Abstract
HIV-1 serodiscordant couples may experience increased risks of relationship dissolution; however, longitudinal stability of these relationships is poorly understood. We determined rates and correlates of separation among 469 serodiscordant couples in Nairobi and found that 113 (24 %) separated during 2 years of follow-up. Couples with a female HIV-1 infected partner (F+M–) and no income were more likely to separate than M+F– couples without income (HR = 5.0; 95 % CI 1.1–25.0), and F+M– and M+F– couples with income (HR = 2.4; 95 % CI 1.3–4.5 and HR = 2.3; 95 % CI 1.2–4.8, respectively). High separation rates may be important for couple support services and for conducting discordant couple studies.
Keywords: HIV-1 serodiscordant couples, Relationship dissolution, Separation, HIV-1 transmission, Poverty, Women
Introduction
In recent years, HIV-1 serodiscordant couples have increasingly been evaluated to identify methods to prevent HIV-1 transmission and host factors associated with susceptibility to HIV-1 acquisition or infectivity [1, 2]. While these studies often require that couples report having a stable relationship at enrollment, literature regarding longitudinal stability of these relationships is sparse. Because HIV-discordancy may be associated with stigma, suspicions of infidelity and altered relationship roles, understanding correlates of relationship instability is important in designing programs and interventions for HIV-1 serodiscordant couples. Furthermore, understanding risk factors for separation may aid the design and analysis of discordant couple studies.
Previous research suggests that separation is more likely among couples affected by HIV and that women living with HIV may experience a particularly high risk of abandonment [3–5]. For instance, population-based surveys of HIV-1 infected and uninfected individuals in Malawi found that separation was more likely among participants with HIV and was highest among women with HIV [3]. Additionally, data from Rakai, Uganda demonstrated that separation was more common among HIV-infected women and that HIV-1 discordant couples in which the female partner was infected were at higher risk of relationship dissolution than discordant couples in which the male partner had HIV-1, concordant HIV-1 uninfected couples, or concordant HIV-1 infected couples [5]. That study also found age, household wealth, and outside sexual partners to be associated with separation or divorce among HIV-1 discordant couples.
Identifying risk factors for separation has potential to aid in designing couple support services and will elucidate challenges in conducting observational and clinical studies of HIV-serodiscordant couples. Therefore, we determined the incidence of relationship dissolution among a prospective cohort of HIV-1 discordant couples in Nairobi and evaluated risk factors for separation, with a particular focus on the gender of HIV-1 infected partners. We hypothesized that HIV-serodiscordant couples in which the female partner had HIV-1 would have the greatest risk of separation.
Methods
Participant Recruitment and Follow-up
HIV-1 serodiscordant couples were recruited from voluntary counseling and testing sites in Nairobi, Kenya between September 2007 and May 2009 for a study evaluating immunologic correlates of HIV-1 transmission, and were followed quarterly for up to 2 years. Couples were defined as sexual partners of the opposite gender who were married or in a steady sexual relationship, had been living together, and considered each other their primary partner. Stable couples were eligible to participate if they reported ≥3 sex acts within 3 months of enrollment and if they planned to maintain the relationship for at least 2 years. Couples were ineligible if the HIV-1 infected partner was using antiretroviral therapy or had a history of clinical AIDS (WHO stage IV) since these factors substantially reduce the risk of HIV-1 transmission.
At study visits, clinical staff administered questionnaires to assess socio-demographic characteristics, sexual behaviors, and medical histories. Questionnaires were administered to each partner in a couple separately to ensure confidentiality and limit potential misclassification. All participants provided written informed consent, and the study received ethical approval from the institutional review boards at the University of Washington and the University of Nairobi.
Classification of Relationship Dissolution and Couplelevel Covariates
At each follow-up visit, participants were asked if their relationship status had changed since the previous visit. If participants answered ‘yes’, they were asked if they had been formally separated or divorced and relationship dissolution was defined as either partner in a couple reporting divorce or separation during follow-up.
