Abstract
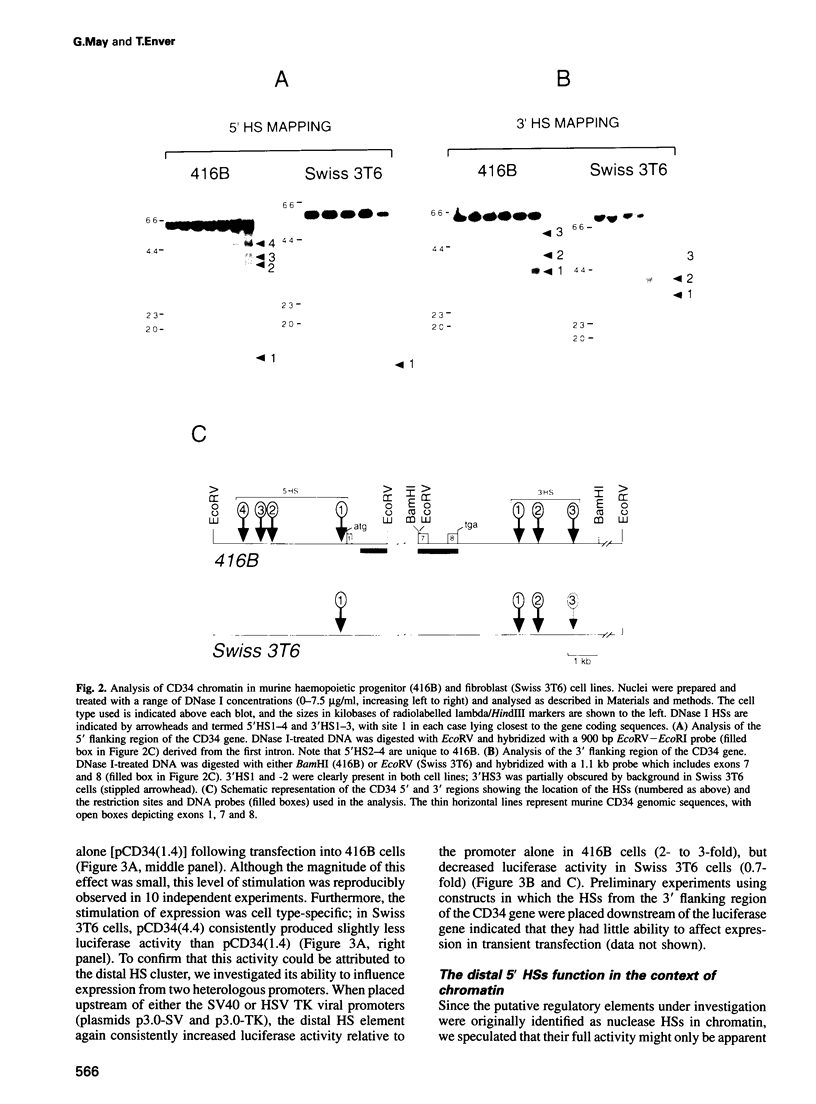
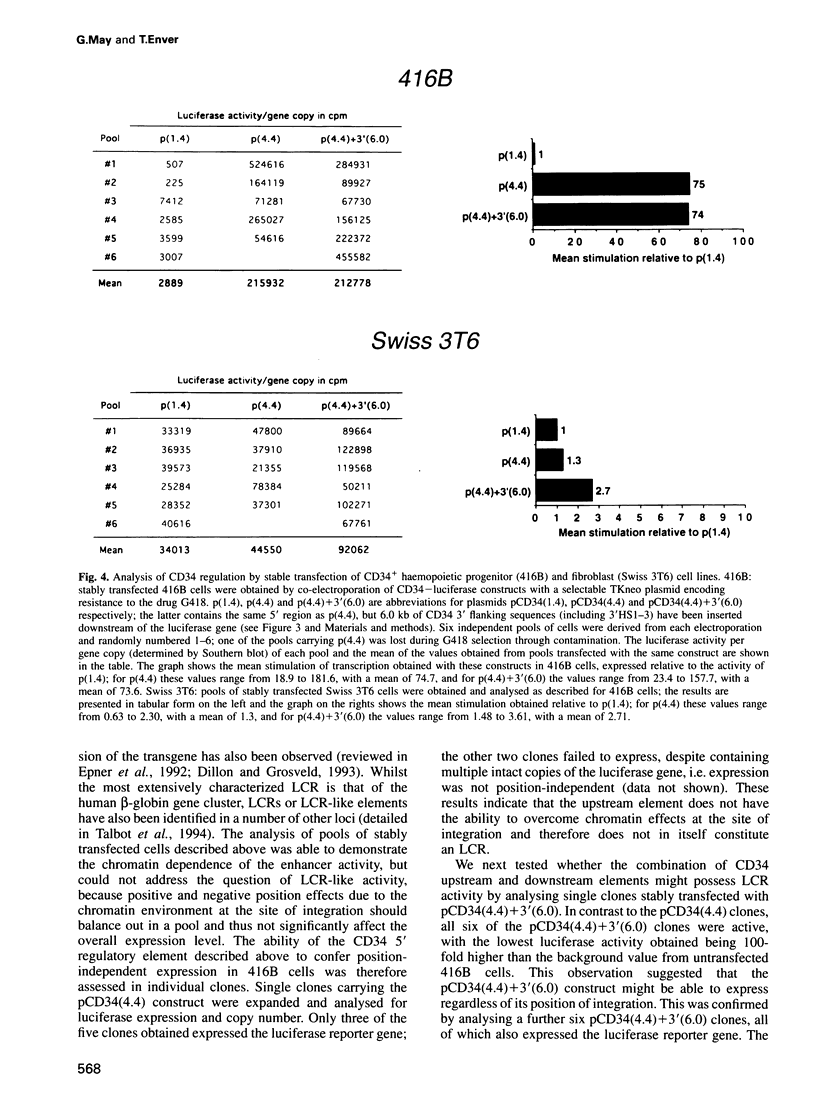
The ability to target heterologous gene expression to haemopoietic stem cells will allow biological manipulation of this compartment and facilitate gene therapy of blood disorders. To identify regulatory elements with this potential, we have analysed the transcriptional regulation of the murine stem cell antigen CD34. Within haemopoiesis CD34 is expressed in stem and early progenitor cells, but it is also expressed in certain non-haemopoietic cell types, including fibroblasts. Comparison of CD34 chromatin in haemopoietic progenitor cells (416B and M1) and fibroblasts (Swiss 3T6) revealed several DNase I hypersensitive regions, one of which, a cluster of sites centred 3 kb upstream of exon 1, was specific to haemopoietic progenitors. This element stimulated expression by approximately two orders of magnitude in CD34+ haemopoietic progenitors, but not in CD34+ fibroblasts or in CD34- haemopoietic cells (18.8). Enhancer function was dependent upon chromosomal integration, although position-independent expression was not obtained. However, largely position-independent expression was conferred by addition of a downstream element which was hypersensitive in all the cell types analysed. We conclude that the murine CD34 gene is regulated by a chromatin-dependent, upstream enhancer element which acts in conjunction with a downstream domain-controlling element to confer high level gene expression in haemopoietic progenitor cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumheter S., Singer M. S., Henzel W., Hemmerich S., Renz M., Rosen S. D., Lasky L. A. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993 Oct 15;262(5132):436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- Berenson R. J., Andrews R. G., Bensinger W. I., Kalamasz D., Knitter G., Buckner C. D., Bernstein I. D. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest. 1988 Mar;81(3):951–955. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson R. J., Bensinger W. I., Hill R. S., Andrews R. G., Garcia-Lopez J., Kalamasz D. F., Still B. J., Spitzer G., Buckner C. D., Bernstein I. D. Engraftment after infusion of CD34+ marrow cells in patients with breast cancer or neuroblastoma. Blood. 1991 Apr 15;77(8):1717–1722. [PubMed] [Google Scholar]
