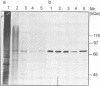
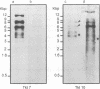
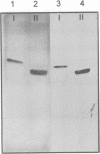
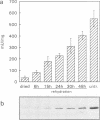
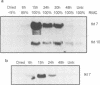
Abstract
Transketolases, key enzymes of the reductive and oxidative pentose phosphate pathways, are responsible for the synthesis of sugar phosphate intermediates. Here we report the first molecular analysis of transketolase genes from plants. Three distinct classes of transketolase-encoding cDNA clones were isolated from the desiccation-tolerant resurrection plant Craterostigma plantagineum. One class represented by the transcript tkt3 is constitutively expressed in leaves and roots under all physiological conditions tested. By biochemical analysis and protein sequencing of purified transketolase, it was shown that tkt3 is expressed in three enzymatically active isoforms. An intriguing discovery was that accumulation of the two other transketolase transcripts, tkt7 and tkt10, is preferentially associated with the rehydration process of the desiccated plant; whereas tkt10 is only expressed in leaves, tkt7 was detected in leaves and roots. This observation suggests a possible role for these transketolases in the conversion of sugars, which are a major phenomenon in the rehydration process. Despite an abundant level of tkt7 and tkt10 transcripts in rehydrating leaves, proteins could not be isolated. This is due in part to a translational control mechanism acting on the loading of mRNAs to polysomes.
Full text
PDF


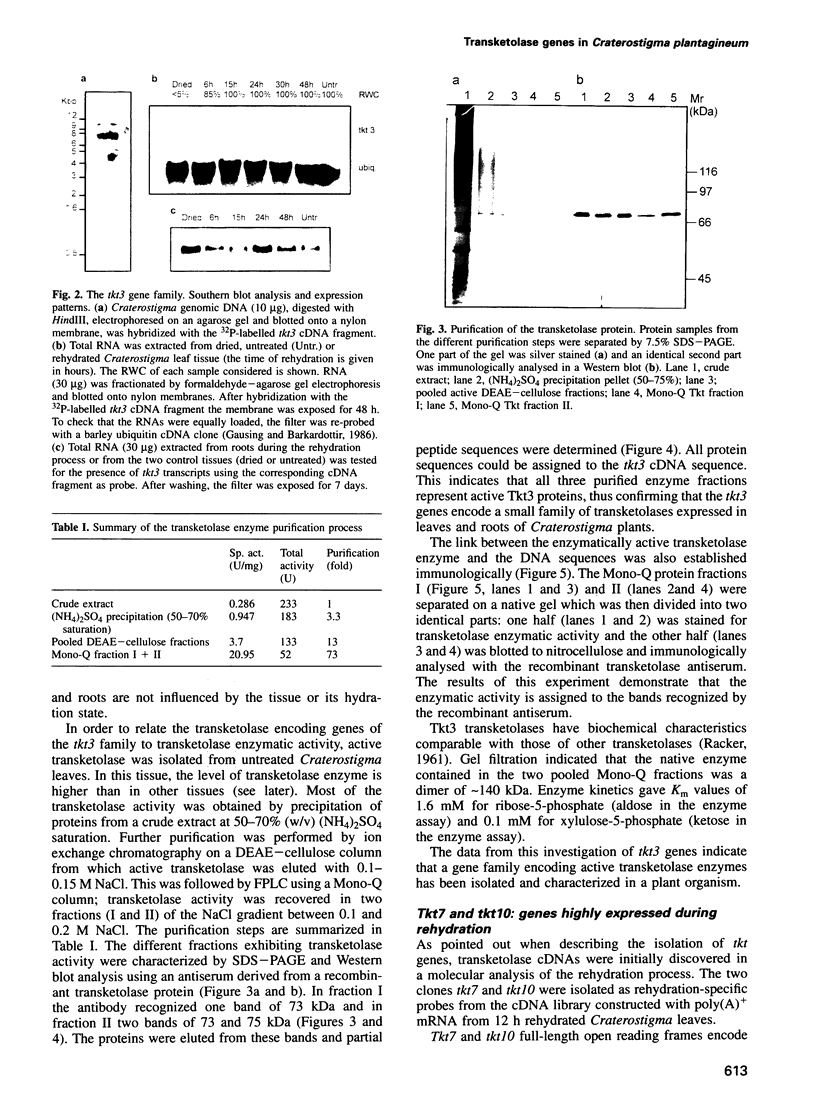
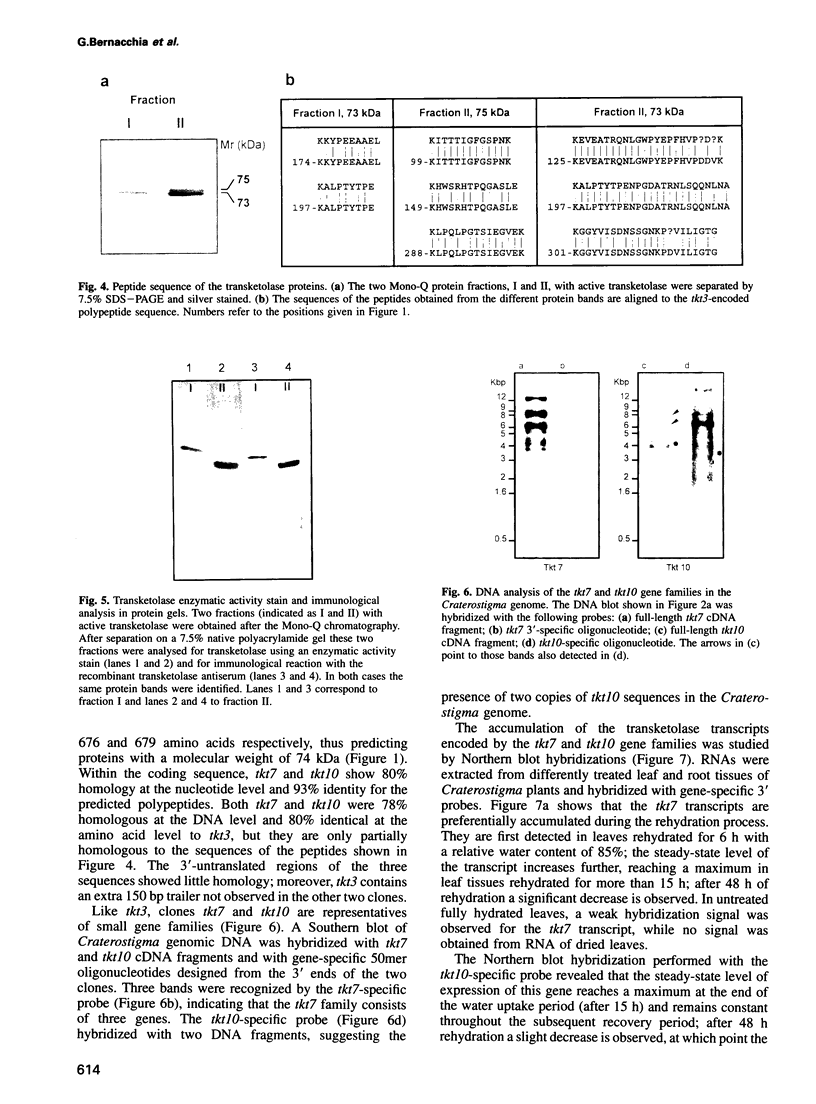
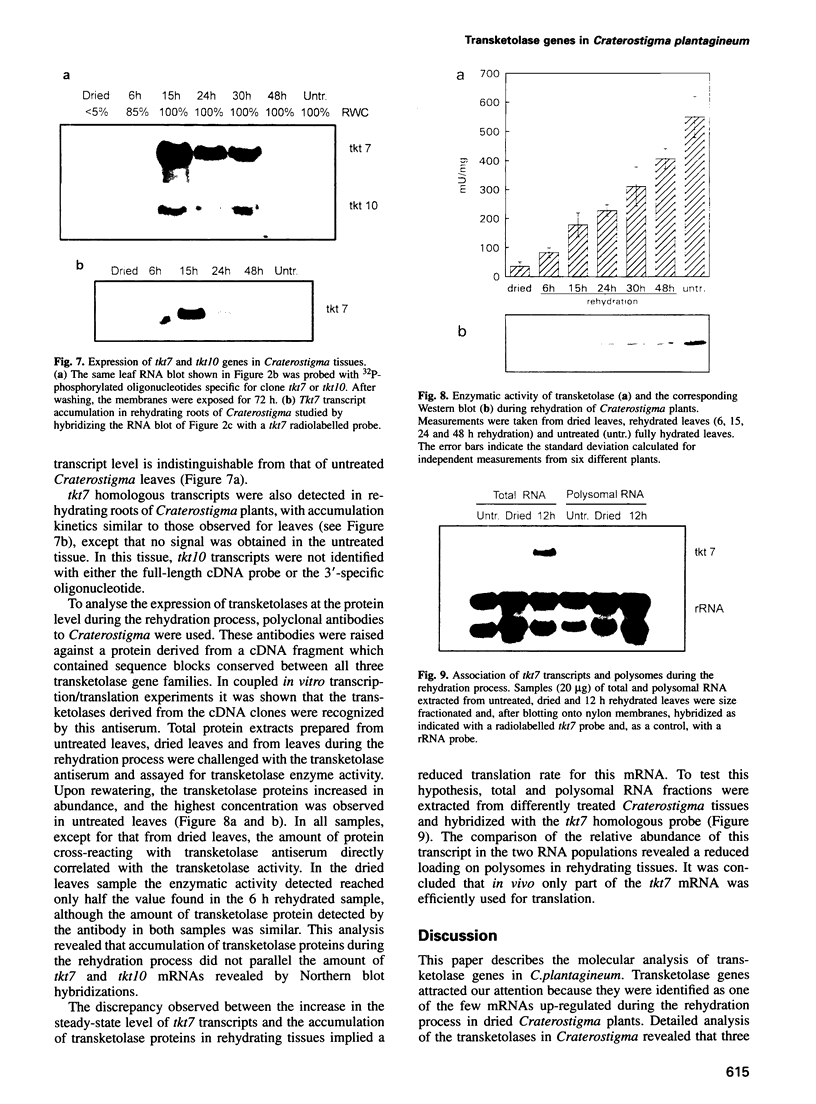



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels D., Engelhardt K., Roncarati R., Schneider K., Rotter M., Salamini F. An ABA and GA modulated gene expressed in the barley embryo encodes an aldose reductase related protein. EMBO J. 1991 May;10(5):1037–1043. doi: 10.1002/j.1460-2075.1991.tb08042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D., Hanke C., Schneider