INTRODUCTION
Cardiovascular (CV) diseases in general and heart failure (HF) in particular are major contributors to death and morbidity in the Western world, where they also are recognized as important drivers of health care expenditure. The health and economic burden of these disorders is projected to increase with the aging of populations around the world1-3. Based on accumulating evidence that chronic overactivity of the renin-angiotensin-aldosterone system (RAAS) plays a fundamental role in HF pathophysiology, drugs inhibiting key components of the RAAS have become a cornerstone of contemporary CV drug therapy4-6. For example, angiotensin-converting enzyme inhibitors (ACEi) reduce biosynthesis of angiotensin-II (Ang-II), one of the strongest vasoconstrictors, pro-hypertrophic and pro-fibrotic hormones in man. Moreover, ACEi may prevent proteolysis of bradykinin (BK), thus enhancing BK-mediated vasodilatory effects that may counteract the profound vasoconstriction seen in HF patients7. Excessive levels of Ang-II have been implicated in many CV diseases, and additional to ACEi, the detrimental actions of Ang-II can be abrogated by direct angiotensin receptor (ATR) blockers (ARB). However, despite encouraging results from many clinical trials, ACEi and ARBs-based pharmacotherapy is still far from optimal. ACEi may lose their efficacy over time due to redundant Ang-II-generating pathways and the so-called “aldosterone escape”8, while conventional ARBs do not possess the BK-enhancing properties of ACEi and are considered less effective in HF compared to ACEi4, 5.
The natriuretic peptides (NPs), consisting of atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), C-type natriuretic peptide and urodilatin (URO) are predominantly generated by the heart, vasculature, kidney and central nervous system in response to wall stress and number of other stimuli. Importantly the NPs, particularly ANP and BNP, represents the body's own blood pressure (BP) lowering system. Besides promoting vasodilation, NPs counteract pathological growth, fibrosis and dysfunction of heart, kidneys, brain and the vasculature. Current NP-augmenting strategies include the design of a number of synthetic NPs as well as inhibition of neprilysin (NEP), the key enzyme responsible for NP breakdown. Dual-acting angiotensin receptor neprilysin inhibitors (ARNi) are under scientific scrutiny for the treatment of hypertension and HF.
This review summarizes the current knowledge on RAAS blockade and NP-augmenting drugs as single or combined strategies in HF. We will discuss challenges that have been met with some of these compounds, as well as novel therapeutic agents currently being evaluated which could strengthen our pharmacological armamentarium for HF.
RENIN-ANGIOTENSIN ALDOSTERONE SYSTEM
The RAAS is fundamental in the overall regulation of CV homeostasis through the actions of important hormones, which regulate vascular tone, and specifically blood pressure (BP) through vasoconstriction and renal sodium and water retention. These hormones, specifically Ang-II and aldosterone, also possess direct actions that are important in HF by mediating cardiomyocyte hypertrophy and cardiac fibrosis with activation of collagen synthesis and fibroblast proliferation (Figure 1)9-11. RAAS is as well causally involved in the pathophysiology of cardiorenal syndrome in HF, which carries a particularly poor prognosis. Thus, blockade of RAAS has become a central therapeutic strategy for HF by employing RAAS modulating drugs such as ACEi, ARBs and mineralcorticoid receptor antagonists (MRA)4.
Figure 1. Simplified schematic of the renin-angiotensin-aldosterone system (RAAS).
A multitude of stressor signals induce the angiotensin gene. The prohormone angiotensinogen is cleaved by the protease Renin to the direct precursor angiotensin-I (Ang-I), and further to biologically active angiotensin-II (Ang-II). These steps can be inhibited by Renin-inhibitors or ACE-inhibitors (ACEi), respectively, but important alternative Ang-II-generating pathways exist. Alternative splicing of Ang-I and prohormones Ang-(1-12) or Ang-(1-9) by neprilysin (NEP) results in generation of Ang-(1-7). Binding of mature Ang-II to the type-1 angiotensin receptor (ATR-1) activates intracellular signaling cascades that exert adverse biological effects within the cardiovascular system such as pathological cardiac hypertrophy, vascular remodeling and renal fibrosis.
To date these agents have had a positive impact upon HF with improvements in symptoms, outcomes and survival. Indeed their use is increasingly widespread and their use is moving from symptomatic HF into earlier stages of mild and asymptomatic myocardial dysfunction to delay the progression of HF. Recently, a pivotal trial was completed with the mineralcorticoid receptor antagonist (MRA) epleronone in patients with systolic HF and mild symptoms12. Importantly MRAs compared to placebo reduced both the risk of death and risk of hospitalization thus delaying disease progression and providing further momentum to this continuously expanding therapeutic modality. In addition, the MRA spironolactone is under investigation in the ongoing TOPCAT trial for efficacy in HF with preserved ejection fraction (HFPEF), a disease entity for which no specific treatment recommendations exist4, 13.
Direct renin inhibition upstream of ACE, well-known for decades as a RAAS-blocking concept, prevents the generation of Ang-I and thus, Ang-II. The first-in-class drug aliskiren is currently being evaluated in two clinical HF trials, the Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) and the Aliskiren Trial of Minimizing Outcomes for Patients with Heart Failure (ATMOSPHERE)14-16. ASTRONAUT set out to evaluate the primary and secondary composite endpoints of CV death or HF re-hospitalization at 6 and 12 months, respectively, in 1,782 patients recently hospitalized with HF and reduced systolic LV function. The very recently published study showed that aliskiren in addition to standard therapy failed to reduce primary or secondary endpoints but led to significantly larger decrease from baseline in NT-proBNP levels16. Subgroup analysis demonstrated increased all-cause mortality at 12 months for patients with a history of diabetes randomized to aliskiren while non-diabetics showed net benefit compared to placebo. The reason for such a bidirectional effect of aliskiren on all-cause mortality depending on the presence or absence of diabetes deserves further evaluation. In a similar but considerably larger HF cohort (planned enrolment, n=7,000), ATMOSPHERE will compare effects on mortality and morbidity by aliskiren, enalapril or dual aliskiren+enalapril treatment. Recent evidence suggest similar rates of angioedema with aliskiren and ACEi17.
Perhaps the latest advance in RAAS blockade in HF has been the development of innovative AT1 receptor antagonists which possess dual actions that go beyond simple antagonism of Ang-II binding. Like conventional ARBs, these molecules target the superfamily of G-protein-coupled receptors (GPCRs). Activation of GPCRs by an agonist leads to intracellular dissociation of a heterotrimeric G protein into Ga and G13 subunits, resulting in activation of second messenger mediated cellular responses. The 13-arrestins are a second group of proteins which activate specific signaling pathways in a G-protein-independent manner. Studies have shown that some ligands can selectively activate either G-protein or 13-arrestin pathways18. We recently reported the actions of a novel 13-arrestin-biased ligand for the angiotensin II type 1 (AT1R), TRV12002719. TRV120027 antagonizes G-protein signaling like an ARB, but, unlike conventional AT1 receptor antagonists, it activates 13-arrestin and downstream signals. In rodents, TRV12007 has vasodilating effects similar to a conventional ARB, but, unlike an ARB, enhanced cardiac contractility while decreasing myocardial oxygen consumption20. In a large animal model of HF, TRV12007, in combination with furosemide, has potent renal and systemic vasodilating properties and preserved GFR despite a reduction in BP21. Currently, TRV12007 has entered early trials in acute decompensated HF (ADHF).
Together, although efficient RAAS blockade can be achieved at multiple levels, ACEi and MRA remain the cornerstone of contemporary HF pharmacotherapy4. Despite initial enthusiasm due to their greater tolerability compared to ACEi, ARBs offer little cardioprotection at least post-MI, and do therefore no longer appear on the “A-list” of recommended medical therapy for HF4, 22. Novel ARBs, including contractility-enhancing compounds seem promising. RAAS blockade afforded by direct renin inhibitors may eliminate some of the shortcomings of current strategies but no clinical outcome data in HF are available yet. Moreover, regarding their safety profile important adverse effects do not seem to occur less frequently than with ACEi17.
