Abstract
EMBO J (2013) 32: 3130–3144 doi: ; DOI: 10.1038/emboj.2013.233; published online November 01 2013
Amphisomes are intermediate organelles, formed during autophagy through the fusion between autophagosomes and endosomes. Complex multivesicular vacuoles that resemble amphisomes have been observed in various cell types, but whether they have cellular roles other than being a precursor structure is still enigmatic. While autophagy-related (ATG) proteins interact with the endocytic pathways in other processes different from autophagy, Patel and colleagues now report that these factors come together to generate amphisome-like compartments that regulate mucin secretion in goblet cells.
ATG and endosomal proteins have been linked to secretion, and the specific loss of them impairs the function of different secretory cell types (Jung et al, 2008; DeSelm et al, 2011; Ushio et al, 2011; Sasidharan et al, 2012). ATG proteins have also been shown to interact with the endocytic pathway in few situations that do not involve autophagy. For example in phagocytic cells, the surface of bacteria-containing phagosomes acquires LC3/Atg8 through the concerted action of a subpopulation of ATG proteins. This process, which has been termed LC3-associated phagocytosis (LAP), promotes the fusion of phagosomes with lysosomes (Sanjuan et al, 2007). Something similar occurs during entotic cell death, an engulfment programme leading to the elimination of cells into lysosomes. The entotic vacuole membranes surrounding the internalized cells also recruit LC3 through a mechanism that depends on several ATG proteins, but not on autophagosome formation (Florey et al, 2011).
In their work aimed to understand the function of ATG proteins in goblet cells, Patel et al (2013) show that the autophagy and endocytic machinery converge at the amphisomes to promote the secretion of mucins. In the gastrointestinal tract, secretory cells have a crucial role in providing the mucus barrier that protects against intestinal pathogens. Mucins, the main components of the mucus, are produced in goblet cells where large polymers of these highly glycosylated proteins are packed into secretory granules that accumulate at the apical surface. The release of these mucin granules relies on a series of cellular events that are tightly coordinated. Patel et al (2013) show that knockout mice lacking ATG5 in the intestinal epithelium, that is, Atg5VC mice, exhibit both a dramatic accumulation of mucin granules in goblet cells and a diminished mucus secretion. Taking advantage of a newly developed in vitro system to culture and differentiate intestinal epithelial stem cells into secretory goblet cells, the authors also demonstrate that the ablation of other ATG proteins causes the same phenotype showing that the autophagy machinery is required for mucin secretion in these specialized cells (Patel et al, 2013). Interestingly, ATG proteins affect the functionality of another gastrointestinal secretory lineage, the Paneth cells. Paneth cells homozygous for the atg16L1 risk allele, associated with Crohn disease, produce less secretory granules than in controls (Cadwell et al, 2008). This suggests that although ATG proteins regulate secretion in the two most abundant secretory lineages in the intestinal tract, two different mechanisms are probably involved.
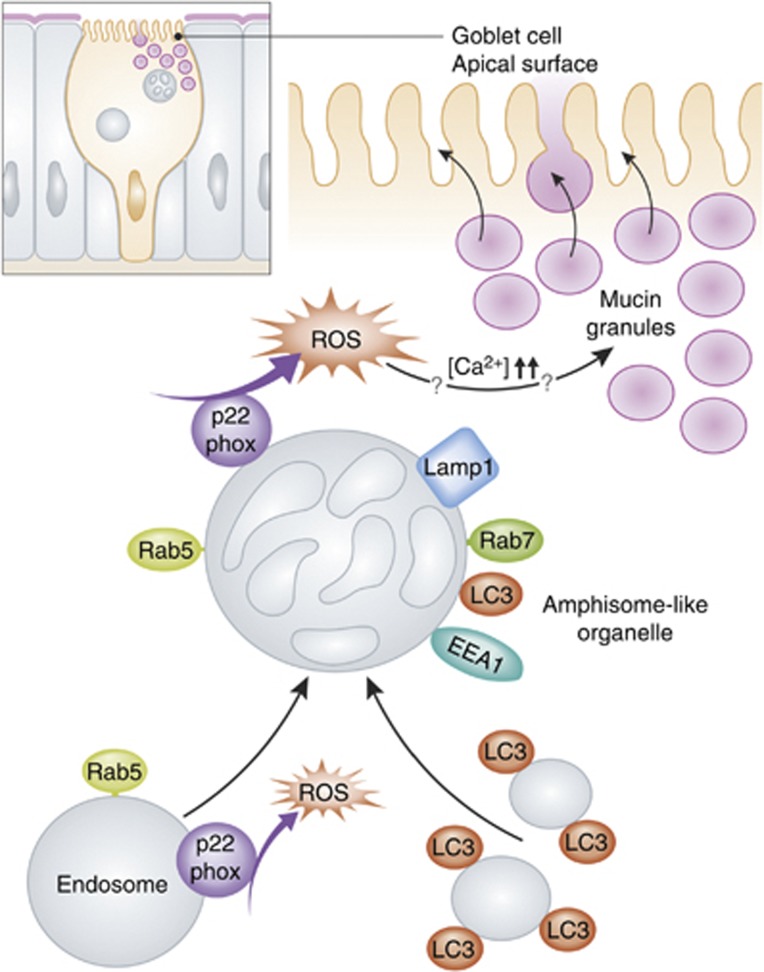
A microarray analysis of mRNA from Atg5VC mouse colonic epithelial cells revealed a possible alteration in the endocytic pathway. Indeed, blocking endocytosis also provoked an accumulation of mucin granules. While LC3B has been previously found on the surface of secretory granules (Ushio et al, 2011; Ishibashi et al, 2012), immuno-electron microscopy of wild-type mouse intestinal tissue revealed a distribution of LC3B not on mucin granules, but on multivesicular vacuoles positive for several endosomal proteins (Patel et al, 2013). Because of the morphological and molecular characteristics of these compartments, it appears that the ATG proteins together with the endocytic pathway regulate secretion in goblet cells by converging in what could be a new amphisome-like organelle (Figure 1).