Separation was classified at the couple-level, however, partners completed questionnaires separately. Thus, we created couple-level covariates by combining answers to individual-level questions. These covariates included desire for future children, any shared living children, marital status, primary education, and earned income. We defined desire for future children as one, both, or neither partner wanting additional children. Similarly, we indicated if one, both, or neither partner had attained a primary level of education, defined as ≥8 years of schooling. Earned income was classified as neither versus at least one partner reporting an income. Couples in which either partner reported one or more living children with their study partner were classified as having shared children. For marital status, there were several couples in which only one partner reported being married, which could simply be due to misclassification or could represent underlying relationships characteristics. Thus, marital status was defined as both partners reporting being married or not married, with a third category indicating if reports of marriage differed within the couple. Sexual behavior data was collected separately for both partners in a couple through private face-to-face interviews.
Data Analysis
Baseline correlates of separation were analyzed using Kaplan–Meier curves and Cox proportional hazards (PH) regression. Time to separation was defined as the number of days between enrollment and the first visit at which either partner reported being separated. Couples that never reported separation were censored at the last follow-up visit attended by either partner, and couples experiencing HIV-1 transmission were censored at the last visit before seroconversion was detected. Potential covariates were selected a priori based on literature review and were considered in univariate and multiple variable models. Multiple variable models were adjusted for the number of shared living children and the duration of the relationship based on a priori hypotheses about associations with separation and other predictors. We also evaluated statistical interactions between the infected partner’s gender and other variables. The PH assumption was tested using Schoenfeld residuals and was found to be valid for all covariates.
Results
Description of the Cohort
Table 1 provides baseline characteristics of 469 HIV-1- discordant couples enrolled in this cohort, of which, 301 (64 %) had a female HIV-1 infected partner (F+M–). At enrollment, 448 (95.5 %) couples were married, 12 (2.6 %) couples were not married, and 9 (1.9 %) couples disagreed in their marital status report. The median relationship duration was 5.3 years (inter-quartile range [IRQ]: 2.5, 10.4) and was shorter among F+M– couples (4.9 [IQR: 2.5, 9.2]) than M+F– couples (6.0 [IQR: 2.5, 10.9]). In general, males were older than females, and partners of both genders were older in M+F– couples. Among all HIV-infected participants, women had higher median CD4+T cell counts and plasma HIV-1 viral loads than men (476 vs. 339/µlL and 4.6 vs. 4.8/mL, respectively). Over the course of the study, 12 (3 %) couples experienced an HIV-1 transmission event for a seroconversion rate of 1.5 per 100 person-years.
Table 1.
Couple-level baseline characteristics of HIV-1 serodiscordant couples included in this cohort, by gender of the HIV-1 seropositive partner.a
| Gender of HIV-1 positive partner | ||
|---|---|---|
| Male (N = 168) |
Female (N = 301) |
|
| Live togetherb | ||
| Yes | 165 (98 %) | 288 (96 %) |
| No | 3 (2 %) | 9 (3 %) |
| Disagree | 0 (0 %) | 4 (1 %) |
| Relationship duration (years)c | 6.0 (2.5, 10.1) | 4.9 (2.5, 9.2) |
| Any children together | 138 (82 %) | 235 (78 %) |
| Desire future children | ||
| Both partners | 57 (34 %) | 107 (36 %) |
| One partner | 42 (25 %) | 86 (29 %) |
| No | 67 (40 %) | 106 (35 %) |
| Marriedb | ||
| Yes | 163 (97 %) | 285 (95 %) |
| No | 4 (2 %) | 8 (3 %) |
| Disagree | 1 (1 %) | 8 (3 %) |
| Female partner age (years) | 29 (25, 35) | 28 (24, 34) |
| Male partner age (years) | 36 (32, 42) | 34 (29–39) |
| 1+ partner earns income | 148 (88 %) | 266 (88 %) |
| Less than primary education (<8 years) | ||
| Both | 38 (23 %) | 74 (25 %) |
| None | 63 (38 %) | 116 (39 %) |
| One | 67 (40 %) | 111 (37 %) |
| Plasma HIV-1 RNA levels of infected partner (log10 copies) | 4.8 (4.1, 5.4) | 4.6 (3.7, 5.2) |
| CD4+T cell count of infected partner (cells/mm3) | 340 (232, 502) | 476 (308, 671) |
Medians and interquartile ranges (IQR) are provided for continuous variables. Number and percentages are provided for categorical variables
These couple-level characteristics were coded as “yes” if both partners in a couple reported the characteristic, “no” if neither partner reported the characteristic, and “disagree” if one partner reported “yes” and the other reported “no”
Relationship duration was defined on the couple-level by taking the mean of the relationship duration reported independently by each partner in a couple
Incidence and Correlates of Separation
Among all 466 couples with ≥1 follow-up visit, the mediation duration of follow-up was 1.7 years (IQR: 1.2, 2.0). Of these couples, 113 (24 %) had at least one partner report relationship dissolution during 678 couple-years of follow-up for an overall incidence rate of 16.6 per 100 couple-years. Among 85 participants who reported separation or divorce and responded to an inquiry asking the reason for dissolution, 45 (53 %) responded that HIV-discordance was the primary cause.
