Abstract
Piwi proteins and Piwi-interacting RNAs (piRNAs) repress transposition, regulate translation, and guide epigenetic programming in the germline. Here, we show that an evolutionarily conserved Tudor and KH domain-containing protein, Tdrkh (a.k.a. Tdrd2), is required for spermatogenesis and involved in piRNA biogenesis. Tdrkh partners with Miwi and Miwi2 via symmetrically dimethylated arginine residues in Miwi and Miwi2. Tdrkh is a mitochondrial protein often juxtaposed to pi-bodies and piP-bodies and is required for Tdrd1 cytoplasmic localization and Miwi2 nuclear localization. Tdrkh mutants display meiotic arrest at the zygotene stage, attenuate methylation of Line1 DNA, and upregulate Line1 RNA and protein, without inducing apoptosis. Furthermore, Tdrkh mutants have severely reduced levels of mature piRNAs but accumulate a distinct population of 1′U-containing, 2′O-methylated 31–37 nt RNAs that largely complement the missing mature piRNAs. Our results demonstrate that the primary piRNA biogenesis pathway involves 3′→5′ processing of 31–37 nt intermediates and that Tdrkh promotes this final step of piRNA biogenesis but not the ping-pong cycle. These results shed light on mechanisms underlying primary piRNA biogenesis, an area in which information is conspicuously absent.
Keywords: Miwi, piRNA, Piwi, spermatogenesis, Tdrd
Introduction
The establishment and maintenance of the germline are essential for passing genetic information from one generation to the next. The Piwi proteins, a subfamily of the Piwi/Argonaute family (Cox et al, 1998), play a central role in germline development and gametogenesis (Thomson and Lin, 2009; Siomi et al, 2011). There are three murine Piwi proteins, Mili, Miwi, and Miwi2. Mili and Miwi2 act in concert to mediate DNA methylation of transposons. Spermatogenic arrest is coincident with loss of DNA methylation of mobile genetic elements and their resultant activation in Mili and Miwi2 mutants (Aravin et al, 2007; Kuramochi-Miyagawa et al, 2008). Piwi-interacting RNAs (piRNAs) bind to Piwi proteins and are hypothesized to act as sequence-specific guides to recruit epigenetic machinery to genomic sites complementary to their sequences (Lin and Yin, 2008; Watanabe et al, 2011b; Huang et al, 2013). They are derived from cellular transcripts, including gene-coding mRNAs and retrotransposons, and specific intergenic loci (Aravin et al, 2006; Girard et al, 2006; Grivna et al, 2006a; Lau et al, 2006). Mili functions in both primary and secondary piRNA biogenesis, whereas Miwi2 functions mainly in the secondary biogenesis pathway (also referred to as the ping-pong cycle; Siomi et al, 2011).
In the primary pathway, cellular transcripts are recruited and processed through a mostly uncharacterized mechanism that relies on the scaffolding protein Tdrd1 for proper function (Reuter et al, 2009; Mathioudakis et al, 2012). Mature primary piRNAs are bound by Mili and Miwi (Beyret et al, 2012). Recent reports have demonstrated a complete lack of piRNAs following mutations of Mov10l1 and MitoPLD (the mouse homologue of Drosophila Zuc), implicating these genes in primary piRNA processing (Zheng et al, 2010; Watanabe et al, 2011a). Here, we report that Tdrkh, a Tudor and KH domain-containing protein, interacts with Miwi and Miwi2. Mutation of tdrkh results in male sterility due to meiotic defects with concurrent loss of retrotransposon silencing. piRNA populations are severely reduced in Tdrkh mutants, with piRNA biogenesis blocked at a novel intermediate state resulting in accumulation of a class of previously uncharacterized precursor RNAs. Our data demonstrate that Tdrkh is a component of the primary piRNA processing pathway and facilitates processing and maturation of the 3′ ends of piRNAs.
Results
Tdrkh interacts with Miwi and Miwi2 in vitro and in vitro
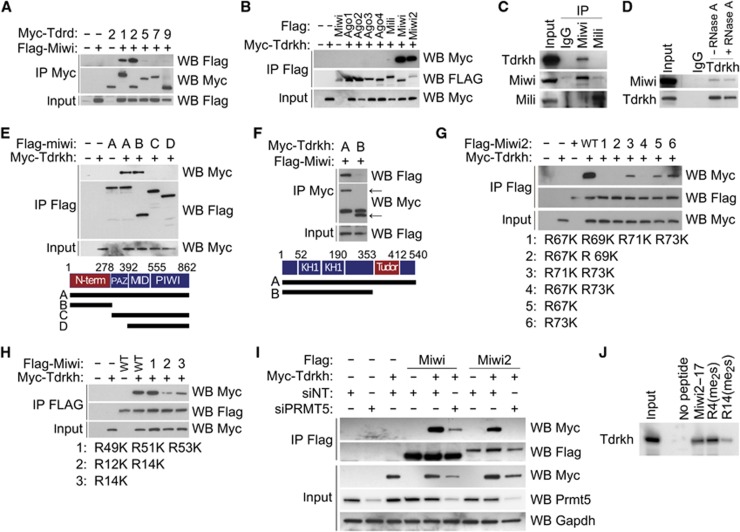
Tdrkh was identified as an interacting partner of mouse Miwi, but not Mili (Chen et al, 2009; Vagin et al, 2009; Wang et al, 2009). Tdrkh and its fly homologue Papi (Liu et al, 2011) are 24% identical and 37% similar, with similarity approaching 56% within the conserved Tudor domain (Supplementary Figure 1). Miwi was preferentially pulled down by Tdrkh when co-transfected in 293T cells with five different Tudor domain-containing proteins (Tdrds; Figure 1A). In the reciprocal experiment, Tdrkh was specifically pulled down by Miwi and Miwi2 (Figure 1B); only a slight interaction with Mili was noted. In adult testes, Tdrkh and Miwi reciprocally co-immunoprecipitated each other (Figure 1C and D), but no in vivo interaction between Mili and Tdrkh was noted (Figure 1C). Furthermore, Tdrkh and Miwi2 co-localize in pre-natal testes (Supplementary Figure 3h). Thus, Tdrkh specifically interacts with Miwi and Miwi2.
Figure 1.
Tdrkh interacts with Miwi and Miwi2. (A) FLAG-tagged Miwi and myc-tagged Tdrd proteins were co-transfected into 293T cells followed by immunoprecipitation of the myc-tagged proteins. Tdrkh is also known as Tdrd2. (B) Myc-tagged Tdrkh was co-transfected with FLAG-tagged Piwi and Ago proteins into 293T cells, followed by immunoprecipitation of the FLAG tag. (C) Co-immunoprecipitation of Miwi and Mili followed by western blot detection of Tdrkh from 2-month-old CD-1 mouse testis extract. (D) Co-immunoprecipitation of Miwi by Tdrkh from 2-month-old CD-1 mouse testis extract treated with or without RNase A. (E) Domain mapping of the Miwi–Tdrkh interaction by co-transfection with FLAG-tagged Miwi truncations and myc-Tdrkh. (F) Domain mapping of the Miwi–Tdrkh interaction by co-transfection with myc-tagged Tdrkh truncations and FLAG-Miwi. Arrows indicate specific western blot bands. (G) Co-immunoprecipitation of myc-Tdrkh by FLAG-Miwi2 containing point mutations to prevent symmetric dimethylation. (H) Co-immunoprecipitation of myc-Tdrkh by FLAG-Miwi containing point mutations to prevent symmetric dimethylation. (I) siRNA-mediated knockdown of PRMT5 prevents co-immunoprecipitation of myc-Tdrkh by FLAG-Miwi and FLAG-Miwi2. (J) Biotinylated Miwi peptides corresponding to Miwi residues 2–17, either with no methylated residues (Miwi2–17) or with symmetric dimethylation on arginine 4 (R4(me2s)) or arginine 14 (R14(me2s)), were incubated with adult C57 testis lysate prepared with 150 mM salt followed by western blot analysis for Tdrkh.
The interaction between Tdrkh and Miwi does not require RNA, because digestion of testicular extracts with RNase A had no effect on the ability of Tdrkh to co-immunoprecipitate Miwi (Figure 1D; Supplementary Figure 2a). Furthermore, mutating residues the KH domains critical for RNA binding had no effect on binding (Supplementary Figure 2b).
The Tudor domain of Tdrkh interacts with Miwi and Miwi2 via symmetrically dimethylated arginines
We then identified protein domains that mediate these interactions by co-immunoprecipitation assay of various Miwi and Tdrkh variants in 293 T cells (see Supplementary Materials). The N-terminus of Miwi interacts with the Tudor domain of Tdrkh (Figure 1E and F). This is consistent with findings that the Tudor domain of Tdrd proteins interacts with the N-terminus of Piwi proteins (Chen et al, 2009; Kirino et al, 2009; Reuter et al, 2009; Vagin et al, 2009).
Some Tdrd proteins are known to interact with Piwi proteins by binding to symmetrically dimethylated arginine residues in the N-terminus of Piwi proteins (Kirino et al, 2009; Reuter et al, 2009; Vagin et al, 2009). We tested whether this mechanism also mediates the interaction between Tdrkh and Miwi/Miwi2. Miwi2 contains four arginines that could be symmetrically dimethylated; they lie in a GRARVRARG motif near the N-terminus of the protein. Mutating these four arginines abolished the interaction between Miwi2 and Tdrkh (Figure 1G). For Miwi, however, mutating arginines in the conserved GRARVRARG motif in its N-terminal domain had no effect on Miwi–Tdrkh interaction (Figure 1H). Miwi contains two additional symmetrically dimethylatable arginines in the 12th and 14th position (Vagin et al, 2009). Mutating them to lysine compromised Miwi–Tdrkh interaction (Figure 1H). Thus, the methylation of arginine residues in the N-terminal domain of Piwi proteins mediates their interaction with Tdrkh.
We then directly tested whether this interaction required symmetric dimethylation. siRNA-mediated knockdown of PRMT5, which catalyses symmetric dimethylation of arginines at RA/RG motifs, abolished the Miwi2-Tdrkh interaction and severely attenuated the Miwi–Tdrkh interaction (Figure 1I). We also performed pulldown assays with biotinylated peptides encompassing residues 2–17 of the N-terminal of Miwi that contains six RA/RG motifs. We analysed arginines 4 and 14, which interact with Tudor domain-containing proteins or are symmetrically dimethylated, respectively (Vagin et al, 2009; Liu et al, 2010). Symmetric dimethylation of R4 enhances the ability of the N-terminal of Miwi to pull down Tdrkh by two-fold (Figure 1I). In contrast, symmetric dimethylation of R14 severely weakened the interaction. This pattern occurred regardless of salt concentration, using either cytoplasmic testicular lysate or Tdrkh overexpressed in rabbit reticulocyte lysate. As a whole, these results strongly argue that symmetric dimethylation of specific arginine residues within the N-terminal domain of Miwi and Miwi2 mediate their interaction with the Tudor domain of Tdrkh.