Figure 1.
Schematic representation for the regulated secretion of mucin granules by amphisome-like structures in goblet cells. ROS generated by NADPH oxidases promote the fusion of LC3-positive vesicles with endosomes marked by Rab5 and containing the NADPH oxidase subunit p22phox. The resulting amphisomes-like organelles are decorated with LC3, endosomal proteins (Rab5, Rab7 and EEA1) and p22phox and localize near the mucin granules. The formation of these copartments probably prolong and/or enhance the production of ROS by the NADPH oxidase, which in turn increases the levels of cytoplasmic calcium through an unknown mechanism leading to the release of the mucin granules.
NADPH oxidases are known to be present in endosomes, and NADPH oxidase-generated reactive oxygen species (ROS) are necessary for LC3 recruitment to phagosomes.(Huang et al, 2009). Patel et al (2013) thus explored whether these enzymes played a role in mucin granule secretion in goblet cells. Indeed, expression of a mutant form of p22phox, a transmembrane subunit of several NADPH oxidase complexes, altered the exocytosis of these carriers. Moreover, p22phox was found to localize to Rab5-positive endosomes and also with the observed amphisome-like structures (Figure 1). Because a mutant form of p22phox also caused a misslocalization of both LC3 and the early-endosomal marker protein EEA1, the obvious conclusion was that ROS production by endosomes is necessary to trigger the formation of the amphisome-like organelles via the acquisition of the ATG machinery (Figure 1). Interestingly, addition of H2O2 that mimics ROS generation was able to induce mucin granule exocytosis in the p22phox mutant cells, showing that ROS was also required to regulate secretion in goblet cells (Patel et al, 2013). Furthermore, H2O2 bypassed as well the mucin granule secretion defect in autophagy and endocytosis-deficient goblet cells through an increase of cytosolic calcium levels (Patel et al, 2013). This, together with the observation that the loss of ATG5 and the block of the endocytic pathway impair the production of ROS has led Patel et al (2013) to propose that amphisome-like organelles are a signalling platform, where NADPH oxidase-driven ROS production promotes the release of the mucin granules.
Amphisomes have been characterized and defined as autophagic vacuoles formed upon fusion between autophagosomes and endosomes. Given that ATG and endosomal proteins converge in multivesicular and/or vacuolar compartments resembling amphisomes in cellular processes independent of autophagy, one could consider to use the term amphisomes to describe a more heterogenous and ampler population of unnamed compartments where part of the autophagy and endosomal machineries co-localize. Based on this consideration, the study by Patel et al (2013) has identified an amphisome-like structure where molecular events interconnect to trigger granule secretion. While their work adds to the still limited number of non-degradative roles of the autophagic pathway, which include unconventional secretion (Subramani and Malhotra, 2013), it is one of the first reports highlighting that amphisomes (or any autophagosomal intermediate structure) could be more than just a transport intermediate, and at least in goblet cells, they could act as a platform where signals integrating some aspects of the cell physiology are elicited.