The strongest correlate of relationship dissolution was not having shared children, which was associated with a 2.5-fold increased risk of separation (hazard ratio [HR] = 2.5; 95 % confidence interval [CI]: 1.7–3.3, p < 0.001; adjusted HR [aHR] = 2.5; 95 % CI 1.4–3.3, p < 0.001). Increased partnership duration was associated with decreased risk of separation but the magnitude and significance of this association diminished after adjusting for shared children (for a 5-year increase, HR = 0.8; 95 % CI 0.7–1.0, p = 0.04 and aHR = 0.9, 0.8–1.2, p = 0.57). Unmarried couples were over three times as likely to report separation than married couples before adjustment (HR = 3.2; 95 % CI 1.3–7.9; p = 0.01, aHR = 1.8; 95 % CI 0.7, 4.8, p = 0.2) and couples that gave discrepant reporting of marital status at enrollment had a 5-fold increased risk of separating during follow-up (HR = 5.4; 95 % CI 2.2–13.3; p < 0.001; aHR = 4.7; 95 % CI 1.8–12.1, p = 0.001).
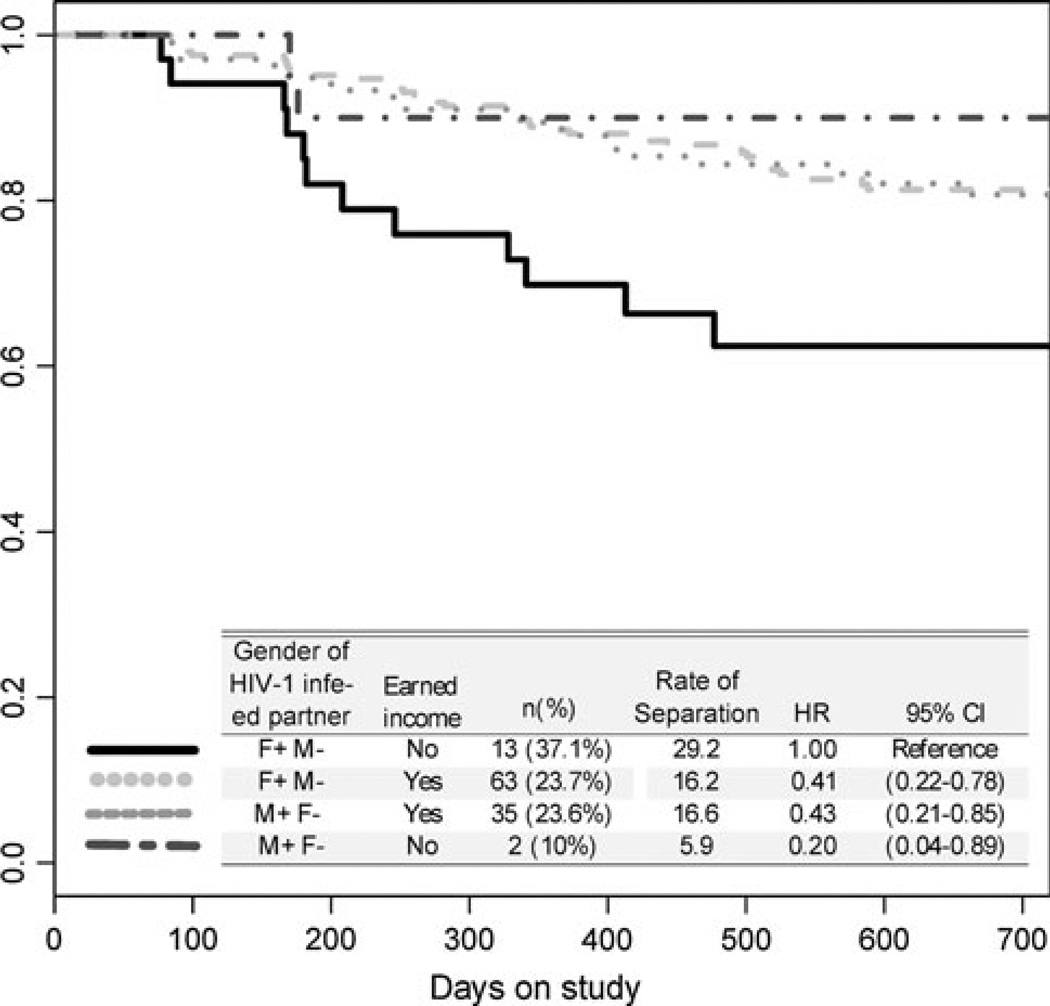
When considered independently, gender of the HIV-infected partner and earned income by either partner were not associated with separation. However, significant interaction between these variables (p = 0.03) indicated that F+M– couples without an income were at the highest risk of separation (Fig. 1). Specifically, 13 of 35 (37 %) F+M–couples without an income separated during the study, compared to 2 of 20 (10 %) M+F– couples without an income (HR = 5.0; 95 % CI 1.1–25.0). Among couples reporting any earned income, separation was reported by 63 of 226 (24 %) F+M– couples (HR = 2.4; 95 % CI 1.3–4.5) and 35 of 148 (24 %) M+F– couples (HR = 2.3; 95 % CI 1.2–4.8). The interaction between gender of the HIV-1 seropositive partner and earned income remained significant after adjusting for the number of shared children and relationship duration (p = 0.03).