K., Michel D., Salamini F. A desiccation-related Elip-like gene from the resurrection plant Craterostigma plantagineum is regulated by light and ABA. EMBO J. 1992 Aug;11(8):2771–2778. doi: 10.1002/j.1460-2075.1992.tb05344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray E. A. Molecular Responses to Water Deficit. Plant Physiol. 1993 Dec;103(4):1035–1040. doi: 10.1104/pp.103.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Gibson J. L., McCue L. A., Tabita F. R. Identification, expression, and deduced primary structure of transketolase and other enzymes encoded within the form II CO2 fixation operon of Rhodobacter sphaeroides. J Biol Chem. 1991 Oct 25;266(30):20447–20452. [PubMed] [Google Scholar]
- Crowe L. M., Mouradian R., Crowe J. H., Jackson S. A., Womersley C. Effects of carbohydrates on membrane stability at low water activities. Biochim Biophys Acta. 1984 Jan 11;769(1):141–150. doi: 10.1016/0005-2736(84)90017-8. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dure L., 3rd A repeating 11-mer amino acid motif and plant desiccation. Plant J. 1993 Mar;3(3):363–369. doi: 10.1046/j.1365-313x.1993.t01-19-00999.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forde B. G., Kreis M., Bahramian M. B., Matthews J. A., Miflin B. J., Thompson R. D., Bartels D., Flavell R. B. Molecular cloning and analysis of cDNA sequences derived from poly A+ RNA from barley endosperm: identification of B hordein related clones. Nucleic Acids Res. 1981 Dec 21;9(24):6689–6707. doi: 10.1093/nar/9.24.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaff D. F. Desiccation-tolerant flowering plants in southern Africa. Science. 1971 Dec 3;174(4013):1033–1034. doi: 10.1126/science.174.4013.1033. [DOI] [PubMed] [Google Scholar]
- Gausing K., Barkardottir R. Structure and expression of ubiquitin genes in higher plants. Eur J Biochem. 1986 Jul 1;158(1):57–62. doi: 10.1111/j.1432-1033.1986.tb09720.x. [DOI] [PubMed] [Google Scholar]