Tdrkh is associated with mitochondria in the germline
Tdrkh is expressed quite ubiquitously and is highly enriched in the brain and the testis (Supplementary Figure 3a). In situ RNA hybridization (Supplementary Figure 4a) and immunofluorescence (Supplementary Figure 3b) revealed its expression in spermatogonia, spermatocytes, and round spermatids, but not in elongating spermatids (Supplementary Figure 3b). Tdrkh is cytoplasmic and localized in punctuate structures. Immunofluorescence microscopy revealed that Tdrkh is extensively co-localized with the mitochondrial marker CoxIV (Supplementary Figure 3c). Biochemical fractionation further revealed that Tdrkh is highly enriched in the mitochondrial matrix (Supplementary Figure 3d). Immuno-electron microscopy confirmed the mitochondrial localization of Tdrkh and observed enrichment within the intermitochondrial cement (IMC), a form of the nuage (Supplementary Figure 3e). In sum, these data show that Tdrkh is a mitochondrion-associated protein.
Tdrkh foci overlap with pi-bodies and piP-bodies
Recent studies have described distinct cytoplasmic organelles as sites of piRNA biogenesis and function (Aravin et al, 2009; Shoji et al, 2009). Pi-bodies contain Mili, Tdrd1, and Mvh and localize to IMC in pro-spermatogonia and postnatal germ cells. IMC and associated pi-bodies are required for primary piRNA biogenesis (Watanabe et al, 2011a). PiP-bodies are larger structures containing Miwi2, Tdrd9, Mael, and P-body components. Cross-talk between pi- and piP-bodies is thought to be responsible for ping-pong-based piRNA biogenesis. As Tdrkh also localizes to IMC, we examined potential co-localization of Tdrkh and piRNA pathway components. In 18dpc testis sections, Tdrkh shows identical granular cytoplasmic localization as in adults and is often adjacent to and partially co-localizes with the pi-body components Mili (Supplementary Figure 3f) and Tdrd1 (Supplementary Figure 3g). Tdrkh was also found to overlap with cytoplasmic Miwi2 (Supplementary Figure 3h) and Mael (Supplementary Figure 3i) in piP-bodies, and occasionally co-localizes with the P-body markers Ge-1 (Supplementary Figure 3j) and Ddx6 (Supplementary Figure 3k). This suggests that Tdrkh can alternatively interact with both the Mili and Miwi2 protein complexes.
Tdrkh is required for male meiosis
To investigate the function of Tdrkh in spermatogenesis, we generated a Tdrkh-null allele by gene targeting (Figure 2A; Supplementary Figure 5a–d). Tdrkh−/− females generated litters of normal size and appearance upon crossing with C57 males, but Tdrkh−/−males are sterile, even though vaginal plugs are present in the mated females. Testes from 8-week-old homozygous nulls were ∼1/4 the size of control littermates (Figure 2B; Supplementary Figure 5e). Histological analysis revealed that spermatogenesis in tdrkh mutant animals was arrested in meiosis; no haploid spermatid was observed (Figure 2C).
Figure 2.
Tdrkh is required for male fertility. (A) Western blot analysis of tdrkh +/+, +/−, and −/− animal testis lysate. (B) Two-month-old testis from tdrkh +/− and −/− animals, ticks=0.1″. (C) H&E staining of 2-month-old +/− and −/− testis sections, scale bar=50 μm. (D) Quantitation of 14dpp spermatocyte spreads, N(+/−)=1821, N(−/−)=848. (E) Labelling of spermatocyte spreads from 14dpp +/− and −/− testis stained with Sycp1 and Sycp3. L, leptotene; Z, zygotene; P, pachytene, scale bar=10 μm. (F) Double labelling of 14dpp spermatocyte spreads with Rad51 (red) and Sycp3 (green). L, leptotene; Z, zygotene; Z/P, zygotene/pachytene; P, pachytene. Scale bar=10 μm. (G) Double labelling of 14dpp spermatocyte spreads with γH2A.X (red) and Sycp3 (green). L, leptotene; Z, zygotene; P, pachytene, scale bar=10 μm. (H) Staining of 14dpp germ cell spreads. Spermatogonia stained with anti-phospho-S25 53BP1, spermatocytes stained with total anti-53BP1, scale bar=50 μm. (I) Staining of 2-month-old CD-1 testis sections with anti-phospho-S25 53BP1 (green) and laminin (red), scale bar=75 μm. (J) TUNEL labelling (green) of 2-month-old +/− and −/− testis sections, scale bar=75 μm. (K) Western blot analysis of 11dpp Tdrkh +/− and −/− lysates. C, UV-irradiated CCE ES cell extract.
To determine the sub-stage of meiotic arrest, we stained spermatocyte spreads from tdrkh+/− and tdrkh−/−littermates with Sycp3 and Sycp1 at 14dpp, a time point at which a substantial portion of spermatocytes have progressed to pachytene stage (36%, Figure 2D). No pachytene spermatocytes were observed in tdrkh−/−spreads (Figure 2C–E). Mutant spermatocytes showed major defects in forming synaptonemal complexes, as lateral elements marked by Sycp3 were severely fragmented in the zygotene stage and transverse elements marked by Sycp1 did not assemble properly (Figure 2E). Thus, the tdrkh−/− spermatocytes are arrested at the zygotene stage.
Tdrkh deletion causes massive DNA damage without apoptosis
We next performed staining with Rad51 to determine whether double-stranded break (DSB) foci had formed properly in tdrkh−/−mutants. In tdrkh+/− males, Rad51 foci were numerous in leptotene stage, coalescing onto Sycp3-labelled chromatin in zygotene stage, and become 2–3 sites/chromosome of crossover in pachytene stage (Figure 2F). In contrast, tdrkh−/− spreads ranged from a relatively normal appearance to widespread Rad51 staining along chromosomes. This was often continuous with, yet separate from, Sycp3-positive regions (Figure 2F).
Staining for γH2A.X revealed further defects in mutant spermatocytes. γH2A.X foci appear in leptotene as DSBs form, and disappear by zygotene as the majority of DSBs resolve (Figure 2G; staining is retained on the XY body, however). Tdrkh−/− spermatocytes display extremely high levels of γH2A.X staining. Mutant leptotene spermatocytes initiate γH2A.X foci similarly to the controls, but these foci spread extensively and do not resolve in zygotene stage (Figure 2G). These data indicate defects in recognition of homologous pairing and a high level of DNA damage. Tdrkh mutants activate a robust DNA damage response. 53BP1 is a DNA damage checkpoint protein with sensation and transduction functions. In 14dpp tdrkh mutant spermatocyte spreads, we observed a re-distribution from a diffuse nuclear pattern to a distinct punctuate pattern indicative of 53BP1 activation in spermatogonia (Figure 2H, upper panels) and spermatocytes (Figure 2H, lower panels). In wild-type adult testes, 53BP1 expression largely mirrors γH2A.X, with nuclear-wide expression in leptotene, which dissipates in zygotene, and is restricted to the sex body in pachytene spermatocytes (Figure 2I). In contrast, tdrkh mutants have high and abnormal activation of 53BP1, indicating a widespread DNA damage response (Figure2I).
We next asked whether this response led to apoptosis. Surprisingly, TUNEL staining in 11dpp and 2-month-old animals did not reveal apoptosis in the tdrkh mutant (Figure 2J). No activation of Caspase-3 or PARP cleavage was detected in the mutant, either (Figure 2K). This is highly surprising, as nearly all piRNA pathway mutants display robust TUNEL labelling (Kuramochi-Miyagawa et al, 2004; Carmell et al, 2007; Shoji et al, 2009; Frost et al, 2010; Watanabe et al, 2011a). Thus, Tdrkh may have additional downstream functions in response to DNA damage. Alternatively, spermatogenic arrest in Tdrkh mutants may be a result of a hyperactive chromosomal asynapsis checkpoint and not a result of DNA damage. Thirteen-month-old tdrkh mutant testes are highly atrophic and depleted of mature cells (Supplementary Figure 5f). Thus, abnormal cells are eliminated during ageing, likely through a necrotic process, and are not replaced as the animal ages. Interestingly, Tdrkh mutant spermatogonia were occasionally positive for the mitotic marker phospho-H3 (Supplementary Figure 5g). It is unclear if this indicates ongoing mitosis or arrest during mitosis. In sum, our data are consistent with overall arrest of mitosis and meiotic progression, resulting in the reduced size of tdrkh−/− testes.
Tdrkh represses Line-1 retrotransposon expression
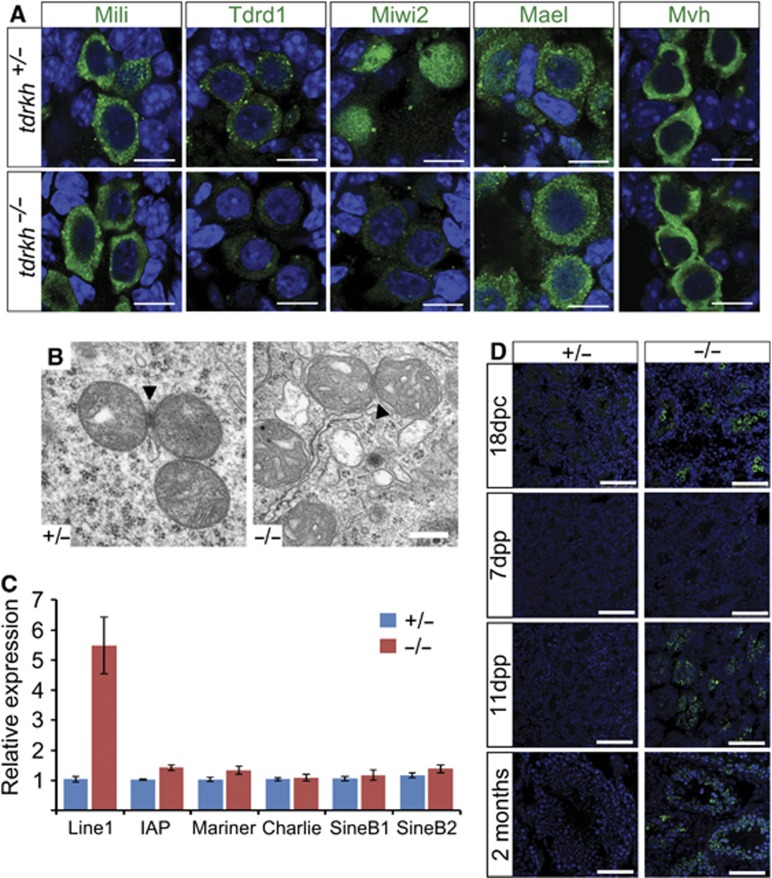
One mechanism through which DNA damage could occur is by elevated transposon activity as in other piRNA pathway mutants (Siomi et al, 2011). To investigate if Tdrkh represses transposition, we first examined localization of pi- and piP-body markers in 18dpc testes of tdrkh+/− and tdrkh−/− littermates. Pi-body markers Mili and Mvh are localized similarly in tdrkh+/− and tdrkh−/− testes (Figure 3A). In contrast, Tdrd1 is lost from most pi-bodies in tdrkh−/− testes. The remaining pi-bodies contain weaker Tdrd1 staining. Thus, Tdrkh may recruit Tdrd1 to intact pi-bodies (Figure 3A). The piP-body components Miwi2 and Mael are in the nucleus and the cytoplasm, respectively (Figure 3A). In tdrkh−/− testes, however, Miwi2 becomes exclusively cytoplasmic and displays weak piP-body staining (Figure 3A). Mael remains diffusely cytoplasmic, with its signal in piP-bodies similarly weaker (Figure 3A). These results imply that Tdrkh is required for proper function of the Miwi2 in the nucleus and the piP-body. Electron microscopy on 11dpp tdrkh+/− and tdrkh−/− testes did not reveal ultrastructural changes in tdrkh−/− testes. In particular, IMC was still observed (Figure 3B), which indicates that Tdrkh does not contribute to the structural integrity of IMC.