NATRIURETIC PEPTIDE SYSTEM
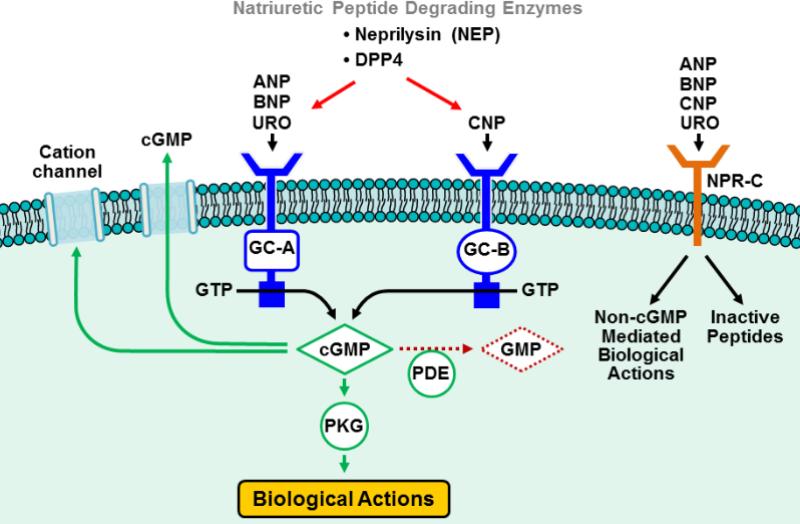
The natriuretic peptide system (NPS) (Figure 2) has emerged as an increasingly important autocrine, paracrine and endocrine system linked to particulate guanylyl cyclase (GC) receptors, the second messenger cGMP and its effector molecule protein kinase G23. Originally discovered by DeBold and co-workers, who reported that the heart synthesized and released a factor that both augmented natriuresis by the kidney but also possessed BP lowering properties24. This cardiac factor was identified as atrial natriuretic peptide (ANP) and recent studies have reported that genetic variations of the ANP gene, which increases circulating levels of ANP, protects against human hypertension25. Based upon these renal and vascular actions of ANP an intravenous drug, known as carperitide, has been approved for HF in Japan. Studies have also well established that ANP mediates its action via the GC-A receptor which is widely expressed throughout a number of tissues and especially in the adrenal cortex in which ANP is a potent inhibitor of aldosterone independent of its robust renin inhibitory actions26, 27. In the kidney, alternative processing of the ANP precursor, proANP by an unknown protease generates an ANP-like peptide called urodilatin (URO), which regulates renal sodium and water handling28. Indeed studies evaluating the effects of synthetic URO (ularitide) in ADHF patients (SIRIUS I29 and SIRIUS II30) have shown favourable effects on hemodynamic, neurohumoral and symptomatic profiles without any compromise in renal function despite a modest and dose-dependent decrease in BP. Moreover, a larger Phase III trial is currently ongoing, evaluating the efficacy and safety of ularitide in ADHF patients (TRUE-AHF)31. To complement ANP is the cardiac hormone B-type natriuretic peptide (BNP), which is approved as nesiritide in the United States and Canada for the treatment of ADHF. Like ANP, BNP is a ligand for the GC-A receptor and possesses similar pleiotropic actions which include natriuresis, aldosterone suppression and vasodilatation. While the seminal VMAC clinical trial32 lead to the US Food and Drug Administration (FDA) approval of nesiritide for ADHF, two subsequent meta-analyses suggested that nesiritide administration may be associated with increased short-term risk of death33 and worsening renal function34 in ADHF patients. However several other studies including ADHERE (Acutely Decompensated Heart Failure Registry)35-39 failed to demonstrate these adverse associations with nesiritide therapy. Thus, the ASCEND-HF clinical trial was designed to address these safety and efficacy concerns that were raised since its approval. In the ASCEND trial, nesiritide improved symptoms in the European, but not the United States ADHF patient cohort and was not superior to conventional therapy in improving mortality in ADHF patients40. Notably, these neutral findings could have been related to excessive hypotension with doses that are potently vasodilating and thus offsetting the beneficial renal actions of nesiritide. To underscore the importance of the GC-A receptor beyond the actions discussed above and relevant to HF are the anti-hypertrophic and anti-apoptotic actions which may contribute to long-term favourable anti-remodeling actions if a GC-A agonist can be given chronically23, 41. Indeed, in a recently completed human trial in mild systolic HF, 8 weeks of BNP administered twice daily by subcutaneous injection improved symptoms and reduced LV mass as determined by MRI42.
Figure 2. Simplified schematic of the natriuretic peptide system (NPS).
ANP, BNP and URO stimulate Cyclic GMP (cGMP) production by binding to the guanylyl cyclase (GC) receptor A, while CNP generates cGMP by binding to the GC-B receptor. Cyclic GMP modulates the activity of cGMP-dependent protein kinase G (PKG) to exert its pluripotent cardiac, vascular and renal biological actions. Cyclic GMP also regulates phosphodiesterases (PDEs) and cation channels. The cGMP signal is terminated by a variety of PDEs that hydrolyze cGMP to GMP. The NPs are removed from the circulation and inactived by the clearance receptor (NPR-C) and also degraded by a variety of peptidases including neprilysin (NEP) and dipeptidyl peptidase IV (DPPIV). In addition to the clearance capacity of NPR-C from the circulation, evidence has promoted the concept that the NPR-C mediates non-cGMP regulated biological actions.
C-type natriuretic peptide (CNP) is the third member of the natriuretic peptide family and is produced in endothelial cells43, 44 and renal epithelial cells45, 46. CNP mediates it's biological action through the activation of the GC-B receptor as well as potentially the non-cGMP mediated receptor, NPR-C26, 47-50. While having an important action to promote bone growth, evidence has supported that CNP has important CV actions as well. These include hyperpolarization of vascular smooth muscle49, anti-thrombotic actions50, promotion of re-endothelialization51 and potent anti-fibrotic properties48, 52, 53. The use of CNP as a HF therapeutic has been limited by both its rapid enzymatic degradation as well as by its lack of renal enhancing actions. A designer CNP-based NP has been engineered which is now in clinical trials for HF and this will be discussed below.
A key component of the NPS is the ectoenzyme neutral endopeptidase (NEP), which is also known as neprilysin. This membrane bound enzyme is widely expressed but is most abundant in the kidney. NEP serves as the principal mechanism for enzymatic removal of the native natriuretic peptides with susceptibility to degradation greatest for CNP>ANP>BNP54. Furthermore, many other substrates for NEP exist, some of them with opposing physiological actions. These include endothelin-1, kinin peptides, opioid peptides, Substance P, amyloid beta protein, and gastrin55-57. Importantly, NEP hydrolyzes Ang I to angiotensin 1-7 (ANg 1-7)58, and since Ang 1-7 opposes the action of Ang-II, the hydrolysis of Ang I to Ang 1-7 by NEP potentially has beneficial CV effects. Inhibition of NEP (NEPi) has been advanced as a therapeutic modality. If NEP only targeted NPs, NEPi would augment the vasodilating and natriuretic actions afforded by increased levels of these peptides. However, NEP's ability to catabolize numerous substrates also means that sole NEPi yields broader effects than anticipated, and explains why NEPi is best combined with the inhibition of other vasoactive peptides. Candoxatril was the first potent, orally available NEP inhibitor. Candoxatril mediated a dose-dependent increase in plasma ANP, natriuresis, and cGMP in humans but also increased circulating Ang-II59. Importantly, Candoxatril's effects on BP in hypertensive patients were not clinically meaningful. Candoxatril was also investigated in HF. In a canine model of severe HF, which is characterized by both NP elevation and RAAS activation, candoxatril was natriuretic and suppressed aldosterone60. In human HF, candoxatril increased ANP and BNP levels, promoted natriuresis and decreased clearance of exogenously administered ANP61. However, systemic and pulmonary vascular resistances were not altered.
Early strategies to enhance the salutary actions of the NP system have clearly met challenges. Clinical efficacy of recombinant drugs such as nesiritide, carperitide or ularitide has been limited by hypotension and their short bioavailability. For the class of single-acting NEPi, as discussed below, their effectiveness to promote the endogenous NPs and to improve overall cardiorenal function was only finally realized when combined with RAAS modulators.
DESIGNER NATRIURETIC PEPTIDES
Therapeutic use of the native NPs has been highly attractive given their diverse intrinsic protective properties which include natriuresis, diuresis, RAAS suppressing, inhibition of fibrosis, vasodilatation and angiogenesis. In an effort to overcome the shortcomings of recombinant NPs outlined above, the concept of designer NPs has emerged as an innovative advancement in drug discovery for the treatment of various CV diseases. Designer NPs are novel peptides that have been engineered through modifications in their amino acid structures or through use of genetically altered forms of native NPs. The rationale behind this concept is to produce chimeric NPs whose pharmacological and beneficial biological profiles go beyond those of the native NPs while minimizing undesirable effects.
