vectors. Blood. 1992 Jan 15;79(2):356–364. [PubMed] [Google Scholar]
- Challita P. M., Kohn D. B. Lack of expression from a retroviral vector after transduction of murine hematopoietic stem cells is associated with methylation in vivo. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2567–2571. doi: 10.1073/pnas.91.7.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks G. M., Kohn D. B. Growth factors increase amphotropic retrovirus binding to human CD34+ bone marrow progenitor cells. Blood. 1993 Dec 1;82(11):3290–3297. [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Fraser C. C., Szilvassy S. J., Eaves C. J., Humphries R. K. Proliferation of totipotent hematopoietic stem cells in vitro with retention of long-term competitive in vivo reconstituting ability. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1968–1972. doi: 10.1073/pnas.89.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama F., Shih J. P., Awgulewitsch A., Warr G. W., Clark S. C., Ogawa M. Clonal proliferation of murine lymphohemopoietic progenitors in culture. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5907–5911. doi: 10.1073/pnas.89.13.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. T., Lemischka I. R. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990 Feb;4(2):220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- Kohn D. B., Weinberg K. I., Nolta J. A., Heiss L. N., Lenarsky C., Crooks G. M., Hanley M. E., Annett G., Brooks J. S., el-Khoureiy A. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med. 1995 Oct;1(10):1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Nolta J. A., Hanley M. B., Kohn D. B. Sustained human hematopoiesis in immunodeficient mice by cotransplantation of marrow stroma expressing human interleukin-3: analysis of gene transduction of long-lived progenitors. Blood. 1994 May 15;83(10):3041–3051. [PubMed] [Google Scholar]
- Nolta J. A., Smogorzewska E. M., Kohn D. B. Analysis of optimal conditions for retroviral-mediated transduction of primitive human hematopoietic cells. Blood. 1995 Jul 1;86(1):101–110. [PubMed] [Google Scholar]
- Rill D. R., Santana V. M., Roberts W. M., Nilson T., Bowman L. C., Krance R. A., Heslop H. E., Moen R. C., Ihle J. N., Brenner M. K. Direct demonstration that autologous bone marrow transplantation for solid tumors can return a multiplicity of tumorigenic cells. Blood. 1994 Jul 15;84(2):380–383. [PubMed] [Google Scholar]
- Spits H., Lanier L. L., Phillips J. H. Development of human T and natural killer cells. Blood. 1995 May 15;85(10):2654–2670. [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- van Beusechem V. W., Kukler A., Heidt P. J., Valerio D. Long-term expression of human adenosine deaminase in rhesus monkeys transplanted with retrovirus-infected bone-marrow cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7640–7644. doi: 10.1073/pnas.89.16.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]