Figure 3.
Tdrkh represses Line1 expression. (A) Localization of pi-body (Mili, Tdrd1), piP-body (Miwi2, Mael), and pan-nuage (Mvh) components in 18dpc tdrkh +/− and −/− testis sections, scale bar=10 μm. (B) Electron microscopy of 11dpp tdrkh +/− and −/− spermatocytes. Arrowheads indicate nuage/intermitochondrial cement. Scale bar=300 nm. (C) RT-qPCR analysis of transposon expression in three independent pairs of 12dpp testis for each genotype. Error bars indicate standard deviation. (D) Line1 Orf1p expression (green) in 18dpc, 7dpp, 11dpp, and adult tdrkh +/− and −/− testis sections. Scale bar=75 μm.
We then assessed transposon RNA expression in tdrkh+/− and tdrkh−/− testes during development. Immunostaining revealed that the Line1 protein Orf1p is barely detectable in 18dpc tdrkh+/− gonocytes due to the establishment of DNA methylation (Aravin et al, 2009). In contrast, 18dpc tdrkh−/− gonads display drastic upregulation of Orf1p staining. At 7dpp, Orf1p expression decreases in tdrkh+/− testes, consistent with its RNA decrease as revealed by qPCR analysis (Supplementary Figure 6b). At 11dpp, immediately before the terminal arrest of mutant spermatocytes, Orf1p remained undetectable in tdrkh+/− testes but was drastically upregulated in the mutant testes and persists at high levels in the arrested tubules at 2 months (Figure 3D). Notably, only Line1 RNA but not other transposon RNAs is upregulated (Figure 3C; Supplementary Figure 6a). Thus, deletion of tdrkh results in robust Line1 expression during embryogenesis when DNA would be methylated and again during meiosis. Interestingly, we failed to observe cytoplasmic inclusions indicative of Line1 particle formation (for instance, Shoji et al, 2009) in our EM analysis of 11dpp tdrkh−/− animals (Figure 3B, data not shown), suggesting that mature particles may not form.
Deletion of Tdrkh results in CpG hypomethylation at Line1 promoters
To examine whether the activation of Line1 expression in tdrkh−/− testes is due to the loss of DNA methylation, we performed bisulphite sequencing to determine the methylation of transposonic DNA sequences. Line1 consist of five distinct subfamilies; two of them, L1MdA and L1MdGf, are capable of re-integration. DNA from tdrkh−/− postnatal spermatogonia shows severe defects in CpG methylation of L1MdA sequences and obvious defects in L1MdGf sequences (Supplementary Figure 7a). No change was observed in methylation of IAP sequences (Supplementary Figure 7b). Thus, DNA hypomethylation in tdrkh−/− testes is specific to Line1.
Embryonic piRNA biogenesis is compromised in Tdrkh −/− mice
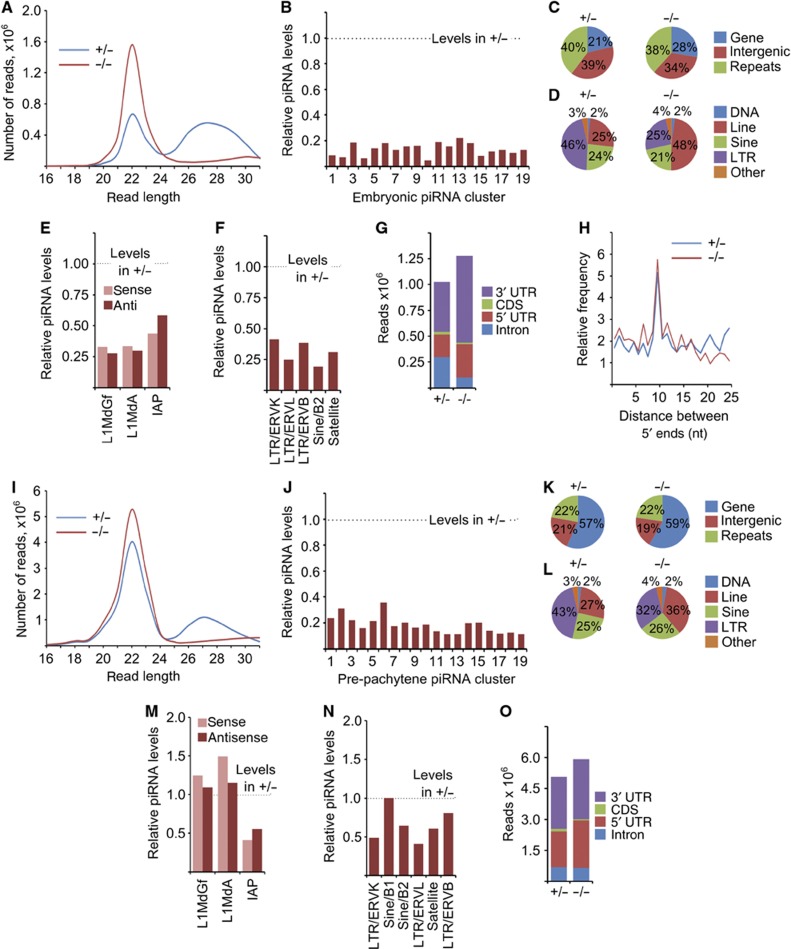
DNA methylation of repetitive elements is thought to be guided in a sequence-specific manner by piRNAs (Siomi et al, 2011). The observed DNA demethylation phenotype indicates that Tdrkh may play an important role in piRNA biogenesis. To address this hypothesis, we first analysed global piRNA populations at 18dpc, when germ cell-specific DNA methylation is active and Tdrkh partially co-localizes with Mili and Miwi2 (Supplementary Figure 3f and h). Known mutations in piRNA pathway, such as tdrd1, tdrd9, and mael, result only in skewing of otherwise mostly normal piRNA populations (Aravin et al, 2009; Reuter et al, 2009; Shoji et al, 2009). However, deletion of tdrkh causes nearly complete elimination of global piRNA levels (Figure 4A; Supplementary Table 1). In all, 25–31 bp reads were reduced by ∼80% in the mutant library, indicating severe defects in piRNA biogenesis. Concordantly, unique piRNAs mapped to the top 19 embryonic piRNA-generating clusters were decreased by 85% compared to wild-type testes (Figure 4B).
Figure 4.
Tdrkh functions in primary piRNA biogenesis. (A) Size distribution of small RNA libraries from 18dpc tdrkh +/− and −/− testes, without normalization. (B) Ratio of 18dpc piRNA levels in Tdrkh mutant testes derived from the top 19 embryonic piRNA clusters, relative to tdrkh +/− levels. (C) Percentage of total library reads mapping to indicated categories in 18dpc libraries. (D) Percentage of repeat-associated reads mapping to indicated transposon family in control (upper) and mutant (lower) 18dpc libraries. (E) Ratio of sense and antisense 18dpc piRNAs mapping to specified transposons, relative to control levels. (F) Ratio of 18dpc piRNA reads mapping to indicated transposon families, relative to control levels. (G) Levels of piRNAs mapping to indicated mRNA region, expressed as a percentage of total reads in each genotype. (H) Distances between 5′ complementary ends of piRNA mapping to IAP consensus sequence were measured to calculate a ping-pong biogenesis signature. (A–H) 18dpc libraries. (I) Size distribution of small RNA libraries from 11dpp tdrkh +/− and −/− testes, without normalization. (J) Ratio of 11dpp piRNA levels in Tdrkh mutant testes derived from the top 19 post-natal pre-pachytene piRNA clusters, relative to control reads. (K) Percentage of total library reads mapping to indicated categories in 11dpp libraries. (L) Percentage of repeat-associated reads mapping to indicated transposon family in control (upper) and mutant (lower) 11dpp libraries. (M) Ratio of sense and antisense 11dpp piRNAs mapping to specified transposons, relative to control levels. (N) Ratio of 11dpp piRNA reads mapping to indicated transposon families, relative to control levels. (O) Levels of 11dpp piRNAs mapping to indicated mRNA region, expressed as a percentage of total reads in each genotype. (I–O) 11dpp libraries.
We then focused on the repeat-associated 18dpc piRNA population. While the percentage of repeat-associated reads within the residual piRNA population was unchanged (Figure 4C), the majority of these reads were shifted to Line1-derived piRNAs in the tdrkh-mutant library (Figure 4D). Levels of sense and antisense piRNAs mapped to Line1 and IAP were proportionally decreased (Figure 4E). piRNAs mapped to the five most abundant classes of transposons were also reduced (Figure 4F; Supplementary Table 2). These data suggest that the residual piRNA population responds to increased transposon activity and that loss of transposon piRNAs is reflective of a global decrease in primary piRNA biogenesis.
Further analysis of residual 18dpc piRNA populations revealed abrupt changes in mRNA-derived piRNAs. Reads of 5′ and 3′UTR-derived piRNAs were increased by ∼1.8-fold in tdrkh mutants. In contrast, reads of the small population of piRNAs from coding sequences were further reduced by 40% and intron-derived piRNAs decreased by 60% (Figure 4G). These results suggest a role for Tdrkh in processing of piRNAs from transcripts, possibly through regulation of Tdrd1 localization (Figure 3A).
We also noted a 2.3-fold increase in the reads of 22-bp RNAs in mutant testes, which is highly consistent with the 2.4-fold increase in reads mapped to mature miRNAs (Figure 4A; Supplementary Table 1). A similar change was observed in 11dpp libraries even though no change in abundant miRNAs was observed by northern blotting (Supplementary Figure 8a). Hence, this increase is likely an artifact of library construction. Therefore, normalization to either total reads or miRNAs is inappropriate. As such, we did not perform normalization between libraries so that piRNA levels can be directly compared.
Secondary piRNA biogenesis is intact in tdrkh mutant testes
We next asked whether secondary piRNA biogenesis was intact in these mutants. Secondary piRNA biogenesis, a.k.a. the ping-pong cycle, generates new piRNAs from transposons bound and cleaved by Piwi proteins (Brennecke et al, 2007; Gunawardane et al, 2007; Aravin et al, 2008). The signature of ping-pong piRNA biogenesis is a 10-nt overlap in 5′ ends formed as a result of sequences with a uridine in the first position pairing with complementary sequences possessing an adenine at the 10th position. We therefore measured the relative frequency of overlap from 5′ ends of complementary IAP and Line1-derived piRNAs in 18dpc libraries. This revealed enrichment in sequences with a 10-bp overlap. This enrichment was unchanged in the mutant library (Figure 4H; Supplementary Figure 8c), suggesting that secondary piRNA biogenesis is intact in tdrkh mutants. This argument is further supported by the lack of sense/antisense bias observed in residual Line1-derived piRNAs (Figure 4D). Such a bias would be expected if ping-pong-based piRNA biogenesis had collapsed. In sum, our data indicate that secondary piRNAs are generated independently of Tdrkh.
Tdrkh is required for proper pre-pachytene piRNA biogenesis
We then analysed piRNA populations at 11dpp, a later time point immediately preceding meiotic arrest when heterozygotes and mutants still possess identical cell populations. Deletion of tdrkh also causes collapse of pre-pachytene piRNA populations (Figure 4I; Supplementary Table 1). Uniquely mapped piRNAs from the 19 top pre-pachytene piRNA-generating clusters are reduced, on average, by 82% (Figure 4J). This again suggests a major role for Tdrkh in piRNA biogenesis.