CD-NP (Cenderitide)
The most advanced designer NP to date was designed by investigators in the Cardiorenal Research Laboratory at Mayo Clinic and first reported in 200862. This novel 37 amino acid (AA) hybrid NP named CD-NP (Figure 3), which is now known as cenderitide, consists of the mature form of native human CNP fused with the15 AA C-terminus of Dendroaspis natriuretic peptide (DNP), which was first isolated from the venom of the green mamba63. This unique first generation designer NP62 retains the anti-fibrotic48, 52, 53, anti-proliferative 64and anti-hypertrophic65, 66 effects and venodilatation67 of CNP as well as natriuretic and diuretic effects of DNP68, which are very desirable properties for drugs to combat a number of CV diseases including HF. Importantly, CD-NP also has anti-proliferative actions in cultured human cardiac fibroblasts and stimulates cGMP production in these same cells to a greater extent than equimolar concentrations of BNP62. In vitro studies have demonstrated CD-NP is the first NP to activate both the GC-A and GC-B receptor at physiological doses69 and is more resistant to proteolytic degradation than ANP, BNP and CNP70. In normal canines, intravenous (IV) infusion of CD-NP activates plasma cGMP and had natriuretic, diuretic, RAAS suppressing actions and unloaded the heart with minimal effects on mean atrial pressure (MAP)62. Further, when compared to conventional recombinant BNP (nesiritide) therapy, an equimolar dose of CD-NP significantly increased GFR and was less hypotensive than BNP62. Moreover, infusion of CD-NP in experimental HF induced by rapid ventricular pacing also had significant cardiac unloading effects, increases in GFR, renal perfusion, diuresis and natriuresis, and reductions in plasma renin activity together with a modest reduction in MAP62. In healthy human subjects, CD-NP infusion increased urinary and plasma cGMP levels, suppressed plasma aldosterone, induced a significant diuretic and natriuretic responses and a minimal, yet significant reduction in MAP71. In March 2011, cenderitide received a fast track designation from the FDA and currently is in Phase II clinical trials targeting post-acute HF patients using chronic subcutaneous infusion technology72.
Figure 3. Amino acid structures of designer natriuretic peptides.
CD-NP (cenderitide) consists of amino acids from native human CNP (green) and DNP (light blue). CU-NP consists of amino acids from native human CNP (green) and URO (pink). ANX-042 consists of amino acids from native human BNP (red) and 16 amino acids (yellow) from the C-terminus of the alternative spliced transcript of BNP.
CU-NP
Building the on the encouraging findings of cenderitide in both experimental and human studies and designer NP technology, a humanized version of cenderitide, called CU-NP, was created. CU-NP is an engineered NP (Figure 3), consisting of the 17 AA ring of native human CNP linked to both the C- and N- termini of urodilatin, which is a 32 AA cleavage product of intrarenal processed proANP73. Although CU-NP is in the early stages of drug development, initial experimental studies have demonstrated that IV infusion of CU-NP activates cGMP in canine HF and exerts renal-enhancing, cardiac-unloading and RAAS-suppressing actions without excessive hypotension74. CU-NP has also direct anti-hypertrophic effects through the inhibition of the sodium-hydrogen exchanger 1(NHE-1)/calcineurin pathway75.
ANX-042
Another strategy for drug discovery is the biology of alternative RNA splicing which may provide unique opportunities to identify drug targets and therapeutics. We recently reported an alternative spliced transcript for BNP (AS-BNP)76. This alternative spliced BNP transcript is present in failing human hearts and is reduced following mechanical unloading. The transcript would generate a unique 34 AA C-terminus while maintaining the remaining structure of native mature BNP. Importantly, unlike BNP, this novel peptide failed to stimulate cGMP in vascular cells or to vasorelax pre-constricted arterial rings. From this structure, we designed a shortened 42 AA peptide from AS-BNP, which is currently known as ANX-042 (Figure 3), and demonstrated its ability to stimulate cGMP, like BNP, in canine glomerular isolates and cultured human mesangial cells but lacking similar effects in vascular cells. In a canine-pacing model of HF, systemic infusion of ANX-042 did not alter MAP but increased GFR, suppressed plasma renin and Ang-II, while inducing natriuresis and diuresis. Importantly in 2012, ANX-042 was approved as an investigational new drug (IND) from the FDA and now has begun a first-in-human clinical trial as a designer renal-enhancing and non-hypotensive NP which could make ANX-042 a potential novel renal-selective agent for HF.
In summary, the natriuretic peptides represent the most important endogenous counterpart to RAAS by conferring cardiac, renal and vascular protection. Therapeutic augmentation of the NP system in HF has been attempted directly employing a broad range of recombinant and engineered NPs, or indirectly by preventing NP degradation (through NEPi). In particular degradation-resistant NPs including designer NPs have shown encouraging early results and are now under evaluation in clinical trials. NEPi as monotherapy to augment NPs has largely produced neutral effects in clinical studies, and therefore its greatest potential presumably lies in the combination with blockers of the RAAS and other neurohormonal systems that are causally inflicted in HF.
RAAS BLOCKADE COMBINED WITH NPS AUGMENTATION
Dual ACE/NEP (vasopeptidase) inhibition in HF
As previously described, the RAAS and NPS have a yin/yang relationship with each system, serving as a counter-regulatory constraint on the activity of the other77. This physiological relationship provides the potential to achieve greater benefits with modulation of both systems than manipulation of individual systems. Specifically, the beneficial effects of inhibition of the RAAS may potentially be augmented by enhancement of NP activity. Conversely, the disappointing clinical effects of NEP inhibitors as monotherapy78 may be overcome by combination with RAAS blockade.
Single molecular entities have been developed combining NEP inhibition (NEPi) with both ACEi and ARBs as single molecules (Table).
Table.
Important clinical and preclinical studies of combined NEP inhibitors in cardiovascular disease
| Drug | Study or model characteristics | Study endpoints | Key results (NEPi drugs vs comparator) |
|---|---|---|---|
| ACEi+NEPi (vasopeptidase inhibitors) | |||
| Sampatrilat79 | Patients with hypertension | BP, plasma renin activity, urinary cGMP excretion | Good antihypertensive effect. No increase of plasma renin activity |
| Sampatrilat80 | Patients with resistant hypertension | BP, plasma renin activity at 8 wks | Sustained antihypertensive effect superior to ACEi. No increase of plasma renin activity |
| Sampatrilat81 | Preclinical. Rats with HF post-MI | LV hemodynamics and remodeling at 5 wks | Reduced mortality and LV remodeling, improved hemodynamics |
| Omapatrilat82 | Preclinical. Hamsters with HF due to dilated cardiomyopathy. | Survival, LV remodeling and function at 8 wks. | Reduced mortality and LV remodeling, improved hemodynamics |
| Omapatrilat83 | Omapatrilat vs enalapril in patients with hypertension (OCTAVE) | BP control at 24 wks | Antihypertensive effect superior to ACEi, but increase of angioedema |
| Omapatrilat84 | Omapatrilat vs lisinopril in NYHA class II-IV systolic HF (IMPRESS) | Exercise capacity at 12 wks, HF death/morbidity at 24 wks | Reduced composite of mortality and HF hospitalizations. No angioedema and fewer AE than ACEi |
| Omapatrilat85 | Omapatrilat vs enalapril in in systolic HF NYHA 2-4 (OVERTURE) | Composite of mortality and HF hospitalizations at 62 wks | No difference in primary endpoint |
| ACEi+NEPi+ECEi | |||
| Benazepril+daglutril86 | Preclinical. Rats with HF post-MI | LV hemodynamics and remodeling after 4 wks treatment | Better preserved LV structure and function than ACEi or ECEi/NEPi alone |
| NEPi+ECEi | |||
| CGS2630387 | Preclinical. Rats with HF post-MI | LV hemodynamics and remodeling after 30 days treatment | Reduced LV remodeling and filling pressures, increased LV function compared to NEPi alone |
| Daglutril (SLV-306)88 | Preclinical. Rats with hypertension (salt sensitive Dahl rats) | LV remodeling and neurohormonal activation at 6 wks | Reduced LV remodeling and ET-1 levels similar to ACEi |
| Daglutril (SLV-306)89 | Patients with ADHF | Acute hemodynamics after single-bolus dose | Reduced LV filling pressures, but no clear dose-response |
| SLV-33890 | Rats with renovascular (2K1C) hypertension | LV remodeling and BP at 12 wks | BP-independent inhibition of cardiac fibrosis |
| ARB+NEPi (ARNi) | |||
| LCZ-69691 | Preclinical and clinical. Double transgenic hypertensive rats. Healthy human controls. | BP effects in hypertensive rats. Pharmacokinetics in healthy humans. | Double transgenic rats: sustained BP reductions. Humans: well-tolerated, effective blocker of AR and NEP |
| Valsartan/candoxatril92 | Preclinical study in normal rats. | BP effects, tracheal plasma extravasation | Antihypertensive effect of ARNi similar to omapatrilat. No observation of tracheal plasma extravasation /angioedema |
| LCZ-69693 | Patients with mild to moderate hypertension. | BP lowering at 8 wks | Greater reductions in blood pressure with LCZ-696 than ARB alone. Safety endpoints met |
| LCZ-69694 | Patients with HF and preserved EF and elevated NT-proBNP (PARAMOUNT) | NT-proBNP changes, symptoms, LV remodeling | Reduced NT-proBNP and left atrial size, improved NYHA-class |
| LCZ-69695 | Patients with stable chronic HF and reduced EF (PARADIGM-HF) | Death or HF hospitalization | Ongoing (estimated completion date april 2014) |
NEPi indicates neprilysin inhibitor; ACEi, angiotensin-converting enzyme inhibitor; BP, blood pressure; cGMP, cyclic GMP; wks, weeks; HF, heart failure; MI, myocardial infarction; LV, left ventricle; NYHA, New York Heart Association (functional class); AE, adverse events; ET-1, endothelin-1; 2K1C, 2-kidney 1 -clip model; ARNi, angiotensin receptor neprilysin inhibitor; EF, ejection fraction, NT-proBNP, N-terminal pro brain natriuretic peptide.