- Blom van Assendelft G., Hanscombe O., Grosveld F., Greaves D. R. The beta-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989 Mar 24;56(6):969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- Brown J., Greaves M. F., Molgaard H. V. The gene encoding the stem cell antigen, CD34, is conserved in mouse and expressed in haemopoietic progenitor cell lines, brain, and embryonic fibroblasts. Int Immunol. 1991 Feb;3(2):175–184. doi: 10.1093/intimm/3.2.175. [DOI] [PubMed] [Google Scholar]
- Burn T. C., Satterthwaite A. B., Tenen D. G. The human CD34 hematopoietic stem cell antigen promoter and a 3' enhancer direct hematopoietic expression in tissue culture. Blood. 1992 Dec 15;80(12):3051–3059. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cray C., Keane R. W., Malek T. R., Levy R. B. Regulation and selective expression of Ly-6A/E, a lymphocyte activation molecule, in the central nervous system. Brain Res Mol Brain Res. 1990 Jun;8(1):9–15. doi: 10.1016/0169-328x(90)90003-v. [DOI] [PubMed] [Google Scholar]
- Cross M., Dexter T. M. Growth factors in development, transformation, and tumorigenesis. Cell. 1991 Jan 25;64(2):271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Scott D., Teich N. M. Isolation and characterisation of a bipotential haematopoietic cell line. Nature. 1979 Feb 8;277(5696):471–474. doi: 10.1038/277471a0. [DOI] [PubMed] [Google Scholar]
- Dillon N., Grosveld F. Transcriptional regulation of multigene loci: multilevel control. Trends Genet. 1993 Apr;9(4):134–137. doi: 10.1016/0168-9525(93)90208-y. [DOI] [PubMed] [Google Scholar]
- Enver T., Brice M., Karlinsey J., Stamatoyannopoulos G., Papayannopoulou T. Developmental regulation of fetal to adult globin gene switching in human fetal erythroid x mouse erythroleukemia cell hybrids. Dev Biol. 1991 Nov;148(1):129–137. doi: 10.1016/0012-1606(91)90323-u. [DOI] [PubMed] [Google Scholar]
- Epner E., Kim C. G., Groudine M. What does the locus control region control? Curr Biol. 1992 May;2(5):262–264. doi: 10.1016/0960-9822(92)90379-o. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Ford A. M., Healy L. E., Bennett C. A., Navarro E., Spooncer E., Greaves M. F. Multilineage phenotypes of interleukin-3-dependent progenitor cells. Blood. 1992 Apr 15;79(8):1962–1971. [PubMed] [Google Scholar]