Repeat-associated piRNAs show more complex changes in the 11dpp tdrkh mutant. Line1-associated sense piRNAs are elevated in the mutant library (Figure 4M) and increased from 27 to 36% of repeat-associated piRNAs (Figure 4K and L). We confirmed upregulation of Line1 piRNAs by northern blotting (Figure 5F) and RNase protection assays (Figure 5G). Levels of Sine element piRNAs are unchanged, whereas satellite, IAP, and other LTR transposons are decreased in tdrkh mutants (Figure 4M and N; Supplementary Table 2).
Figure 5.
Tdrkh facilitates piRNA maturation from 31 to 36 nt Mili-bound piRNA intermediates. (A) End labelling and small RNA analysis of Mili-bound small RNAs in 11dpp tdrkh+/− and tdrkh−/− testes reveals the accumulation of piRNA intermediates in the mutant testes. (B) Size distribution of Mili-associated RNA libraries from 11dpp tdrkh+/− and tdrkh−/− testes, without normalization. (C) Nucleotide distribution at the first position in 24–31 nt and 32–40 nt Mili-associated RNAs from tdrkh+/− and tdrkh−/− testes. (D, E) Alignments between mature and precursor piRNAs within a 1-kb window of piRNA clusters on chromosome 10 (D) and chromosome 8 (E). All mature piRNAs that perfectly map to an intermediate are shown, and the alignment of the pair is presented in the plots. Note that all alignments posses perfectly aligned 5′ ends. Mature piRNA=black, intermediate=red. (F) Northern blot analysis of total 11dpp RNA using an antisense probe that recognizes a mature 27-nt Line1 piRNA (upper panel), an antisense probe that recognizes its 31 bp intermediate form (middle), or U6 RNA as a loading control (bottom). (G) RNase protection assay using total 11dpp RNA and a radiolabelled probe that can be protected from RNase digestion by both the mature 27 nt Line1 piRNA and its 31nt intermediate form. * indicates non-digested probe, arrowhead indicates the intermediate piRNA, and the arrow indicates the mature piRNA. (−) in the RNA lane indicates only yeast RNA was present in the hybridization. +/− and −/− indicate genotypes of Tdrkh animals. (H) Northern blot analysis of total 11dpp RNA for expression of a cluster-derived piRNA using an antisense probe, which recognizes the mature and intermediate forms of the piRNA (upper panel), a longer antisense probe that specifically recognizes only the intermediate form (middle panel), or an antisense probe for U6 as loading control (middle, bottom panels). (I) High-resolution northern blotting of total 11dpp +/− and −/− RNA for expression of a cluster-derived piRNA and its intermediate, using the same antisense probe in (H). The mature form is visible in the +/− sample while the intermediate is present in the −/− sample. (J, K) High-resolution northern blot analysis following treatment of total mutant 11dpp RNA with water (C) or with NaIO4 and β-elimination (β). Non-protected 3′ ends will result in RNAs which migrate ∼2 nt faster than control reactions. M, 1nt marker. (J) Northern blot with an antisense probe for the cluster-derived piRNA intermediate studied in (H, I). (K) Northern blot with an antisense probe for the let-7c miRNA. (L) Abundance of 24–31 nt and 32–40 nt Mili-associated intermediate RNA levels in tdrkh−/− testes derived from the top 19 post-natal pre-pachytene piRNA clusters, normalized to 24–31 nt control reads. (M) Ratio of mature (27 nt) and intermediate (31–36 nt) Mili-associated piRNAs between tdrkh+/− and tdrkh−/− testes mapped to L1MdGf, L1MdA, and IAP retrotransposons. (N) Ratio of mature (27nt) and intermediate (31–36nt) Mili-associated piRNAs from tdrkh+/− and tdrkh−/− testes mapped to introns, 5′UTRs, coding regions, and 3′UTRs. (O) Percentage of total library reads that are mapped to indicated categories in tdrkh+/− (upper) and tdrkh−/− (lower) libraries. (P) Percentage of repeat-associated reads mapped to indicated categories in tdrkh+/− (upper) and tdrkh−/− (lower) libraries.
We next examined piRNAs that mapped to mRNAs. Overall, mRNA-derived piRNA levels were slightly elevated in tdrkh mutants. While there was no change in intronic piRNAs, tdrkh mutant piRNAs that mapped to 5′ and 3′UTRs were slightly increased. In contrast, piRNAs mapped to coding regions were decreased by 75% as compared to controls (Figure 4O; Supplementary Table 3). This suggests a potential role for Tdrkh in the processing of piRNAs from their precursors.
Tdrkh is intimately involved in piRNA precursor maturation
We next determined the step at which Tdrkh acts in piRNA biogenesis. An effect on nuage formation is unlikely, as IMC was noted in tdrkh−/− spermatocytes (Figure 3B). Consistently, Mili expression at the mRNA (Supplementary Figure 8e) and protein (Supplementary Figure 8f) levels and localization (Figure 3A) are unaffected in tdrkh mutant testis. Levels of putative precursor transcripts mapping to the five pre-pachytene clusters that generate the largest amounts of mature piRNAs were unaffected in tdrkh mutants (Supplementary Figure 8g). Thus, Tdrkh is likely not required for precursor transcription or stability. Tdrkh does not interact with proteins required for primary piRNA biogenesis, such as Mov10L1 and MitoPLD (Supplementary Figure 8h and i). We then asked whether Tdrkh participates in a downstream step of the primary piRNA pathway, by immunoprecipitating Mili-associated RNAs from tdrkh 11dpp mutant and control testes and analysing them by 5′ end labelling. Mili-associated piRNAs are most abundant at 27 nt in tdrkh+/− testes. In tdrkh−/− testes, however, Mili-associated small RNAs are shifted to 30–40 nt (Figure 5A). Since residual piRNAs in total RNA libraries contain a 5′U in the first position (Supplementary Figure 8b and d), Tdrkh is likely involved in processing and maturation of 3′, rather than 5′, piRNA ends.
Mili-associated RNAs in tdrkh mutants are 31–36 nt piRNA intermediates
We then deeply sequenced 18–40 nt Mili-associated RNAs from 11dpp tdrkh+/− and tdrkh−/− testes. The tdrkh+/− library shows a distinct peak at 27 nt, characteristic of Mili-associated piRNAs (Figure 5B). This peak is reduced four-fold in the tdrkh−/− library and is replaced by a 31–36 nt plateau enriched ∼five-fold compared to the tdrkh+/− library. We hypothesized that this 31–36 nt population are piRNA intermediates whose 3′ ends have been incompletely processed.
To test this hypothesis, we characterized sequences from each library as either ‘mature’ or ‘intermediate’ using two separate criteria. First, we classified mature piRNAs and precursors as 24–31 nt and 32–40 nt sequences, respectively. Realizing this might include ‘intermediates’ in the ‘mature’ mutant library, we also performed analyses using a strict definition of 27 nt sequences as ‘mature’ piRNAs and 31–36 nt sequences as ‘intermediates’ to minimize this ambiguity, at the expense of a possible decrease in the sensitivity of our analyses. The 24–31 nt sequences from tdrkh+/− and tdrkh−/− testes share similar preference for a 1′U (Figure 5C). Similarly, 32–40 nt sequences from tdrkh+/− and tdrkh−/− testes are also strongly biased for 1′U, suggesting that they are generated through the same mechanism as mature piRNAs (Figure 5C). This analysis was also performed as a function of piRNA length, which confirms the conclusion (Supplementary Figure 9a).
If 32–40 nt RNAs are piRNA intermediates formed through 3′ end processing, then one would predict a significant degree of overlap between them and mature piRNAs, starting at the same 5′ nucleotides. To test this, we queried the tdrkh+/− 24–31 nt library for perfect matches in the 32–40 nt tdrkh−/− library and recovered a perfect partner for 35% of the tdrkh−/− Mili-associated 32–40 nt sequences. Within this set, we detected matching pairs in which the 5′ ends of mature piRNAs and their precursors were perfectly aligned, leaving the 3′ tail of the precursors overhanging (summarized in Supplementary Figure 9b; for examples, see Figure 5D and E; Supplementary Figure 9c and d). This is an extremely high degree of overlap, considering the random processing of mature piRNA sequences from long precursor sequences, the shifts of library composition in the tdrkh mutant (Figure 4 and see below), and the complexity of piRNA populations in vivo. This overlap strongly implies that Mili-associated 32–40 nt RNAs are intermediates with 3′ end to be processed into mature piRNAs.
We confirmed this 5′ end alignment by analysing cluster-derived, perfectly matching pairs of Mili-associated 24–30 nt RNAs from tdrkh+/− testes and 31–40 nt RNAs from tdrkh−/− testes. Matching pairs had perfectly aligned 5′ ends, with the 32–40 nt RNAs containing overhanging 3′ ends (Figure 5D and E; Supplementary Figure 9e and f). Only 1.8% of cluster-derived mutant intermediates did not have a mature piRNA match. This further supports our hypothesis that Mili-associated 32–40 nt RNAs are piRNA intermediates.
We then queried the nature of the remaining ∼65% of mutant intermediates that did not match to mature piRNAs. Gene-derived intermediates make up the majority of this unmatched population (Supplementary Figure 9g) and contain similar proportions of 5′UTR/3′UTR/CDS/intron sequences as the corresponding population of piRNA-matching gene-derived intermediates (Supplementary Figure 9h). Since these libraries are highly enriched for 1′U and are 3′ methylated (see below), they are likely bona fide intermediates rather than non-specific degradation products.
Putative piRNA intermediates have 2′-O-methylated 3′ ends
We then directly detected these piRNA intermediates. Northern blotting of total 11dpp testicular RNA shows drastic upregulation of Line1 piRNAs in tdrkh−/− testes. Line1-derived small RNAs ranged from ∼25 to 33 nt in tdrkh mutants (Figure 5F), and we readily detected a 31–33 nt Line1-derived piRNA intermediate using a probe specific for this sequence (Figure 5F). Line1 piRNA intermediates were confirmed using an RNase protection probe that recognizes mature Line1 piRNAs and their 31nt precursor (Figure 5G). They are unlikely non-specific degradation products of Line1 transcripts for several reasons. First, Line1 piRNAs from tdrkh mutants are very similar in their length profile to piRNAs and piRNA intermediates from other regions such as piRNA clusters and 3′UTRs (Supplementary Figure 10f–j). Second, a qualitatively identical length profile is observed in control libraries (Supplementary Figure 10j). Third, reads corresponding to Line1 sequences overwhelmingly contain a 1′U regardless of their size (Supplementary Figure 9e and f). In sum, these data support a regulated processing of Line1 transcripts into piRNA intermediates that are then trimmed to mature piRNAs.
We next performed northern blotting for an abundant cluster-derived piRNA. This probe specifically detected mature 26–28 nt piRNAs in tdrkh+/− testes and a size-shifted, 28–32 nt piRNA intermediate in tdrkh−/− testes (Figure 5H). This intermediate could be specifically recognized by a probe for an abundant 31–32 nt precursor of the 27 nt mature piRNA (Figure 5H and I). Thus, piRNA intermediates were readily detected in multiple RNA preparations from 11dpp heterozygous and tdrkh mutant testes, and are not artefacts resulting from RNA binding to Mili non-specifically during the IP step of RNA isolation.