Early dual NEPi/ACEi agents (vasopeptidase inhibitors) such as sampatrilat demonstrated promising effect in HF and hypertension but were discontinued because of poor oral bioavailability79-81, 96. The most extensively studied ACEi/NEPi thus far has been omapatrilat. Omapatrilat demonstrated equal potency of inhibition and affinity for both enzymes. In a pre-clinical HF model omapatrilat prevented cardiac dysfunction and remodeling and improved survival; also, it produced significant BP reductions in low, normal and high renin hypertension models, including spontaneously hypertensive rats82, 97.
OCTAVE was the definitive clinical outcome trial to evaluate the beneficial effects of omapatrilat (versus enalapril)83 in 25,302 untreated or uncontrolled hypertensives. OCTAVE demonstrated improved systolic BP control with omapatrilat and more patients achieving target BP compared to enalapril. The trial however, reported an increase in prevalence of angioedema in omapatrilat-treated patients, 2.2 vs. 0.7%. The mechanism underlying this rare, but potentially life-threatening, adverse event is presumably related to enhanced BK levels achieved with blockade of zinc-containing metalloproteinases such as NEP, and potentially aminopeptidase-P (APP) and dipeptidyl peptidase-4 (DPP4) as well83, 98. Omapatrilat has also been studied in patients with systolic chronic HF. A 573 patient Phase IIB study (IMPRESS)84 compared omapatrilat 40mg/day to lisinopril 20mg/day for 24 weeks. Omapatrilat reduced the composite endpoint of death, HF admission or discontinuation of study treatment for worsening HF compared to lisinopril and produced a greater improvement in NYHA Class III-IV patients. Furthermore, there appeared to be greater preservation of renal function with omapatrilat. There was no significant angioedema signal observed; indeed there were fewer overall adverse events with omapatrilat compared to lisinopril. These favourable findings led to a major outcome study, OVERTURE85, which randomised 5,770 NYHA Class II-IV systolic HF patients to enalapril 10mg twice daily or omapatrilat 40mg once daily for a mean duration of 14.5 months. The primary endpoint (death or hospitalization for HF requiring IV therapy) was not significantly different compared to enalapril. A twice daily regimen of omapatrilat may have resulted in a smoother pharmacokinetic and pharmacodynamic profile (particularly large post-dose falls in systemic BP with once-daily omapatrilat) and this may have translated into fewer major primary endpoint events.
Vasopeptidase inhibition unfortunately exemplifies yet another therapeutic strategy that despite strong scientific rationale and positive early trials has not translated into better pharmacotherapy for patients with HF.
Triple inhibitors of ACE, NEP and ECE
Endothelin-1 (ET-1) is a pluripotent vasoconstrictor and multifunctional neurohormone that contributes in the progression of HF and many other CV diseases99-101. ET-1 plasma levels strongly predict mortality in CV disease and ET-1 production markedly increases in HF102-104. A multitude of ET-1 receptor antagonists (ERA) have been tested in acute and chronic HF settings, but the majority failed to improve outcomes. While the concept of ERA in HF is now widely considered as futile, with the exception of HF due to certain forms of pulmonary arterial hypertension, abrogation of ET-1 biosynthesis by ET-converting enzyme (ECE)-inhibition is a strikingly lesser explored avenue. In experimental HF ECE-inhibition (ECEi) improved cardiorenal function together with reduction of other key neurohormones such as Ang-II, renin and aldosterone105-107. ECE may also cleave NPs to a physiologically relevant degree, accordingly its targeting through ECEi might simultaneously augment and suppress NPs and ET-1 levels, respectively108. In human acute HF, ECEi induced favourable hemodynamic changes similar to ET receptor blockade and on top of ACEi, but no long-term data exist109. Dual ECE/NEP inhibition reduced adverse LV remodeling and dysfunction in a post-MI HF model87. The ECE/NEP inhibitor SLV-306 (daglutril) was shown to acutely lower LV filling pressures in human HF, and given over time, to reverse elevated plasma ET-1 levels and pathological cardiac remodeling similar to ACE-inhibition in rats with LV hypertrophy88, 89 (Table). Daglutril also abrogated big-ET-mediated BP increases and enhanced NP levels in healthy humans110. More recently, SLV-338, a similar dual ECE/NEP inhibitor prevented experimental hypertension-induced cardiac fibrosis independently of BP lowering90. Triple ACE/ECE/NEP inhibitors have been designed to suppress biosynthesis of Ang-II and ET-1 and to augment vasodilators including BK, NPs and adrenomedullin. In rats with HF post-MI, ACE/ECE/NEP inhibition improved LV structure and function more than either ACE or ECE/NEP inhibition alone86. Although encouraging, these initial results would need to be evaluated in randomized prospective clinical trials.
Unfortunately, further clinical development of triple ACE/ECE/NEP inhibitors appears to have been abandoned, perhaps due to previous concerns about safety with vasopeptidase inhibitors. The largely negative results from ET-receptor antagonist HF trials may further have tempered enthusiasm for the field. In addition, unlike with RAAS, it appears that for the case of ET the scientific community has made considerably less distinction between the modalities of receptor antagonism and inhibition of biosynthesis (by ECEi).
Dual angiotensin-receptor/ NEP inhibitors (ARNi)
Based on the above considerations on vasopeptidase inhibitors, newer agents combining NEPi with not an ACEi but ARB have been developed, again as single molecules (ARNi) (Table). The rationale for these agents is that ARBs are less likely to interfere with BK metabolism and thus less likely to contribute to cough and angioedema. LCZ-696 is a fixed dose combination of valsartan and AHU-377 (NEPi pro-drug) in a 1:1 ratio and is the first and most clinically advanced compound in this new class91. Pre-clinically, LCZ-696 was able to lower BP in double transgenic (renin, Ang-II over-expression) rats with associated increases in plasma cGMP, renin concentration and activity and Ang-II levels indicating that appropriate receptors were targeted as per expected pharmacological actions91. Furthermore, enhanced tracheal plasma extravasation was not observed with the ARB/NEPi valsartan/candoxatril92, suggesting minimal risk of angioedema with the ARB/NEPi combination, further confirmed by recent patient data on ARB and ACEi17.
A large Phase II placebo-controlled study of LCZ-696 has recently been undertaken in patients with mild to moderate hypertension93. The key findings after 8 weeks of follow-up were significantly greater reductions in office and ambulatory BP with LCZ-696 compared to the equivalent dose of valsartan alone. Importantly LCZ-696 was well tolerated and there were no cases of angioedema reported. Neurohormonal biomarker assessment confirmed the expected augmentation of plasma ANP and cyclic GMP as well as plasma renin in the LCZ-696 cohorts.
LCZ-696 may also have considerable potential in the setting of systolic chronic HF analogous to the attempt to establish omapatrilat as standard background RAAS blocker (replacing ACEi) in this setting in the OVERTURE study conducted a decade earlier. The PARADIGM-HF study95 is an ongoing efficacy and safety assessment of LCZ-696 in patients with stable chronic HF (left ventricular ejection fraction <40%). Prior to randomization to either LCZ-696 20mg bid or enalapril 10mg bid, a single-blind run-in period is undertaken. Patients receive (sequentially) enalapril 10mg bid, LCZ-696 100mg bid and then LCZ-696 200mg bid over a duration of between 5 to 8 weeks. Patients post-randomization are then followed until 2,410 primary outcome events (CV death or HF hospitalization) have been achieved.
There are a number of interesting design features built in to PARADIGM-HF that are worthy of comment. Specifically, the single-blind run-in period is designed to firstly switch patients to a standard comparator agent, enalapril, and to establish that they are able to tolerate a dose equivalent to that achieved in the SOLVD-treatment study111 that forms the basis for ongoing use of ACE-inhibitors in systolic HF (mean of 16.6mg/day). Patients are then evaluated with regard to tolerability of LCZ-696 at progressively increasing doses, prior to randomization. The other important design feature of PARADIGM-HF is that, unlike in OVERTURE, patients receive twice daily dosing of LCZ-696. This was deliberately designed to minimise the potential (as alluded to earlier) for large drops in BP and other potential hemodynamic disturbance following administration of the full daily dose given on a once daily basis.