- Ford A. M., Watt S. M., Furley A. J., Molgaard H. V., Greaves M. F. Cell lineage specificity of chromatin configuration around the immunoglobulin heavy chain enhancer. EMBO J. 1988 Aug;7(8):2393–2399. doi: 10.1002/j.1460-2075.1988.tb03084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Epner E., Driscoll M. C., Enver T., Brice M., Papayannopoulou T., Groudine M. A deletion of the human beta-globin locus activation region causes a major alteration in chromatin structure and replication across the entire beta-globin locus. Genes Dev. 1990 Oct;4(10):1637–1649. doi: 10.1101/gad.4.10.1637. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Thompson C., Elder J. T., Groudine M. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1359–1363. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown J., Molgaard H. V., Spurr N. K., Robertson D., Delia D., Sutherland D. R. Molecular features of CD34: a hemopoietic progenitor cell-associated molecule. Leukemia. 1992;6 (Suppl 1):31–36. [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- He X. Y., Cockerill P. N., Cen D., Davis B. R. Transcriptional regulation and chromatin structure of the human CD34 gene promoter region. Blood. 1994 Apr 1;83(7):1822–1830. [PubMed] [Google Scholar]
- Keshet E., Lyman S. D., Williams D. E., Anderson D. M., Jenkins N. A., Copeland N. G., Parada L. F. Embryonic RNA expression patterns of the c-kit receptor and its cognate ligand suggest multiple functional roles in mouse development. EMBO J. 1991 Sep;10(9):2425–2435. doi: 10.1002/j.1460-2075.1991.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K. D., Lindwall G., Maher S. E., Bothwell A. L. Characterization of promoter elements of an interferon-inducible Ly-6E/A differentiation antigen, which is expressed on activated T cells and hematopoietic stem cells. Mol Cell Biol. 1990 Oct;10(10):5150–5159. doi: 10.1128/mcb.10.10.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. S., Ito T., Fackler M. J., Smith O. M., Collector M. I., Sharkis S. J., May W. S. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994 Aug 1;84(3):691–701. [PubMed] [Google Scholar]
- Matthews W., Jordan C. T., Wiegand G. W., Pardoll D., Lemischka I. R. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991 Jun 28;65(7):1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- Nocka K., Majumder S., Chabot B., Ray P., Cervone M., Bernstein A., Besmer P. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice--evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989 Jun;3(6):816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Parslow T. G., Granner D. K. Chromatin changes accompany immunoglobulin kappa gene activation: a potential control region within the gene. Nature. 1982 Sep 30;299(5882):449–451. doi: 10.1038/299449a0. [DOI] [PubMed] [Google Scholar]