We then analysed whether these intermediates were 2′-O-methylated on their 3′ ends, since mature piRNAs contain this modification to protect them from degradation (Horwich et al, 2007; Ohara et al, 2007; Saito et al, 2007; Kirino and Mourelatos, 2007a, 2007b). 2′-O-methylation is catalysed by Hen1 and is closely associated with 3′ trimming activity (Kawaoka et al, 2011). We treated total 11dpp testicular RNA with NaIO4 followed by β elimination. We found that the cluster-derived piRNA precursor described above is 2′-O-methylated (Figure 5J), as are the Line1 piRNA precursor and its corresponding mature piRNA (Supplementary Figure 9i). In contrast, miRNA let-7c is not 2′-O-methylated and correspondingly shifts by ∼2nt (Figure 5K). This explains the stability of the piRNA intermediates cloned from Mili-associated RNAs and suggests a close association between their biogenesis, trimming, and 3′ end modification.
31–36 nt piRNA intermediates complement missing mature piRNAs
We asked whether the deficiencies of mature piRNA sequences in tdrkh mutants could be rescued if the intermediate RNAs were to be properly processed. Deletion of tdrkh causes a significant reduction in the number of cluster-derived 24–31 nt reads (Figure 5L). This reduction is ‘rescued’ by including mutant 32–40 nt sequences. A more severe depletion, using the strict criteria of ‘mature’ and ‘intermediate’ piRNAs defined above, is also negated by including 31–36 nt intermediate RNAs (Supplementary Figure 10a). These data, combined with the 5′ end analysis in Figure 5D and E, demonstrate that many 31–36 nt Mili-associated RNAs are derived from piRNA clusters and imply that they are normally processed into mature piRNAs.
We next analysed reads that are mapped to repeat elements and genes. In the tdrkh mutant, mature piRNAs mapped to Line1 elements were slightly increased and piRNAs mapped to IAP sequences were decreased by 50% (Figure 5M). 31–36 nt intermediates that are mapped to both Line1 elements were highly upregulated (Figure 5P), consistent with our northern and RNase protection assay results (Figure 5F and G). Note that IAP transcripts are not elevated in the tdrkh mutant (Figure 3C), and inclusion of piRNA intermediates ‘rescues’ mutant IAP piRNA levels only back to those observed in control testes (Figure 5M). Both mature piRNAs and piRNA intermediates display an overwhelming sense bias (Supplementary Figure 10b).
Gene-derived Mili-associated 27 nt 11dpp piRNAs are severely reduced in tdrkh mutants. This is true for reads derived from introns, 5′ and 3′ UTRs, and coding sequences (Figure 5N). Mature sequences outnumber intermediate sequences by only 1- to 1.5-fold in the control library (Supplementary Figure 10c), whereas intermediates outnumber mature sequences by 10- to 20-fold in the mutant library (Figure 5N), suggesting a buildup of incompletely processed intermediates in the tdrkh mutant.
Tdrkh deletion results in re-wiring of transcript networks entering the piRNA pathway
We then asked how the 24–31 nt and 32–40 nt populations change upon deletion of tdrkh. In control libraries, mature piRNAs mapped to genes, repeats, and intergenic regions were highly enriched compared to 32–40 nt RNAs (Figure 5O). Upon deletion of tdrkh, 32–40 nt intermediates become the predominant species of RNAs mapped to these regions. Moreover, the total amount of RNAs mapped to genic regions increases by 25% at the expense of reads mapped to intergenic regions in tdrkh mutants (Figure 5O). This is accompanied by striking shifts in the genes producing these reads (Supplementary Table 4). Qualitatively identical results were obtained with our stricter criteria (Supplementary Figure 10d). This shows that tdrkh deletion causes shifts in the types of transcripts entering the piRNA pathway and might reflect the inappropriate localization of Tdrd1 in tdrkh mutants.
Reads mapped to repeat elements are again enriched in 24–31 nt sequences over 32–40 nt sequences in the control library; this ratio is flipped in the mutant library (Figure 5O). Similar results are observed using our stringent definitions of mature and intermediate piRNAs (Supplementary Figure 10e). Together, these data (Figure 5O and P) indicate that although the proportion of repeat-associated sequences does not significantly change, there is a substantial change in both the wiring and processing of the sequences routed into the piRNA pathway following tdrkh deletion.
Mili-associated piRNA intermediates display origin-associated size biases
While performing quality control checks, we queried perfectly matching mRNA-associated reads from the mutant Mili-associated library for their size distribution (Supplementary Figure 10f, control library shown in Supplementary Figure 10g). While intron-derived reads are evenly distributed, those mapped to 5′ UTRs and 3′UTRs show high enrichment for 35nt and 31nt reads. 5′ UTR reads also display a pronounced peak at 27 nt. In contrast, reads mapped to coding regions generate mature piRNAs enriched for 26 nt sequences and show an extreme preference for 31nt and 36 nt intermediates (Supplementary Figure 10f).
We found no evidence of over representation of specific mRNAs that may account for these size biases. Intermediates were produced from thousands of mRNAs. The same top five genes generated the most 5′ and 3′UTR-derived intermediates in tdrkh mutants; they accounted for <8% of the clones of the total 3′UTR population (‘clones’ refers to how many kinds of specific sequences are observed with no weight given to their frequency; ‘reads’ refers to how many clones are present as well as their frequency). The top five genes that generate coding sequence intermediates account for only 2.1% of the total number of coding sequence clones in the mutant (Supplementary Table 4). These data indicate that origin-associated signatures are likely a general phenomenon but are not due to differential processing of only a few transcripts, and reflect the observed re-wiring of transcripts entering the piRNA pathway following tdrkh deletion.
Origin-specific size biases are apparent in cluster- and retrotransposon-mapped reads as well. Control Mili-associated RNAs from piRNA clusters show a clear 27–28 nt size range (Supplementary Figure 10h), whereas tdrkh mutants display prevalent enrichment of 33 and 37 nt species. Line1 and IAP reads from tdrkh mutants likewise display unique size preferences, distinct from genic and cluster reads and from each other (Supplementary Figure 10i). These trends are clearly visible in control libraries as well, showing that they are not dependent on Tdrkh expression (Supplementary Figure 10j). In sum, these data indicate that different piRNA precursors and mRNAs generate specifically sized intermediates in producing mature piRNAs with distinct size biases.
We attempted to identify any possible signal in the piRNA intermediates that may guide 3′ end cleavage or explain their size biases. We identified a marked preference for pyrimidine residues at the 3′ end of intermediates (65.8% of sequences) but not any obvious motif beyond this preference (Supplementary Figure 10k). Considering that the cleavage signal may have been removed during processing, we extended the 3′ ends of the intermediates by 50nt but were again unable to identify any sort of regulatory motif that may guide intermediate generation.
Mili-associated RNAs include a unique 22 nt species
A secondary, 22 nt peak was observed in both Mili IP libraries (Figure 5B). 81% of these 22 nt sequences start with a 1′U (Supplementary Figure 11a) and 50% of them are mapped to longer RNAs present in the Mili-associated libraries. More than 75% of these sequences map to 3′UTRs (Supplementary Figure 11b). The 3′UTR-derived sequences are overwhelmingly directional to their matching piRNAs or intermediates (Supplementary Figure 11c) and are highly enriched for 1′U (Supplementary Figure 11d). In addition, the 5′ ends of >40% of the 22nt reads were located within 12nt of the start of their matching piRNAs and intermediates (Supplementary Figure 11e and f) and revealed a pronounced peak at 0 nt. This indicates that 22nt piRNAs and their matches often start at the same nucleotide. This may indicate that they are degradation products of piRNAs or their intermediates. However, this can only account for 5–6% of the 22 nt Mili-associated RNA population. As these sequences contain a 1′U and are highly specific for 3′UTRs, they may possibly be involved in piRNA biogenesis or a distinct sub-population of piRNAs.
Discussion
In this study, we have reported the crucial role of Tdrkh in the piRNA pathway and spermatogenesis as well as the discovery of a key intermediate step of piRNA biogenesis. We have shown that Tdrkh is a mitochondrial-associated protein that partially co-localizes with pi-bodies and piP-bodies. Our data indicate that Tdrkh is crucial for the final steps of piRNA biogenesis by participating in the processing of 31–37 nt intermediates into mature piRNAs. Cytologically, Tdrkh could achieve this role by acting in pi-bodies and piP-bodies, and in transferring piRNA precursors or intermediates to or between these granules (Figure 6). Furthermore, we have shown that Tdrkh is involved in epigenetic silencing of transposons. Similar to other piRNA pathway mutants (Siomi et al, 2011), disruption of epigenetic silencing of transposons leads to their expression in gonocytes and during meiosis. This activation is correlated with spermatogenic arrest. Finally, our data indicate that Tdrkh might integrate the piRNA pathway with the DNA damage response.
Figure 6.
A model of Tdrkh function in piRNA biogenesis and the Piwi-piRNA pathway. Mili-associated piRNA precursors (red line) are selected and loaded into Mili complexes and processed into intermediates. Tdrkh recruits or facilitates activity of a 3′ exonuclease, ‘trimmer’, in the nuage, which proceeds to trim the intermediates to mature piRNA length. In addition, in embryonic testes, Tdrkh may function in transferring mature piRNAs or piRNA intermediates between Mili and Miwi2 complexes. Mili- and Miwi2-piRNA complexes proceed to enact epigenetic regulation, transposon (Tn) repression, translational regulation, and possibly surveillance of genomic integrity. In response to indefensible attacks on genomic integrity, Tdrkh may function in sensing or transducing apoptotic responses to mitochondria. See Discussion for details.
Tdrkh is required for meiotic progression
This requirement is evident by meiotic arrest at the pre-pachytene stage in the tdrkh mutant, accompanied by substantial chromosomal asynapsis, appearance of DNA damage markers, and activation of 53BP1, a checkpoint protein involved in sensing and transducing DNA damage. Surprisingly, however, no apoptosis was detected in Tdrkh mutants. This stands in stark contrast to the piRNA pathway mutants Mili, Miwi2, Tdrd1, Tdrd5, Tdrd9, MitoPLD, and Mov10l1, which display widespread apoptosis (Kuramochi-Miyagawa et al, 2004; Chuma et al, 2006; Carmell et al, 2007; Shoji et al, 2009; Frost et al, 2010; Yabuta et al, 2011; Watanabe et al, 2011a). It suggests a potential role for Tdrkh in integrating and transducing DNA damage signals, a role supported by its mitochondrial localization. The massive chromosomal disorganization we observed in tdrkh mutants might result in the activation of the chromosomal asynapsis checkpoint in addition to the DNA damage checkpoint. The lack of apoptosis in tdrkh mutant testes could be explained if the output from asynapsis checkpoint activation is the dominant driver of spermatogenic arrest, rather than DNA damage resulting from Line1 overexpression. Alternatively, Tdrkh may be involved in transducing a signal indicating such damage. Presently, it is not known whether Line1 elements undergo re-integration in piRNA pathway mutants. The generation of Spo11-independent double-stranded DNA breaks in the mouse maelstrom mutant (Soper et al, 2008) could result from defects in piRNA pathway-independent roles for Mael in microtubule and spindle assembly, the miRNA pathway, and/or defects in chromatin remodelling (Costa et al, 2006; Sato et al, 2011). With no causative evidence, the link between meiotic arrest and transposon reactivation remains correlative. Future work will delineate the contributions of these distinct pathways in response to Tdrkh deletion.