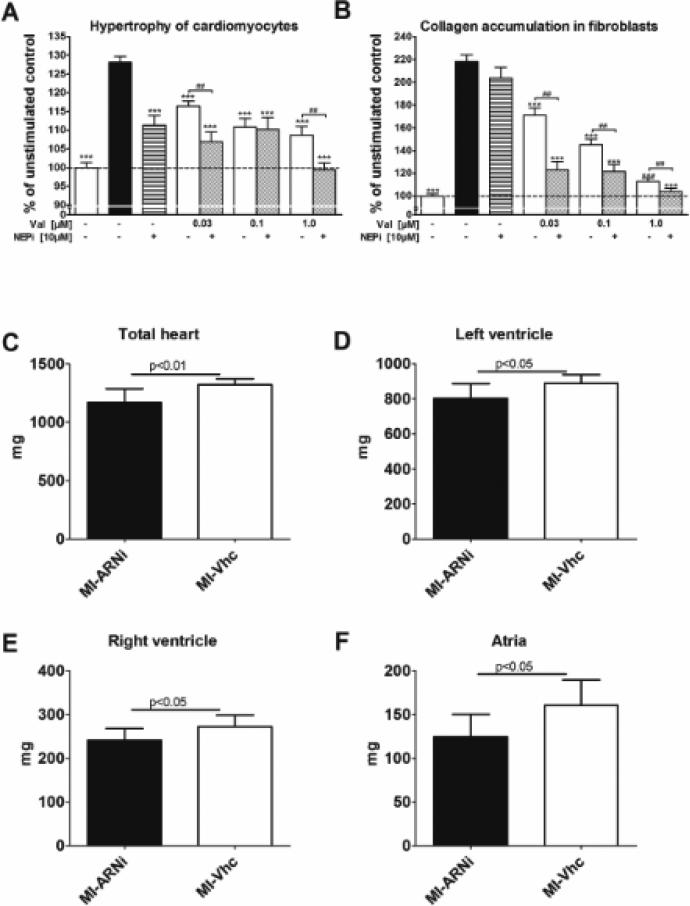
We recently evaluated putative anti-fibrotic and anti-hypertrophic efficacy of ARNi in cultured neonatal rat cardiac fibroblasts and myocytes using 3H-proline and 3H-leucine incorporation, respectively, as described112. Cells were stimulated with Ang-II (100 nM) and co-treated with increasing doses of the ARB Valsartan (Val) in the presence and absence of LBQ-657, the active metabolite of NEPi prodrug AHU-377. ARNi (i.e. Val+NEPi) provided dose-dependent, superior anti-hypertrophic (Figure 4 A) and anti-fibrotic (B) effects compared to Val alone. NEPi alone had only modest effect in cardiomyocytes, and, predictably no discernable effect in fibroblasts113. We further explored the potential utility of ARNi to modulate cardiac remodeling after myocardial infarction (MI). One week after experimental induction of MI in rats, animals were randomized to four weeks of PO treatment with ARNi (LCZ-696; n=11) or vehicle (n=6). At endpoint, ARNi-treated rats exhibited significantly reduced cardiac hypertrophy (Figure 4 C-F).
Figure 4. Cardiac anti-remodeling effects of ARNi in vitro and in vivo.
A, B. Effects of ARB (Valsartan, Val) versus ARNI (Val+NEPi) on Ang-II-stimulated neonatal rat cardiomyocytes hypertrophy (A) and cardiac fibroblasts collagen synthesis (B) as determined by 3H-leucine and 3H-proline incorporation, respectively. Data is shown as percentage of unstimulated control (=100%). ARB dose-dependently attenuated Ang-II mediated effects in both cell-types, with further additional reduction in ARNi-treated cells. Overall, ARNI (Val+NEPi) provided dose-dependent, superior anti-hypertrophic (Figure 4 A) and anti-fibrotic (B) effects compared to ARB alone. The effects of NEPi alone in cardiomyocytes were modest, and negligible in fibroblasts. Unstimulated (negative) and Ang-II-stimulated (positive) controls are the first open and solid columns, respectively, from the left. Dashed line demarks level of unstimulated control. ***P<0.001 vs Ang-II stimulated control; ##P<0.01 ARB+NEPi vs ARB for equal dose of Val, unpaired t-test. C-F. Effects of ARNi on cardiac remodeling after myocardial infarction (MI). One week after surgical ligation of the left anterior descending coronary artery in outbred male Sprague–Dawley rats (250 g), animals were randomized to treatment with ARNi (LCZ-696, 68 mg/kg body weight administered PO) with ARNi (n=11; solid columns) or vehicle (n=6; open columns). After four weeks of treatment, ARNi-treated rats exhibited significantly smaller weights of all cardiac chambers, consistent with reduced cardiac hypertrophy (Figure 4 C-F).
There is considerable further therapeutic potential for ARNi. One obvious area worthy of exploration is that of HFPEF. HFPEF is a heterogenous disorder that is often driven by hypertension and chronic ischemia, two conditions where ARB/NEPi may be efficacious. Furthermore, NPs have direct anti-fibrotic effects in cell culture and this has also been observed in vivo114. HFPEF is a disease characterized by pathological myocardial fibrosis and thus the augmented anti-fibrotic activity of a combined ARB/NEP inhibitor may be of particular benefit in this setting.
The recently published PARAMOUNT-study94 was a phase-2 parallel-group, double-blinded RCT comparing LCZ-696 with valsartan in 301 patients with HFPEF and elevated plasma levels of NT-proBNP. Patients assigned to LCZ-696 showed a greater reduction in NT-proBNP at 12 weeks of follow-up, the primary endpoint. More patients on LCZ-696 exhibited improved NYHA functional class and of note, reduced LA size compared to valsartan, consistent with reverse LA remodeling. Whether the latter was due to greater reductions of LV and LA wall stress by LCZ-696, or rather reflected distinct drug effects on total arrhythmia burden (over 40% of subjects had a history of atrial fibrillation) is debatable, since LV filling pressures estimated by Doppler echocardiography were not different. Of note, attenuation of atrial remodeling was also seen in our experimental study (Figure 4 F). Another encouraging signal was that two important risk groups, namely diabetic patients and those with the highest BP exhibited greater reductions of plasma NT-proBNP by LCZ-696 than valsartan, although the study was underpowered to detect subgroup differences115. At the same time, no enhanced risk of angioedema or other adverse events were reported in PARAMOUNT. Besides LCZ-696, novel single-molecule ARNi are under preclinical evaluation116. No data are currently available.
Development of the new drug class of ARNi and initial results hold promise as a breakthrough in the search for better medical therapies for HF. Beyond HF the ongoing evaluation of ARNi should and hopefully will be extended to post-MI LV systolic dysfunction and diabetic nephropathy.
Future Directions
Today there continues to be a high clinical need for novel therapeutic agents that optimally control HF and major predisposing CV diseases such as hypertension and coronary artery disease. To date, therapeutic strategies targeting the RAAS constitute first-line HF pharmacotherapy and underscore the deleterious effects of this system in HF pathogenesis and progression. Therapeutic augmentation of the NPS by inhibition of NP breakdown or administration of synthetic NPs is another promising area under current investigation. Novel concepts in HF seek to maximize the beneficial properties of the NPS coupled with counteracting RAAS or ET in order to achieve optimal end-organ protection, including the most recent new class of compounds, ARNi. As such, with the promising results of most recent clinical trials examining ARNi, we believe new therapeutic opportunities lie ahead.
Supplementary Material
Acknowledgments
Sources of Funding
Supported by grants from the NIH: R01 HL 36634, P01 HL 76611 and the Mayo Foundation, Rochester, MN, USA; and a National Health and Medical Research Council of Australia program grant ID 546272. TVL is supported by post-doctoral research grant 2011062 from the South-Eastern Norwegian Health Authority.