- Rosnet O., Marchetto S., deLapeyriere O., Birnbaum D. Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene. 1991 Sep;6(9):1641–1650. [PubMed] [Google Scholar]
- Salles G., Chen C. Y., Reinherz E. L., Shipp M. A. CD10/NEP is expressed on Thy-1low B220+ murine B-cell progenitors and functions to regulate stromal cell-dependent lymphopoiesis. Blood. 1992 Oct 15;80(8):2021–2029. [PubMed] [Google Scholar]
- Satterthwaite A. B., Burn T. C., Le Beau M. M., Tenen D. G. Structure of the gene encoding CD34, a human hematopoietic stem cell antigen. Genomics. 1992 Apr;12(4):788–794. doi: 10.1016/0888-7543(92)90310-o. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Sinclair A. M., Dzierzak E. A. Cloning of the complete Ly-6E.1 gene and identification of DNase I hypersensitive sites corresponding to expression in hematopoietic cells. Blood. 1993 Nov 15;82(10):3052–3062. [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Spooncer E., Boettiger D., Dexter T. M. Continuous in vitro generation of multipotential stem cell clones from src-infected cultures. Nature. 1984 Jul 19;310(5974):228–230. doi: 10.1038/310228a0. [DOI] [PubMed] [Google Scholar]
- Talbot D., Collis P., Antoniou M., Vidal M., Grosveld F., Greaves D. R. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature. 1989 Mar 23;338(6213):352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- Talbot D., Descombes P., Schibler U. The 5' flanking region of the rat LAP (C/EBP beta) gene can direct high-level, position-independent, copy number-dependent expression in multiple tissues in transgenic mice. Nucleic Acids Res. 1994 Mar 11;22(5):756–766. doi: 10.1093/nar/22.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott S. J., Lassar A. B., Weintraub H. A novel myoblast enhancer element mediates MyoD transcription. Mol Cell Biol. 1992 Nov;12(11):4994–5003. doi: 10.1128/mcb.12.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Tojo A., Aoki N., Shibuya M. Characterization of the promoter region of the human c-kit proto-oncogene. Jpn J Cancer Res. 1993 Nov;84(11):1136–1144. doi: 10.1111/j.1349-7006.1993.tb02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H., Galli S. J., Geissler E. N. Cloning and functional analysis of the mouse c-kit promoter. Biochem Biophys Res Commun. 1993 Mar 31;191(3):893–901. doi: 10.1006/bbrc.1993.1301. [DOI] [PubMed] [Google Scholar]
- van de Rijn M., Heimfeld S., Spangrude G. J., Weissman I. L. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]