Roles for the piRNA pathway beyond epigenetic repression of transposons
Our data with that of others imply that repression of retrotransposons is not the sole function of the piRNA pathway. For instance, MitoPLD promotes proper genetic imprinting through biogenesis of piRNAs that mediate methylation of the paternally imprinted Rasgrf1 gene locus in trans (Watanabe et al, 2011b). MitoPLD, Mili, Miwi2, Dnmt3l, Tdrkh, and Mov10L1 mutants all exhibit meiotic arrest at the zygotene phase (Bourc’his and Bestor, 2004; Kuramochi-Miyagawa et al, 2004; Carmell et al, 2007; Zheng et al, 2010; Watanabe et al, 2011a), despite the fact that Mili and Miwi2 mutants upregulate both Line1 and IAP while Tdrkh, Mov10L1, and MitoPLD mutants only upregulate Line1 elements. These differences in transposon methylation could result from divergent effects of these mutations on piRNA generation. It is not likely that upregulation of Line1 alone is responsible for arrest at the zygotene phase, because mutations in Tdrd1, Tdrd5, and Tdrd7 also result in upregulation of Line1 but do not cause spermatogenic arrest until the haploid spermatid phase (Chuma et al, 2006; Tanaka et al, 2011; Yabuta et al, 2011). Moreover, repeat-associated piRNAs are unaffected in post-natal Tdrd1 mutant testes. Shifts in piRNA populations arise from increased mRNA-derived piRNAs at the expense of non-transposon intergenic sequences (Reuter et al, 2009), implying the existence of additional functions beyond epigenetic piRNA-dependent Line1 repression. Compellingly, round spermatids from tdrd5 mutants can even generate fertile offspring when injected into oocytes (Yabuta et al, 2011), showing that upregulation of Line1 does not necessarily cause catastrophic loss of genomic integrity.
In fact, the upregulation of Line1 mRNA (Figure 3C and D) as well as upregulation of corresponding sense Line1-associated piRNAs (Figures 4M, 5F; Supplementary Figure 10b) at 11dpp could occur for several reasons. The increase in Line1-associated piRNAs could be an adaptive response to upregulation of Line1 mRNA. The second half of this response should lead to post-transcriptional degradation of remaining Line1 mRNA (Aravin et al, 2007, 2008); however, this part of the response is blunted in tdrkh mutants. We posit that the combination of decreased primary piRNA biogenesis, the absence of ping-pong-based piRNA biogenesis at this specific time point, and the continued production of Line1 mRNA may prevent the limited piRNA response observed from sufficiently clearing remaining Line1 mRNAs. Furthermore, Line1 protein complexes partner with Line1-coding mRNAs and may protect them from degradation. Alternatively, this mechanism of piRNA-dependent post-transcriptional mRNA degradation may be specific to Miwi-associated transcripts (Reuter et al, 2011) and is therefore not yet be active at this time point, and Tdrkh itself may also be required for this manner of degradation. Thus, there are multiple potential reasons for these observations which will require future studies to resolve.
These data suggest other roles for the piRNA pathway beyond piRNA-guided DNA methylation of transposon promoters exist and likely play key functions in spermatogenesis. One of these additional roles is translational regulation. Piwi proteins and piRNAs are associated with polysomes (Grivna et al, 2006a, 2006b; Unhavaithaya et al, 2009), and Tdrd7 has been shown to play important roles in promoter methylation-independent translational repression of Line1 (Tanaka et al, 2011). piRNA pathway-mediated post-transcriptional regulation extends far beyond transposon regulation, as significant percentages of mammalian and Drosophila piRNAs map to 3′UTRs of coding genes (Robine et al, 2009). In Drosophila, sense-strand 3′UTR-derived piRNAs can regulate translation of complementary genes (Saito et al, 2009) and promote mRNA degradation during the maternal-zygotic transition (Rouget et al, 2010). Other roles include Tdrd6 and Tdrd7 functioning in RNP remodelling and chromatoid body formation (Vasileva et al, 2009; Tanaka et al, 2011) and Miwi in regulating stability of key spermiogenic mRNAs (Deng and Lin, 2002; Vourekas et al, 2011) and piRNA-independent regulation of mRNA stability (Vourekas et al, 2011).
Other potential roles could also include the regulation of DNA damage and asynapsis responses that are activated in Mili, Miwi2, Tdrkh, and other piRNA pathway mutants, as well as mitotic checkpoint control. All of these mutants display reduced testes size and progressive loss of germ cells that are not replaced. Aged tdrkh mutant testes display a similar phenotype. This is consistent with mitotic arrest, and subsequent elimination, of spermatogonia. Consistently, we observed activation of checkpoint proteins in tdrkh mutant spermatogonia (Figure 2H) and residual mitotic marker staining (Supplementary Figure 5g). The simplest explanation is that deletion of tdrkh (and other Piwi pathway proteins) results in mitotic arrest. Tdrkh−/− germ cells are gradually lost during ageing, whereas in other piRNA pathway mutants these cells are actively culled by apoptosis. The lack of apoptosis in tdrkh mutants, with its mitochondrial localization, strongly suggests a role for Tdrkh in this process.
Tdrkh as a gatekeeper of apoptosis
As chromosomal asynapsis is accompanied by transposon expression, it has been difficult to distinguish whether one of these defects is casual of the other, and which one results in spermatogenic arrest. Remarkably, Tdrkh mutants do not appear to execute apoptotic programs despite widespread DNA damage and checkpoint activation. The piRNA pathway may function through Tdrkh to transduce this damage response to induce apoptosis. The lack of apoptosis in the tdrkh mutant suggests a role for Tdrkh in this transduction, and the mitochondrial localization of Tdrkh perfectly positions it for inducing a mitochondrial-mediated cell death response. This may reflect an ancestral function of the Piwi pathway (Padmanabhan et al, 2012). Alternatively, the widespread chromosomal asynapsis observed in the tdrkh mutant may induce the asynapsis checkpoint. Normally this checkpoint does not trigger apoptosis until the pachytene stage of meiosis (Burgoyne et al, 2009), and some checkpoint activation scenarios do not induce apoptosis at all (Jaramillo-Lambert et al, 2010); in this model, piRNAs may be required for chromosomal synapsis and defects in piRNA biogenesis lead to asynapsis.
Tdrkh functions in the primary, but not in secondary, piRNA biogenesis pathway
Our data reported here indicate that Tdrkh functions in the primary piRNA biogenesis pathway. Tdrkh is required for production of proper levels of piRNAs derived from piRNA clusters (Figures 4B, J, and ; Supplementary Figure 10a) and mRNAs (Figure 5N), which make up ∼80% of the piRNA population in 11dpp testes. In tdrkh mutants, piRNA precursors are not fully processed to mature piRNAs and accumulate as 31–40 nt intermediates. This limited size range implies that Tdrkh functions in a 3′ maturation step after a major processing event, consistent with recent findings suggesting that 3′ processing by an exonuclease is required for piRNA maturation (Kawaoka et al, 2011). This places Tdrkh downstream of other known components in the primary piRNA processing pathway. The order of precursor and intermediate loading into Piwi proteins, 5′ end formation, and 3′ processing of precursors into intermediates is unknown. 5′ end formation of primary piRNAs can be catalysed through the endonuclease activity of MitoPLD (Ipsaro et al, 2012; Nishimasu et al, 2012) and Miwi (Reuter et al, 2011). Mili can generate 5′ ends of secondary piRNAs (and possibly primary piRNAs; De Fazio et al, 2011; Reuter et al, 2011). Precursors are cleaved to form intermediates through a yet-to-be-identified mechanism and, once bound to Piwi proteins, are trimmed to their mature form by a 3′→5′ exonuclease (‘Trimmer’) (Kawaoka et al, 2011). Tdrkh may facilitate this latter process (Figures 5A and 6) by recruiting Trimmer to Piwi proteins or to the nuage (similar to how it recruits Tdrd1). Deletion of Tdrkh would then impede this process and sharply compromise piRNA biogenesis.
It is also possible that Tdrkh directly promotes Trimmer activity. In the absence of Tdrkh, Trimmer is highly inefficient, allowing Hen1 to prematurely methylate the 3′ ends of intermediates. This stops their processing into mature piRNAs by preventing further exonuclease activity, causing the observed buildup of piRNA intermediates. This hypothesis is highly consistent with the robust coupling of 3′end resection and Hen1-mediated 3′ end methylation (Kawaoka et al, 2011) and nicely integrates our experimental findings and bioinformatic evidence.
Distinct sizing of piRNA intermediates suggests multiple mechanisms of generating mature piRNAs
Different regions of mRNAs generate 27–28 nt piRNAs with similar frequency. However, UTRs and coding regions are specifically enriched for 35 and 31 nt intermediates, respectively, whereas intronic-derived intermediates display no size preference. Likewise, IAP and Line1 retrotransposons show distinctly sized intermediates in both tdrkh mutants and controls. Furthermore, cluster-derived intermediates are different in size from both transposon-based and genic-based intermediates. The basis for these size differences is not clear but is highly suggestive of distinct mechanisms for handling and processing piRNA precursors into intermediates.
Similarities and differences between piRNA-like RNA populations and intermediates
There have been reports of several piRNA-related species. The mouse MSY-RNAs (Xu et al, 2009) are a 25–31 nt species enriched for 1′A, contain several piRNA-like characteristics, and have unknown functions. Tdrd1 immunoprecipitates from Zebrafish revealed Tdrd1-associated transcripts (TATs) (Huang et al, 2011b) which appear to be semi-cleaved piRNA targets, are antisense to the piRNA that presumptively target them, and display a ping-pong signature. If TATs are cleaved in a ping pong-like manner, then they would also then serve as precursors for secondary piRNAs. TATs have not been reported from Tdrd1 immunoprecipitation experiments performed in mice (Reuter et al, 2009; Vagin et al, 2009).
In contrast, presumptive primary piRNA processing intermediates have been identified in Drosophila (Saito et al, 2010). The Mov10L1 homologue Armi binds a family of piRNA intermediate-like molecules (piR-ILs) that are dependent on the Yb-body protein Yb (Qi et al, 2010; Saito et al, 2010). piR-ILs carry mature tj and idefix piRNA sequences. Importantly, piR-ILs do not contain a preference for a 1′U and are undetectable by northern blots based on the EDC-crosslinking protocol. In these regards, piR-ILs are divergent from the Mili-associated piRNA intermediates that we found to accumulate in the absence of Tdrkh. This is consistent with the upstream roles of Armi (Olivieri et al, 2010; Saito et al, 2010) and Mov10L1 (Zheng et al, 2010) in primary piRNA biogenesis. piR-ILs likely represent earlier intermediates, which are not yet competent for loading into Piwi proteins and do not have mature 5′ ends. In contrast, Tdrkh-dependent intermediates contain processed 5′ ends and are awaiting complete 3′ end maturation.