Footnotes
Disclosures
HK has served on the steering committee of the EMPHASIS-HF trial. Mayo Clinic has licensed cenderitide (CD-NP) and CU-NP to Nile Therapeutics and ANX-042 to Anexon Inc. Dr. Burnett is the chair of the scientific advisory board of Nile Therapeutics and is a scientific advisor for Anexon Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luepker RV, Steffen LM, Jacobs DR, Jr., Zhou X, Blackburn H. Trends in blood pressure and hypertension detection, treatment, and control 1980 to 2009: the Minnesota heart survey. Circulation. 2012;126:1852–1857. doi: 10.1161/CIRCULATIONAHA.112.098517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radford MJ, Arnold JM, Bennett SJ, Cinquegrani MP, Cleland JG, Havranek EP, Heidenreich PA, Rutherford JD, Spertus JA, Stevenson LW, Goff DC, Grover FL, Malenka DJ, Peterson ED, Redberg RF. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Failure Society of America. Circulation. 2005;112:1888–1916. doi: 10.1161/CIRCULATIONAHA.105.170073. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 5.Schroten NF, Gaillard CA, van Veldhuisen DJ, Szymanski MK, Hillege HL, de Boer RA. New roles for renin and prorenin in heart failure and cardiorenal crosstalk. Heart Fail Rev. 2012;17:191–201. doi: 10.1007/s10741-011-9262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Lueder TG, Krum H. RAAS Inhibitors and Cardiovascular Protection in Large Scale Trials. Cardiovasc Drugs Ther. 2013;27:171–179. doi: 10.1007/s10557-012-6424-y. [DOI] [PubMed] [Google Scholar]
- 7.Regoli D, Plante GE, Gobeil F., Jr. Impact of kinins in the treatment of cardiovascular diseases. Pharmacol Ther. 2012;135:94–111. doi: 10.1016/j.pharmthera.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–492. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 9.Funder JW. Reconsidering the roles of the mineralocorticoid receptor. Hypertension. 2009;53:286–290. doi: 10.1161/HYPERTENSIONAHA.108.119966. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Xu X, Liu JJ, Lin YX, Gao GD. Angiotensin II receptors subtypes mediate diverse gene expression profile in adult hypertrophic cardiomyocytes. Clin Exp Pharmacol Physiol. 2007;34:1191–1198. doi: 10.1111/j.1440-1681.2007.04694.x. [DOI] [PubMed] [Google Scholar]
- 11.Von Lueder TG, Atar D. Pathophysiology and clinical manifestation. In: Krum H, von Lueder TG, editors. Advances in heart failure management. Future Medicine Ltd.; 2012. Chapter 2. [Google Scholar]
- 12.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [Google Scholar]
- 13.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972. e910. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Krum H, Massie B, Abraham WT, Dickstein K, Kober L, McMurray JJ, Desai A, Gimpelewicz C, Kandra A, Reimund B, Rattunde H, Armbrecht J. Direct renin inhibition in addition to or as an alternative to angiotensin converting enzyme inhibition in patients with chronic systolic heart failure: rationale and design of the Aliskiren Trial to Minimize OutcomeS in Patients with HEart failuRE (ATMOSPHERE) study. Eur J Heart Fail. 2011;13:107–114. doi: 10.1093/eurjhf/hfq212. [DOI] [PubMed] [Google Scholar]
- 15.Gheorghiade M, Albaghdadi M, Zannad F, Fonarow GC, Bohm M, Gimpelewicz C, Botha J, Moores S, Lewis EF, Rattunde H, Maggioni A. Rationale and design of the multicentre, randomized, double-blind, placebo-controlled Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT). Eur J Heart Fail. 2011;13:100–106. doi: 10.1093/eurjhf/hfq209. [DOI] [PubMed] [Google Scholar]
- 16.Gheorghiade M, Bohm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 17.Toh S, Reichman ME, Houstoun M, Ross Southworth M, Ding X, Hernandez AF, Levenson M, Li L, McCloskey C, Shoaibi A, Wu E, Zornberg G, Hennessy S. Comparative risk for angioedema associated with the use of drugs that target the Renin-Angiotensin-aldosterone system. Arch Intern Med. 2012;172:1582–1589. doi: 10.1001/2013.jamainternmed.34. [DOI] [PubMed] [Google Scholar]
- 18.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein- biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boerrigter G, Lark MW, Whalen EJ, Soergel DG, Violin JD, Burnett JC., Jr. Cardiorenal actions of TRV120027, a novel ss-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ Heart Fail. 2011;4:770–778. doi: 10.1161/CIRCHEARTFAILURE.111.962571. [DOI] [PubMed] [Google Scholar]
- 20.Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW. Selectively engaging beta-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 21.Boerrigter G, Soergel DG, Violin JD, Lark MW, Burnett JC., Jr. TRV120027, a novel beta-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circ Heart Fail. 2012;5:627–634. doi: 10.1161/CIRCHEARTFAILURE.112.969220. [DOI] [PubMed] [Google Scholar]
- 22.Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ. 2011;342:d2234. doi: 10.1136/bmj.d2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhn M. Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res. 2003;93:700–709. doi: 10.1161/01.RES.0000094745.28948.4D. [DOI] [PubMed] [Google Scholar]
- 24.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 25.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 27.Burnett JC, Jr., Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984;247:F863–866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 28.Forssmann W, Meyer M, Forssmann K. The renal urodilatin system: clinical implications. Cardiovasc Res. 2001;51:450–462. doi: 10.1016/s0008-6363(01)00331-5. [DOI] [PubMed] [Google Scholar]
- 29.Mitrovic V, Luss H, Nitsche K, Forssmann K, Maronde E, Fricke K, Forssmann WG, Meyer M. Effects of the renal natriuretic peptide urodilatin (ularitide) in patients with decompensated chronic heart failure: a double-blind, placebo-controlled, ascending-dose trial. Am Heart J. 2005;150:1239. doi: 10.1016/j.ahj.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Mitrovic V, Seferovic PM, Simeunovic D, Ristic AD, Miric M, Moiseyev VS, Kobalava Z, Nitsche K, Forssmann WG, Luss H, Meyer M. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur Heart J. 2006;27:2823. doi: 10.1093/eurheartj/ehl337. [DOI] [PubMed] [Google Scholar]
- 31.TRUE-AHF US National Library of Medicine. Clinical trials.gov [online] 2012 http://clinicaltrials.gov/ct2/show/NCT01661634.
- 32.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 33.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 34.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 35.Arora RR, Venkatesh PK, Molnar J. Short and long-term mortality with nesiritide. Am Heart J. 2006;152:1084–1090. doi: 10.1016/j.ahj.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 37.Kurien S, Warfield KT, Wood CM, Miller WL. Effects of standard heart failure therapy and concomitant treatment with intravenous furosemide or inotropes (dobutamine, dopamine, and/or milrinone) on renal function and mortality in patients treated with nesiritide. Am J Cardiol. 2006;98:1627–1630. doi: 10.1016/j.amjcard.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 38.Arora S, Clarke K, Srinivasan V, Gradman A. Effect of nesiritide on renal function in patients admitted for decompensated heart failure. QJM. 2007;100:699–706. doi: 10.1093/qjmed/hcm089. [DOI] [PubMed] [Google Scholar]
- 39.Witteles RM, Kao D, Christopherson D, Matsuda K, Vagelos RH, Schreiber D, Fowler MB. Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre-existing renal dysfunction a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol. 2007;50:1835–1840. doi: 10.1016/j.jacc.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 40.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr., Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 41.Kato T, Muraski J, Chen Y, Tsujita Y, Wall J, Glembotski CC, Schaefer E, Beckerle M, Sussman MA. Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and Akt. J Clin Invest. 2005;115:2716–2730. doi: 10.1172/JCI24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC., Jr. Novel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptide. J Am Coll Cardiol. 2012;60:2305–2312. doi: 10.1016/j.jacc.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC., Jr. Presence of C- type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol. 1992;263:H1318–1321. doi: 10.1152/ajpheart.1992.263.4.H1318. [DOI] [PubMed] [Google Scholar]
- 44.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”. J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattingly MT, Brandt RR, Heublein DM, Wei CM, Nir A, Burnett JC., Jr. Presence of C-type natriuretic peptide in human kidney and urine. Kidney Int. 1994;46:744–747. doi: 10.1038/ki.1994.329. [DOI] [PubMed] [Google Scholar]