- Hawkins C. F., Borges A., Perham R. N. A common structural motif in thiamin pyrophosphate-binding enzymes. FEBS Lett. 1989 Sep 11;255(1):77–82. doi: 10.1016/0014-5793(89)81064-6. [DOI] [PubMed] [Google Scholar]
- Janowicz Z. A., Eckart M. R., Drewke C., Roggenkamp R. O., Hollenberg C. P., Maat J., Ledeboer A. M., Visser C., Verrips C. T. Cloning and characterization of the DAS gene encoding the major methanol assimilatory enzyme from the methylotrophic yeast Hansenula polymorpha. Nucleic Acids Res. 1985 May 10;13(9):3043–3062. doi: 10.1093/nar/13.9.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochetov G. A. Transketolase from yeast, rat liver, and pig liver. Methods Enzymol. 1982;90(Pt E):209–223. doi: 10.1016/s0076-6879(82)90128-8. [DOI] [PubMed] [Google Scholar]
- Koster K. L., Leopold A. C. Sugars and desiccation tolerance in seeds. Plant Physiol. 1988 Nov;88(3):829–832. doi: 10.1104/pp.88.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindqvist Y., Schneider G., Ermler U., Sundström M. Three-dimensional structure of transketolase, a thiamine diphosphate dependent enzyme, at 2.5 A resolution. EMBO J. 1992 Jul;11(7):2373–2379. doi: 10.1002/j.1460-2075.1992.tb05301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool B. A., Plonk S. G., Martin P. R., Singleton C. K. Cloning of human transketolase cDNAs and comparison of the nucleotide sequence of the coding region in Wernicke-Korsakoff and non-Wernicke-Korsakoff individuals. J Biol Chem. 1993 Jan 15;268(2):1397–1404. [PubMed] [Google Scholar]
- McElfresh K. C., Chourey P. S. Anaerobiosis induces transcription but not translation of sucrose synthase in maize. Plant Physiol. 1988 Jun;87(2):542–546. doi: 10.1104/pp.87.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R., Zilinskas B. A. Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. Plant J. 1994 Mar;5(3):397–405. doi: 10.1111/j.1365-313x.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- Oliver M. J. Influence of Protoplasmic Water Loss on the Control of Protein Synthesis in the Desiccation-Tolerant Moss Tortula ruralis: Ramifications for a Repair-Based Mechanism of Desiccation Tolerance. Plant Physiol. 