Though it remains to be establish whether the organelles described by Patel et al (2013) are indeed amphisomes, especially as they are formed by fusion of endosomes with LC3-positive single-membrane vesicles rather than LC3-positive double-membrane autophagosomes, their study raises some intriguing questions. Are these compartments persistent or will they eventually fuse with lysosomes? Why has the cell opted to signal from amphisomes and not from endosomes, where the NADPH oxidases are normally present? Maybe the answer to these questions is hidden in the transient life of amphisomes. In the most classical signalling pathways, the transduction cascade amplifies the initial cue but it also turn it off subsequently through negative feedback loops. This permits to precisely modulate the signal output temporally (and locally). The amphisome-like structures observed in goblet cells could also act as the molecular switch for the signal-stimulating mucin granule secretion. The ROS generated initially from endosomes would trigger the recruitment of LC3 through vesicle fusion events, and the production of this second messenger will be prolonged and/or enhanced in the resulting amphisomes-like structure, leading to a stimulation of mucin granule exocytosis (Figure 1). The subsequent fusion of the amphisomes with lysosomes could lead to the termination of the signal. Other scenarios, however, cannot be excluded like, for example, the delivery of a protein enhancing the NADPH oxidase activity to the endosomes by the LC3-positive vesicles.
While these are just hypotheses, it is clear that Patel et al (2013) have opened a window on a new and unexplored area of the autophagy field. Future investigations will tell us whether what observed in goblet cells is a unique situation or the intermediate organelles characterizing autophagy can carry out cellular functions different from the one delivering unwanted structures into the lysosome interior for degradation, including to serve as signalling platforms.
Acknowledgments
FR is supported by ECHO (700.59.003), ALW Open Program (821.02.017 and 822.02.014), DFG-NWO cooperation (DN82-303) and ZonMW VICI (016.130.606) grants.
Footnotes
The authors declare that they have no conflict of interest.
References
- Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HWt (2008) A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456: 259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSelm CJ, Miller BC, Zou W, Beatty WL, van Meel E, Takahata Y, Klumperman J, Tooze SA, Teitelbaum SL, Virgin HW (2011) Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell 21: 966–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M (2011) Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol 13: 1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, Glogauer M, Grinstein S, Brumell JH (2009) Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci USA 106: 6226–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi K, Uemura T, Waguri S, Fukuda M (2012) Atg16L1, an essential factor for canonical autophagy, participates in hormone secretion from PC12 cells independently of autophagic activity. Mol Biol Cell 23: 3193–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS (2008) Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metabol 8: 318–324 [DOI] [PubMed] [Google Scholar]
- Patel KK, Miyoshi H, Beatty WL, Head RD, Malvin NP, Cadwell K, Guan J-L, Saitoh T, Akira S, Seglen PO, Dinauer MC, Virgin HW, Stappenbeck TS (2013) Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J 32: 3130–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR (2007) Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450: 1253–1257 [DOI] [PubMed] [Google Scholar]
- Sasidharan N, Sumakovic M, Hannemann M, Hegermann J, Liewald JF, Olendrowitz C, Koenig S, Grant BD, Rizzoli SO, Gottschalk A, Eimer S (2012) RAB-5 and RAB-10 cooperate to regulate neuropeptide release in Caenorhabditis elegans. Proc Natl Acad Sci USA 109: 18944–18949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S, Malhotra V (2013) Non-autophagic roles of autophagy-related proteins. EMBO Rep 14: 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio H, Ueno T, Kojima Y, Komatsu M, Tanaka S, Yamamoto A, Ichimura Y, Ezaki J, Nishida K, Komazawa-Sakon S, Niyonsaba F, Ishii T, Yanagawa T, Kominami E, Ogawa H, Okumura K, Nakano H (2011) Crucial role for autophagy in degranulation of mast cells. J Allergy Clin Immunol 127: e1266. [DOI] [PubMed] [Google Scholar]