- 46.Sangaralingham SJ, Heublein DM, Grande JP, Cataliotti A, Rule AD, McKie PM, Martin FL, Burnett JC., Jr. Urinary C-type natriuretic peptide excretion: a potential novel biomarker for renal fibrosis during aging. Am J Physiol Renal Physiol. 2011;301:F943–952. doi: 10.1152/ajprenal.00170.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A. Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation. 2004;110:1231–1235. doi: 10.1161/01.CIR.0000141802.29945.34. [DOI] [PubMed] [Google Scholar]
- 48.Sangaralingham SJ, Huntley BK, Martin FL, McKie PM, Bellavia D, Ichiki T, Harders GE, Chen HH, Burnett JC., Jr. The aging heart, myocardial fibrosis, and its relationship to circulating C-type natriuretic Peptide. Hypertension. 2011;57:201–207. doi: 10.1161/HYPERTENSIONAHA.110.160796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scotland RS, Cohen M, Foster P, Lovell M, Mathur A, Ahluwalia A, Hobbs AJ. C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc Natl Acad Sci U S A. 2005;102:14452–14457. doi: 10.1073/pnas.0504961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohno N, Itoh H, Ikeda T, Ueyama K, Yamahara K, Doi K, Yamashita J, Inoue M, Masatsugu K, Sawada N, Fukunaga Y, Sakaguchi S, Sone M, Yurugi T, Kook H, Komeda M, Nakao K. Accelerated reendothelialization with suppressed thrombogenic property and neointimal hyperplasia of rabbit jugular vein grafts by adenovirus-mediated gene transfer of C-type natriuretic peptide. Circulation. 2002;105:1623–1626. doi: 10.1161/01.cir.0000014985.50017.6e. [DOI] [PubMed] [Google Scholar]
- 52.Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, Kojima M, Kawano Y, Kangawa K. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003;144:2279–2284. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- 53.Soeki T, Kishimoto I, Okumura H, Tokudome T, Horio T, Mori K, Kangawa K. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2005;45:608–616. doi: 10.1016/j.jacc.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 54.Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J. 1993;291(Pt 1):83–88. doi: 10.1042/bj2910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roques BP, Noble F, Dauge V, Fournie-Zaluski MC, Beaumont A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev. 1993;45:87–146. [PubMed] [Google Scholar]
- 56.Ferro CJ, Spratt JC, Haynes WG, Webb DJ. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation. 1998;97:2323–2330. doi: 10.1161/01.cir.97.23.2323. [DOI] [PubMed] [Google Scholar]
- 57.Campbell DJ. Vasopeptidase inhibition: a double-edged sword? Hypertension. 2003;41:383–389. doi: 10.1161/01.HYP.0000054215.71691.16. [DOI] [PubMed] [Google Scholar]
- 58.Stephenson SL, Kenny AJ. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J. 1987;241:237–247. doi: 10.1042/bj2410237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ando S, Rahman MA, Butler GC, Senn BL, Floras JS. Comparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension. 1995;26:1160–1166. doi: 10.1161/01.hyp.26.6.1160. [DOI] [PubMed] [Google Scholar]
- 60.Martin FL, Stevens TL, Cataliotti A, Schirger JA, Borgeson DD, Redfield MM, Luchner A, Burnett JC., Jr. Natriuretic and antialdosterone actions of chronic oral NEP inhibition during progressive congestive heart failure. Kidney Int. 2005;67:1723–1730. doi: 10.1111/j.1523-1755.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 61.McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79–84. doi: 10.1517/13543784.8.1.79. [DOI] [PubMed] [Google Scholar]
- 62.Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr. Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52:60–68. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps). J Biol Chem. 1992;267:13928–13932. [PubMed] [Google Scholar]
- 64.Doi K, Itoh H, Ikeda T, Hosoda K, Ogawa Y, Igaki T, Yamashita J, Chun TH, Inoue M, Masatsugu K, Matsuda K, Ohmori K, Nakao K. Adenovirus-mediated gene transfer of C-type natriuretic peptide causes G1 growth inhibition of cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1997;239:889–894. doi: 10.1006/bbrc.1997.7576. [DOI] [PubMed] [Google Scholar]
- 65.Rosenkranz AC, Woods RL, Dusting GJ, Ritchie RH. Antihypertrophic actions of the natriuretic peptides in adult rat cardiomyocytes: importance of cyclic GMP. Cardiovasc Res. 2003;57:515–522. doi: 10.1016/s0008-6363(02)00667-3. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, de Waard MC, Sterner-Kock A, Stepan H, Schultheiss HP, Duncker DJ, Walther T. Cardiomyocyte-restricted over-expression of C-type natriuretic peptide prevents cardiac hypertrophy induced by myocardial infarction in mice. Eur J Heart Fail. 2007;9:548–557. doi: 10.1016/j.ejheart.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Wei CM, Aarhus LL, Miller VM, Burnett JC., Jr. Action of C-type natriuretic peptide in isolated canine arteries and veins. Am J Physiol. 1993;264:H71–73. doi: 10.1152/ajpheart.1993.264.1.H71. [DOI] [PubMed] [Google Scholar]
- 68.Lisy O, Jougasaki M, Heublein DM, Schirger JA, Chen HH, Wennberg PW, Burnett JC. Renal actions of synthetic dendroaspis natriuretic peptide. Kidney Int. 1999;56:502–508. doi: 10.1046/j.1523-1755.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- 69.Dickey DM, Burnett JC, Jr., Potter LR. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem. 2008;283:35003–35009. doi: 10.1074/jbc.M804538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dickey DM, Potter LR. Dendroaspis natriuretic peptide and the designer natriuretic peptide, CD-NP, are resistant to proteolytic inactivation. J Mol Cell Cardiol. 2011;51:67–71. doi: 10.1016/j.yjmcc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee CY, Chen HH, Lisy O, Swan S, Cannon C, Lieu HD, Burnett JC., Jr. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol. 2009;49:668–673. doi: 10.1177/0091270009336233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neutel J, Rolston W, Maddock S, Goldsmith S, Koren M, Van Antwerp B, Burnett JC, Jr., Lieu HD. Initial experience with subcutaneous infusion of cenderitide in patients with chronic heart failure. J Am Coll Cardiol. 2012;59:E1037. [Google Scholar]
- 73.Schulz-Knappe P, Forssmann K, Herbst F, Hock D, Pipkorn R, Forssmann WG. Isolation and structural analysis of “urodilatin”, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin Wochenschr. 1988;66:752–759. doi: 10.1007/BF01726570. [DOI] [PubMed] [Google Scholar]
- 74.Lee CY, Boerrigter G, Chen HH, Sandberg S, Heublein D, Harty G, Burnett JC., Jr. Cardiorenal and Neurohumoral Actions of a Novel Designer Natriuretic Peptide, CU-NP, In Canine Experimental Heart Failure. Circulation. 2008;118:S293. [Google Scholar]
- 75.Kilic A, Rajapurohitam V, Sandberg SM, Zeidan A, Hunter JC, Said Faruq N, Lee CY, Burnett JC, Jr., Karmazyn M. A novel chimeric natriuretic peptide reduces cardiomyocyte hypertrophy through the NHE-1-calcineurin pathway. Cardiovasc Res. 2010;88:434–442. doi: 10.1093/cvr/cvq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pan S, Chen HH, Dickey DM, Boerrigter G, Lee C, Kleppe LS, Hall JL, Lerman A, Redfield MM, Potter LR, Burnett JC, Jr., Simari RD. Biodesign of a renal-protective peptide based on alternative splicing of B-type natriuretic peptide. Proc Natl Acad Sci U S A. 2009;106:11282–11287. doi: 10.1073/pnas.0811851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Munagala VK, Burnett JC, Jr., Redfield MM. The natriuretic peptides in cardiovascular medicine. Curr Probl Cardiol. 2004;29:707–769. doi: 10.1016/j.cpcardiol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351:1657–1658. doi: 10.1016/s0140-6736(05)77712-6. [DOI] [PubMed] [Google Scholar]
- 79.Wallis EJ, Ramsay LE, Hettiarachchi J. Combined inhibition of neutral endopeptidase and angiotensin-converting enzyme by sampatrilat in essential hypertension. Clin Pharmacol Ther. 1998;64:439–449. doi: 10.1016/S0009-9236(98)90075-3. [DOI] [PubMed] [Google Scholar]
- 80.Norton GR, Woodiwiss AJ, Hartford C, Trifunovic B, Middlemost S, Lee A, Allen MJ. Sustained antihypertensive actions of a dual angiotensin-converting enzyme neutral endopeptidase inhibitor, sampatrilat, in black hypertensive subjects. Am J Hypertens. 1999;12:563–571. doi: 10.1016/s0895-7061(99)00009-6. [DOI] [PubMed] [Google Scholar]