Mili-associated 22 nt RNAs may be involved in piRNA biogenesis from 3 ′UTRs
While investigating Mili-associated RNAs in our controls and mutants, we discovered a previously uncharacterized 22 nt RNA population. Our bioinformatic analysis suggests a significant overlap between these 22 nt RNAs and Mili-associated piRNAs/precursors. It is possible that these sequences may be an uncharacterized class of Mili-associated miRNAs. Although it is also quite possible that many of these sequences are in fact degradation products of mature piRNAs/precursors, this is unlikely to be the case. The 22 nt sequences are often phased several nucleotides from the 5′end of mature piRNAs. This fact, combined with the heavy 3′UTR bias and 1′U preference of the 22 nt RNAs, makes this explanation unsatisfying. As 22 nt RNAs and piRNAs are almost exclusively sense to each other, it is highly unlikely that they are involved in some manner of uncharacterized piRNA amplification scheme. These sequences could either act as guides for piRNA biogenesis or they might be by-products of 5′end processing of piRNA precursors (which are unaffected in tdrkh mutants) that are not fed into downstream steps of piRNA biogenesis. Alternatively, they could be cleavage products of piRNA-guided 3′UTR processing. Although it is also possible that these 22 nt sequences are degradation products of mature piRNAs/intermediates not found in our data set due to incomplete coverage, our sequencing depth of >60 × 106 mapped reads for each Mili-associated library makes this relatively unlikely. The exact nature of these sequences remains unclear and is currently under study.
Cycling of Tdrkh between pi- and piP-bodies
A previous study suggested that Tdrkh is a mitochondrial protein and examined co-localization in somatic tissues and cell lines (Pagliarini et al, 2008). Here, we show definitively that Tdrkh is a bona fide mitochondrial-associated protein in the male germline. MitoPLD, through generation of signalling intermediates, regulates mitochondrial homeostasis and formation of nuage and IMC (Huang et al, 2011a; Watanabe et al, 2011a). Disruption of IMC leads to deregulated piRNA processing. Mili-containing pi-bodies localize to IMC in pro-spermatogonia. Pi-bodies contain additional proteins such as Mvh and Tdrd1 (Aravin et al, 2009; Shoji et al, 2009). This complex is distinct from Miwi2-containing piP-bodies, which contain Mael and Tdrd9 (Aravin et al, 2009; Shoji et al, 2009). It is thought that these two complexes, acting in concert, are responsible for ping-pong-based generation of piRNAs (Siomi et al, 2011). Consistent with its interaction with Miwi2, Tdrkh often co-localizes with piP-bodies and mutation of tdrkh leads to abnormal Miwi2 localization. In addition, Tdrkh is often found in pi-bodies (Supplementary Figure 3f and g) and is required for proper localization of the pi-body component Tdrd1 (Figure 3A). This profile suggests that Tdrkh functions in both of these organelles, potentially shuttling piRNA precursors or RNA intermediates between these two complexes and loading Miwi2 for nuclear translocation.
Tdrkh interacts with Miwi and Miwi2 via symmetric dimethylation
Here, we show that Tdrkh preferentially interacts with Miwi and Miwi2, consistent with earlier reports (Chen et al, 2009; Vagin et al, 2009). These interactions are mediated by symmetric dimethylation of residues within the N-terminal domain of the Piwi proteins. Miwi2 contains a stretch of dimethylated arginines that recruits the Tudor domain of Tdrkh (Figure 1G). The analogous sequence in Miwi is dispensable for this interaction; instead, a distinct motif in the extreme N-terminus of Miwi recruits Tdrkh (Figure 1H) and provides an exceptionally high local positive charge. Crystal structures of the Tdrkh Tudor domain have revealed a highly structured acidic surface (Chen et al, 2009; Liu et al, 2010). The complementary charges between the Tudor domain of Tdrkh and the N-terminal of Miwi may account for the specificity in the interaction between these Piwi proteins and Tudor domain-containing proteins. Symmetric dimethylation of Miwi-R4 may act to stabilize the Miwi–Tdrkh interaction. Although Miwi-R14 has been shown to be symmetrically dimethylated (Vagin et al, 2009; Liu et al, 2010), it has not been shown to bind Tdrkh. In our hands, this modification disrupts the Miwi–Tdrkh interaction. In vivo, this may serve to properly position Tdrkh within the Miwi complex. Complementary crystal structures show that the Tudor domain of the closely related Tudor domain of Snd1 accommodates these two peptides very differently depending on which arginine is modified (Liu et al, 2010). In the context of whole proteins, rather than highly purified fragments, Tdrkh may only utilize R4 to bind with Miwi, allowing MiwiR14 to serve as a platform to recruit additional Tudor domain-containing proteins. Thus, the structural basis and selectivity for these interactions need to be further explored.
Materials and methods
Generation of tdrkh-null mice
Tdrkh-floxed mice were generated by the University of Connecticut Gene Targeting and Transgenic Facility. Founder lines were crossed with EIIa-Cre transgenic mice to excise the floxed exons 2–4 and generate Tdrkh+/− animals. Heterozygous animals were intercrossed to remove the EIIA transgene.
Antibodies
The Miwi2 and Orf1p antibodies were gifts from Javier Martinez (IMBA) and Sandra Martin (UC Denver), respectively. Miwi antisera were previously described (Deng and Lin, 2002).
In vivo interactions
Mouse testes from indicated genotypes and strains were dissected and snap-frozen in liquid nitrogen until use. Lysates were prepared by dounce homogenization in MCB (50 mM HEPES, 150 mM potassium acetate, 2 mM magnesium acetate, 0.1% Triton X-100, 0.1% NP-40, 1 mM DTT, 10% glycerol pH 7.5), clarified by centrifugation at 14 000 r.p.m., and pre-cleared with protein A sepharose (Pierce). RNase A digestions were performed by adding 500 μg RNase A (Qiagen) and incubation for 3 h at 4°C followed by 30 min at room temperature. Primary antibodies were incubated with lysates overnight at 4°C on a rotator and captured with Protein A sepharose beads (GE Healthcare), washed with MCB, and boiled in 2 × SDS loading buffer.
Small RNA library construction, sequencing, and analysis
Five pairs of testes from each age and genotype were used for small RNA library construction. Small RNAs were purified using a miRVana RNA isolation kit (Ambion), and 15–40 nt RNAs were gel-purified on a TBE-urea gel. Libraries were prepared using an Illumina Small RNA sample preparation kit and sequenced on a Genome Analyzer II (Illumina).
Linker sequences were removed using FASTX-Toolkit ( http://hannonlab.cshl.edu/fastx_toolkit/). Degradation products were removed by mapping with Bowtie, allowing two mismatches to a library consisting of tRNA, rRNA, scRNA, scpRNA, and snoRNA with 25 bp extensions on both 5′ and 3′ ends. Remaining sequences were mapped to the genome (mm9), allowing for up to two mismatches. Sequences were annotated using UCSC mm9 (extracted from http://genome.ucsc.edu). For reads with multiple mapping positions, priority was given to genes over repeats, which in turn were prioritized in the order of LINE, SINE, LTR, low complexity, simple repeat, satellite, DNA, RC, and unknown. Sequences without genic or repeat annotation were labelled as intergenic. Mapping of unique piRNAs to pre-pachytene and embryonic clusters was performed using the cluster coordinates in Watanabe et al (2011a). Analyses were normalized to total number of library reads.
For repeat-associated small RNA analysis, reads were mapped to L1MdGf (D84391), L1MdA (M13002), and IAP (M17551) consensus sequences and to LTR, ERV, and SINE sequences extracted from RepBase allowing up to two mismatches. For complementarity analysis, piRNAs mapped to the Line1 and IAP sequences above were analysed separately. The distance between the 5′ ends of overlapping and complementary piRNAs was counted and represented as a percentage of the total number of overlapping pairs, allowing up to three mismatches. 10nt from the 5′end of piRNAs with an adenine in their tenth position were checked for complementarity to the 5′ 10 nts of sequences with a 1′U.
Reads were re-mapped to mm9 with perfect matches for mature miRNA (sequences extracted from http://www.mirbase.org) and genic annotation. In the case of multiple annotations for a given read, priority was given based on 5′UTR, CDS, 3′UTR, and then introns.
Supplementary Material
Acknowledgments
We thank Mei Zhong at the Yale Stem Cell Center Genomics core for assistance with small RNA library construction and sequencing, and Morven Graham, Kim Zichichi, Christina Horensavitz, and the Yale CCMI EM Core for assistance with electron microscopy. Line1 Orf1p antibody was a gift from S Martin (UC Denver) and Miwi2 was kindly provided by J Martinez (IMBA). The small RNA sequences in this paper have been registered in the NCBI GEO under accession number GSE47151. The data will be released on 1st September 2013. JPS was the recipient of an NICHD Ruth L Kirschstein NRSA. This work was supported by the NIH (R01HD42012) and the G Harold and Leila Mathers Foundation.