1991 Dec;97(4):1501–1511. doi: 10.1104/pp.97.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatkowski D., Schneider K., Salamini F., Bartels D. Characterization of Five Abscisic Acid-Responsive cDNA Clones Isolated from the Desiccation-Tolerant Plant Craterostigma plantagineum and Their Relationship to Other Water-Stress Genes. Plant Physiol. 1990 Dec;94(4):1682–1688. doi: 10.1104/pp.94.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radnis B. A., Rhee D. K., Morrison D. A. Genetic transformation in Streptococcus pneumoniae: nucleotide sequence and predicted amino acid sequence of recP. J Bacteriol. 1990 Jul;172(7):3669–3674. doi: 10.1128/jb.172.7.3669-3674.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Reizer A., Bairoch A., Saier M. H., Jr A diverse transketolase family that includes the RecP protein of Streptococcus pneumoniae, a protein implicated in genetic recombination. Res Microbiol. 1993 Jun;144(5):341–347. doi: 10.1016/0923-2508(93)90191-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäferjohann J., Yoo J. G., Kusian B., Bowien B. The cbb operons of the facultative chemoautotroph Alcaligenes eutrophus encode phosphoglycolate phosphatase. J Bacteriol. 1993 Nov;175(22):7329–7340. doi: 10.1128/jb.175.22.7329-7340.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sprenger G. A. Nucleotide sequence of the Escherichia coli K-12 transketolase (tkt) gene. Biochim Biophys Acta. 1993 Nov 16;1216(2):307–310. doi: 10.1016/0167-4781(93)90161-6. [DOI] [PubMed] [Google Scholar]
- Sullivan M. L., Green P. J. Post-transcriptional regulation of nuclear-encoded genes in higher plants: the roles of mRNA stability and translation. Plant Mol Biol. 1993 Dec;23(6):1091–1104. doi: 10.1007/BF00042344. [DOI] [PubMed] [Google Scholar]
- Sundström M., Lindqvist Y., Schneider G., Hellman U., Ronne H. Yeast TKL1 gene encodes a transketolase that is required for efficient glycolysis and biosynthesis of aromatic amino acids. J Biol Chem. 1993 Nov 15;268(32):24346–24352. [PubMed] [Google Scholar]