- 81.Maki T, Nasa Y, Tanonaka K, Takahashi M, Takeo S. Beneficial effects of sampatrilat, a novel vasopeptidase inhibitor, on cardiac remodeling and function of rats with chronic heart failure following left coronary artery ligation. J Pharmacol Exp Ther. 2003;305:97–105. doi: 10.1124/jpet.102.042747. [DOI] [PubMed] [Google Scholar]
- 82.Trippodo NC, Fox M, Monticello TM, Panchal BC, Asaad MM. Vasopeptidase inhibition with omapatrilat improves cardiac geometry and survival in cardiomyopathic hamsters more than does ACE inhibition with captopril. J Cardiovasc Pharmacol. 1999;34:782–790. doi: 10.1097/00005344-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 83.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 84.Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, Porter CB, Proulx G, Qian C, Block AJ. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615–620. doi: 10.1016/s0140-6736(00)02602-7. [DOI] [PubMed] [Google Scholar]
- 85.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation. 2002;106:920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 86.Mellin V, Jeng AY, Monteil C, Renet S, Henry JP, Thuillez C, Mulder P. Triple ACE-ECE NEP inhibition in heart failure: a comparison with ACE and dual ECE-NEP inhibition. J.Cardiovasc.Pharmacol. 2005;46:390–397. doi: 10.1097/01.fjc.0000175457.48031.8b. [DOI] [PubMed] [Google Scholar]
- 87.Mulder P, Barbier S, Monteil C, Jeng AY, Henry JP, Renet S, Thuillez C. Sustained improvement of cardiac function and prevention of cardiac remodeling after long-term dual ECE-NEP inhibition in rats with congestive heart failure. J.Cardiovasc.Pharmacol. 2004;43:489–494. doi: 10.1097/00005344-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Emoto N, Raharjo SB, Isaka D, Masuda S, Adiarto S, Jeng AY, Yokoyama M. Dual ECE/NEP inhibition on cardiac and neurohumoral function during the transition from hypertrophy to heart failure in rats. Hypertension. 2005;45:1145–1152. doi: 10.1161/01.HYP.0000168944.29525.da. [DOI] [PubMed] [Google Scholar]
- 89.Dickstein K, De Voogd HJ, Miric MP, Willenbrock R, Mitrovic V, Pacher R, Koopman PA. Effect of single doses of SLV306, an inhibitor of both neutral endopeptidase and endothelin-converting enzyme, on pulmonary pressures in congestive heart failure. Am.J.Cardiol. 2004;94:237–239. doi: 10.1016/j.amjcard.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 90.Kalk P, Sharkovska Y, Kashina E, von Websky K, Relle K, Pfab T, Alter M, Guillaume P, Provost D, Hoffmann K, Fischer Y, Hocher B. Endothelin-converting enzyme/neutral endopeptidase inhibitor SLV338 prevents hypertensive cardiac remodeling in a blood pressure-independent manner. Hypertension. 2011;57:755–763. doi: 10.1161/HYPERTENSIONAHA.110.163972. [DOI] [PubMed] [Google Scholar]
- 91.Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010;50:401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 92.Hegde LG, Yu C, Renner T, Thibodeaux H, Armstrong SR, Park T, Cheruvu M, Olsufka R, Sandvik ER, Lane CE, Budman J, Hill CM, Klein U, Hegde SS. Concomitant angiotensin AT1 receptor antagonism and neprilysin inhibition produces omapatrilat-like antihypertensive effects without promoting tracheal plasma extravasation in the rat. J Cardiovasc Pharmacol. 2011;57:495–504. doi: 10.1097/FJC.0b013e318210fc7e. [DOI] [PubMed] [Google Scholar]
- 93.Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 94.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 95.PARADIGM-HF US National Library of Medicine. Clinical trials.gov [online] 2012 http://clinicaltrials.gov/ct2/show/NCT01035255?term=paradigm-HF&rank=1.
- 96.Allikmets K. Sampatrilat Shire. Curr Opin Investig Drugs. 2002;3:578–581. [PubMed] [Google Scholar]
- 97.Weber MA. Vasopeptidase inhibitors. Lancet. 2001;358:1525–1532. doi: 10.1016/S0140-6736(01)06584-9. [DOI] [PubMed] [Google Scholar]
- 98.Nussberger J, Cugno M, Amstutz C, Cicardi M, Pellacani A, Agostoni A. Plasma bradykinin in angio-oedema. Lancet. 1998;351:1693–1697. doi: 10.1016/S0140-6736(97)09137-X. [DOI] [PubMed] [Google Scholar]
- 99.Margulies KB, Hildebrand FL, Jr., Lerman A, Perrella MA, Burnett JC., Jr. Increased endothelin in experimental heart failure. Circulation. 1990;82:2226–2230. doi: 10.1161/01.cir.82.6.2226. [DOI] [PubMed] [Google Scholar]
- 100.Grantham JA, Schirger JA, Wennberg PW, Sandberg S, Heublein DM, Subkowski T, Burnett JC., Jr. Modulation of functionally active endothelin-converting enzyme by chronic neutral endopeptidase inhibition in experimental atherosclerosis. Circulation. 2000;101:1976–1981. doi: 10.1161/01.cir.101.16.1976. [DOI] [PubMed] [Google Scholar]
- 101.Kiowski W, Sutsch G, Hunziker P, Muller P, Kim J, Oechslin E, Schmitt R, Jones R, Bertel O. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346:732–736. doi: 10.1016/s0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- 102.Lerman A, Kubo SH, Tschumperlin LK, Burnett JC., Jr. Plasma endothelin concentrations in humans with end-stage heart failure and after heart transplantation. J.Am.Coll.Cardiol. 1992;20:849–853. doi: 10.1016/0735-1097(92)90183-n. [DOI] [PubMed] [Google Scholar]
- 103.Omland T, Lie RT, Aakvaag A, Aarsland T, Dickstein K. Plasma endothelin determination as a prognostic indicator of 1-year mortality after acute myocardial infarction. Circulation. 1994;89:1573–1579. doi: 10.1161/01.cir.89.4.1573. [DOI] [PubMed] [Google Scholar]
- 104.von Lueder TG, Kjekshus H, Edvardsen T, Øie E, Urheim S, Vinge LE, Ahmed MS, Smiseth OA, Attramadal H. Mechanisms of elevated plasma endothelin-1 in CHF: congestion increases pulmonary synthesis and secretion of endothelin-1. Cardiovasc Res. 2004;63:41–50. doi: 10.1016/j.cardiores.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 105.Wada A, Tsutamoto T, Ohnishi M, Sawaki M, Fukai D, Maeda Y, Kinoshita M. Effects of a specific endothelin-converting enzyme inhibitor on cardiac, renal, and neurohumoral functions in congestive heart failure: comparison of effects with those of endothelin A receptor antagonism. Circulation. 1999;99:570–577. doi: 10.1161/01.cir.99.4.570. [DOI] [PubMed] [Google Scholar]
- 106.Martin P, Tzanidis A, Stein-Oakley A, Krum H. Effect of a highly selective endothelin- converting enzyme inhibitor on cardiac remodeling in rats after myocardial infarction. J.Cardiovasc.Pharmacol. 2000;36:S367–S370. doi: 10.1097/00005344-200036051-00106. [DOI] [PubMed] [Google Scholar]
- 107.Wada A, Ohnishi M, Tsutamoto T, Fujii M, Matsumoto T, Yamamoto T, Wang X, Kinoshita M. Chronic effects of an endothelin-converting enzyme inhibitor on cardiorenal and hormonal function in heart failure. Clin.Sci.(Lond) 2002;103(Suppl 1):254S–257S. doi: 10.1042/CS103S254S. [DOI] [PubMed] [Google Scholar]
- 108.Nakayama K, Emoto N, Suzuki Y, Vignon-Zellweger N, Yagi K, Hirata K. Physiological relevance of hydrolysis of atrial natriuretic peptide by endothelin-converting enzyme-1. Kobe J Med Sci. 2012;58:E12–18. [PubMed] [Google Scholar]
- 109.Love MP, Haynes WG, Gray GA, Webb DJ, McMurray JJ. Vasodilator effects of endothelin- converting enzyme inhibition and endothelin ETA receptor blockade in chronic heart failure patients treated with ACE inhibitors. Circulation. 1996;94:2131–2137. doi: 10.1161/01.cir.94.9.2131. [DOI] [PubMed] [Google Scholar]
- 110.Seed A, Kuc RE, Maguire JJ, Hillier C, Johnston F, Essers H, de Voogd HJ, McMurray J, Davenport AP. The dual endothelin converting enzyme/neutral endopeptidase inhibitor SLV-306 (daglutril), inhibits systemic conversion of big endothelin-1 in humans. Life Sci. 2012;91:743–748. doi: 10.1016/j.lfs.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 111.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 112.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur.Heart J. 2010;31:1771–1779. doi: 10.1093/eurheartj/ehp574. [DOI] [PubMed] [Google Scholar]
- 113.Von Lueder TG, Wang B, Kompa A, Huang L, Webb R, Jordan P, Krum H. ARNi, combined neprilysin and angiotensin receptor inhibition augments anti-fibrotic and anti-hypertrophic effects in vitro. Eur.Heart J. 2012;33:P5834. [Google Scholar]
- 114.Lee CY, Burnett JC., Jr. Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12:131–142. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 115.Cleland JG, Clark AL. Heart failure--does it matter whether LVEF is reduced? Lancet. 2012;380:1363–1365. doi: 10.1016/S0140-6736(12)61349-X. [DOI] [PubMed] [Google Scholar]
- 116.Kurtz TW, Klein U. Next generation multifunctional angiotensin receptor blockers. Hypertens Res. 2009;32:826–834. doi: 10.1038/hr.2009.135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.