Author contributions: JPS and HL designed the experiments; JPS performed the experiments; MC and HZ performed bioinformatic analysis; JPS, MC, HZ, and HL analysed the data; and JPS and HL wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Tuschl T (2006) A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442: 203–207 [DOI] [PubMed] [Google Scholar]
- Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KF, Bestor T, Hannon GJ (2008) A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell 31: 785–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ (2007) Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316: 744–747 [DOI] [PubMed] [Google Scholar]
- Aravin AA, van der Heijden GW, Castaneda J, Vagin VV, Hannon GJ, Bortvin A (2009) Cytoplasmic compartmentalization of the fetal piRNA pathway in mice. PLoS Genet 5: e1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyret E, Liu N, Lin H (2012) piRNA biogenesis during adult spermatogenesis in mice is independent of the ping-pong mechanism. Cell Res 22: 1429–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourc’his D, Bestor TH (2004) Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 431: 96–99 [DOI] [PubMed] [Google Scholar]
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ (2007) Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128: 1089–1103 [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Turner JM (2009) The consequences of asynapsis for mammalian meiosis. Nat Rev Genet 10: 207–216 [DOI] [PubMed] [Google Scholar]
- Carmell MA, Girard A, van de Kant HJ, Bourc’his D, Bestor TH, de Rooij DG, Hannon GJ (2007) MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell 12: 503–514 [DOI] [PubMed] [Google Scholar]
- Chen C, Jin J, James DA, Adams-Cioaba MA, Park JG, Guo Y, Tenaglia E, Xu C, Gish G, Min J, Pawson T (2009) Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi. Proc Natl Acad Sci USA 106: 20336–20341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuma S, Hosokawa M, Kitamura K, Kasai S, Fujioka M, Hiyoshi M, Takamune K, Noce T, Nakatsuji N (2006) Tdrd1/Mtr-1, a tudor-related gene, is essential for male germ-cell differentiation and nuage/germinal granule formation in mice. Proc Natl Acad Sci USA 103: 15894–15899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Y, Speed RM, Gautier P, Semple CA, Maratou K, Turner JM, Cooke HJ (2006) Mouse MAELSTROM: the link between meiotic silencing of unsynapsed chromatin and microRNA pathway? Hum Mol Genet 15: 2324–2334 [DOI] [PubMed] [Google Scholar]
- Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H (1998) A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev 12: 3715–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fazio S, Bartonicek N, Di Giacomo M, Abreu-Goodger C, Sankar A, Funaya C, Antony C, Moreira PN, Enright AJ, O’Carroll D (2011) The endonuclease activity of Mili fuels piRNA amplification that silences LINE1 elements. Nature 480: 259–263 [DOI] [PubMed] [Google Scholar]
- Deng W, Lin H (2002) miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2: 819–830 [DOI] [PubMed] [Google Scholar]
- Frost RJ, Hamra FK, Richardson JA, Qi X, Bassel-Duby R, Olson EN (2010) MOV10L1 is necessary for protection of spermatocytes against retrotransposons by Piwi-interacting RNAs. Proc Natl Acad Sci USA 107: 11847–11852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard A, Sachidanandam R, Hannon GJ, Carmell MA (2006) A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442: 199–202 [DOI] [PubMed] [Google Scholar]
- Grivna ST, Beyret E, Wang Z, Lin H (2006a) A novel class of small RNAs in mouse spermatogenic cells. Genes Dev 20: 1709–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivna ST, Pyhtila B, Lin H (2006b) MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc Natl Acad Sci USA 103: 13415–13420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC (2007) A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 315: 1587–1590 [DOI] [PubMed] [Google Scholar]
- Horwich MD, Li C, Matranga C, Vagin V, Farley G, Wang P, Zamore PD (2007) The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr Biol 17: 1265–1272 [DOI] [PubMed] [Google Scholar]
- Huang H, Gao Q, Peng X, Choi SY, Sarma K, Ren H, Morris AJ, Frohman MA (2011a) piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev Cell 20: 376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Houwing S, Kaaij LJ, Meppelink A, Redl S, Gauci S, Vos H, Draper BW, Moens CB, Burgering BM, Ladurner P, Krijgsveld J, Berezikov E, Ketting RF (2011b) Tdrd1 acts as a molecular scaffold for Piwi proteins and piRNA targets in zebrafish. EMBO J 30: 3298–3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XA, Yin H, Sweeney S, Raha D, Snyder M, Lin H (2013) A major epigenetic programming mechanism guided by piRNAs. Dev Cell 24: 502–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsaro JJ, Haase AD, Knott SR, Joshua-Tor L, Hannon GJ (2012) The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature 491: 279–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo-Lambert A, Harigaya Y, Vitt J, Villeneuve A, Engebrecht J (2010) Meiotic errors activate checkpoints that improve gamete quality without triggering apoptosis in male germ cells. Curr Biol 20: 2078–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka S, Izumi N, Katsuma S, Tomari Y (2011) 3′ end formation of PIWI-interacting RNAs in vitro. Mol Cell 43: 1015–1022 [DOI] [PubMed] [Google Scholar]
- Kirino Y, Kim N, de Planell-Saguer M, Khandros E, Chiorean S, Klein PS, Rigoutsos I, Jongens TA, Mourelatos Z (2009) Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nat Cell Biol 11: 652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino Y, Mourelatos Z (2007a) Mouse Piwi-interacting RNAs are 2′-O-methylated at their 3′ termini. Nat Struct Mol Biol 14: 347–348 [DOI] [PubMed] [Google Scholar]
- Kirino Y, Mourelatos Z (2007b) The mouse homolog of HEN1 is a potential methylase for Piwi-interacting RNAs. RNA 13: 1397–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, Lin H, Matsuda Y, Nakano T (2004) Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 131: 839–849 [DOI] [PubMed] [Google Scholar]
- Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, Hata K, Li E, Matsuda Y, Kimura T, Okabe M, Sakaki Y, Sasaki H, Nakano T (2008) DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev 22: 908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE (2006) Characterization of the piRNA complex from rat testes. Science 313: 363–367 [DOI] [PubMed] [Google Scholar]
- Lin H, Yin H (2008) A Novel Epigenetic Mechanism in Drosophila Somatic Cells Mediated by PIWI and piRNAs. Cold Spring Harb Symp Quant Biol 73: 273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Chen C, Guo Y, Lam R, Bian C, Xu C, Zhao DY, Jin J, MacKenzie F, Pawson T, Min J (2010) Structural basis for recognition of arginine methylated Piwi proteins by the extended Tudor domain. Proc Natl Acad Sci USA 107: 18398–18403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Qi H, Wang J, Lin H (2011) PAPI, a novel TUDOR-domain protein, complexes with AGO3, ME31B and TRAL in the nuage to silence transposition. Development 138: 1863–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathioudakis N, Palencia A, Kadlec J, Round A, Tripsianes K, Sattler M, Pillai RS, Cusack S (2012) The multiple Tudor domain-containing protein TDRD1 is a molecular scaffold for mouse Piwi proteins and piRNA biogenesis factors. RNA 18: 2056–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimasu H, Ishizu H, Saito K, Fukuhara S, Kamatani MK, Bonnefond L, Matsumoto N, Nishizawa T, Nakanaga K, Aoki J, Ishitani R, Siomi H, Siomi MC, Nureki O (2012) Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature 491: 284–287 [DOI] [PubMed] [Google Scholar]
- Ohara T, Sakaguchi Y, Suzuki T, Ueda H, Miyauchi K (2007) The 3′ termini of mouse Piwi-interacting RNAs are 2′-O-methylated. Nat Struct Mol Biol 14: 349–350 [DOI] [PubMed] [Google Scholar]
- Olivieri D, Sykora MM, Sachidanandam R, Mechtler K, Brennecke J (2010) An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila. EMBO J 29: 3301–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan PK, Dumas C, Samant M, Rochette A, Simard MJ, Papadopoulou B (2012) Novel Features of a PIWI-Like Protein Homolog in the Parasitic Protozoan Leishmania. PLoS One 7: e52612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK (2008) A mitochondrial protein compendium elucidates complex I disease biology. Cell 134: 112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Watanabe T, Ku HY, Liu N, Zhong M, Lin H (2010) The Yb body, a major site for Piwi-associated RNA biogenesis and a gateway for Piwi expression and transport to the nucleus in somatic cells. J Biol Chem 286: 3789–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Berninger P, Chuma S, Shah H, Hosokawa M, Funaya C, Antony C, Sachidanandam R, Pillai RS (2011) Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature 480: 264–267 [DOI] [PubMed] [Google Scholar]
- Reuter M, Chuma S, Tanaka T, Franz T, Stark A, Pillai RS (2009) Loss of the Mili-interacting Tudor domain-containing protein-1 activates transposons and alters the Mili-associated small RNA profile. Nat Struct Mol Biol 16: 639, 46 [DOI] [PubMed] [Google Scholar]
- Robine N, Lau NC, Balla S, Jin Z, Okamura K, Kuramochi-Miyagawa S, Blower MD, Lai EC (2009) A broadly conserved pathway generates 3′UTR-directed primary piRNAs. Curr Biol 19: 2066–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouget C, Papin C, Boureux A, Meunier AC, Franco B, Robine N, Lai EC, Pelisson A, Simonelig M (2010) Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature 467: 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Inagaki S, Mituyama T, Kawamura Y, Ono Y, Sakota E, Kotani H, Asai K, Siomi H, Siomi MC (2009) A regulatory circuit for piwi by the large Maf gene traffic jam in Drosophila. Nature 461: 1296–1299 [DOI] [PubMed] [Google Scholar]
- Saito K, Ishizu H, Komai M, Kotani H, Kawamura Y, Nishida KM, Siomi H, Siomi MC (2010) Roles for the Yb body components Armitage and Yb in primary piRNA biogenesis in Drosophila. Genes Dev 24: 2493–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Sakaguchi Y, Suzuki T, Siomi H, Siomi MC (2007) Pimet, the Drosophila homolog of HEN1, mediates 2′-O-methylation of Piwi- interacting RNAs at their 3′ ends. Genes Dev 21: 1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Nishida KM, Shibuya A, Siomi MC, Siomi H (2011) Maelstrom coordinates microtubule organization during Drosophila oogenesis through interaction with components of the MTOC. Genes Dev 25: 2361–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Tanaka T, Hosokawa M, Reuter M, Stark A, Kato Y, Kondoh G, Okawa K, Chujo T, Suzuki T, Hata K, Martin SL, Noce T, Kuramochi-Miyagawa S, Nakano T, Sasaki H, Pillai RS, Nakatsuji N, Chuma S (2009) The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev Cell 17: 775–787 [DOI] [PubMed] [Google Scholar]
- Siomi MC, Sato K, Pezic D, Aravin AA (2011) PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol 12: 246–258 [DOI] [PubMed] [Google Scholar]
- Soper SF, van der Heijden GW, Hardiman TC, Goodheart M, Martin SL, de Boer P, Bortvin A (2008) Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev Cell 15: 285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Hosokawa M, Vagin VV, Reuter M, Hayashi E, Mochizuki AL, Kitamura K, Yamanaka H, Kondoh G, Okawa K, Kuramochi-Miyagawa S, Nakano T, Sachidanandam R, Hannon GJ, Pillai RS, Nakatsuji N, Chuma S (2011) Tudor domain containing 7 (Tdrd7) is essential for dynamic ribonucleoprotein (RNP) remodeling of chromatoid bodies during spermatogenesis. Proc Natl Acad Sci USA 108: 10579–10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson T, Lin H (2009) The biogenesis and function of PIWI Proteins and piRNAs: progress and prospect. Annu Rev Cell Dev Biol 25: 355–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unhavaithaya Y, Hao Y, Beyret E, Yin H, Kuramochi-Miyagawa S, Nakano T, Lin H (2009) MILI, a PIWI-interacting RNA-binding protein, is required for germ line stem cell self-renewal and appears to positively regulate translation. J Biol Chem 284: 6507–6519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin VV, Wohlschlegel J, Qu J, Jonsson Z, Huang X, Chuma S, Girard A, Sachidanandam R, Hannon GJ, Aravin AA (2009) Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev 23: 1749–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileva A, Tiedau D, Firooznia A, Muller-Reichert T, Jessberger R (2009) Tdrd6 is required for spermiogenesis, chromatoid body architecture, and regulation of miRNA expression. Curr Biol 19: 630–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vourekas A, Zheng Q, Alexiou P, Maragkakis M, Kirino Y, Gregory BD, Mourelatos Z (2011) Mili and Miwi target RNA repertoire reveals piRNA biogenesis and function of Miwi in spermiogenesis. Nat Struct Mol Biol 19: 773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Saxe JP, Tanaka T, Chuma S, Lin H (2009) Mili interacts with tudor domain-containing protein 1 in regulating spermatogenesis. Curr Biol 19: 640–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Chuma S, Yamamoto Y, Kuramochi-Miyagawa S, Totoki Y, Toyoda A, Hoki Y, Fujiyama A, Shibata T, Sado T, Noce T, Nakano T, Nakatsuji N, Lin H, Sasaki H (2011a) MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev Cell 20: 364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Tomizawa S, Mitsuya K, Totoki Y, Yamamoto Y, Kuramochi-Miyagawa S, Iida N, Hoki Y, Murphy PJ, Toyoda A, Gotoh K, Hiura H, Arima T, Fujiyama A, Sado T, Shibata T, Nakano T, Lin H, Ichiyanagi K, Soloway PD et al. (2011b) Role for piRNAs and noncoding RNA in de novo DNA methylation of the imprinted mouse Rasgrf1 locus. Science 332: 848–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Medvedev S, Yang J, Hecht NB (2009) MIWI-independent small RNAs (MSY-RNAs) bind to the RNA-binding protein, MSY2, in male germ cells. Proc Natl Acad Sci USA 106: 12371–12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta Y, Ohta H, Abe T, Kurimoto K, Chuma S, Saitou M (2011) TDRD5 is required for retrotransposon silencing, chromatoid body assembly, and spermiogenesis in mice. J Cell Biol 192: 781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Xiol J, Reuter M, Eckardt S, Leu NA, McLaughlin KJ, Stark A, Sachidanandam R, Pillai RS, Wang PJ (2010) Mouse MOV10L1 associates with Piwi proteins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc Natl Acad Sci USA 107: 11841–11846 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.