Fig 1.
Kaplan–Meier curves showing interaction between gender of the HIV-1 infected partner and earned income
Discussion
In this prospective study of HIV-serodiscordant couples in Nairobi, incidence of relationship dissolution was high with 24 % of couples reporting separation during 1–2 years of follow-up. Over half (53 %) of participants who reported separation indicated that it was caused by HIV-1 discordance. Marital status and having shared children were the strongest independent correlates of relationship stability, however, significant interaction between the gender of the HIV-infected partner and income indicated that F+M–couples without any income were ~2–5 times more likely to separate than other couples.
Our finding that separation was most common among low socioeconomic status F+M– couples is consistent with other reports that HIV-infected women may be at an especially high-risk of relationship dissolution and abandonment [3–5]. Together, these studies suggest that F+M–couples may experience greater relationship stress due to gender-related power roles such as financial inequalities, male authority in relationships, and other social norms [6, 7], and that these stressors may be accentuated by poverty. Lower incomes have also previously been associated with increased separation risk [5].
In this study, the incidence of relationship dissolution among HIV-1 serodiscordant couples was high (16.6 per 100 couple-years), and was much greater than the incidence of HIV-1 transmission (1.5 per 100 person-years). These observations could help to identify couples needing extra support (e.g. extra counseling) in 1) programs to reduce the spread of HIV-1 and improve patient care and well-being, and 2) clinical studies involving HIV-1 serodiscordant couples. First, high incidence of relationship dissolution may influence HIV-1 transmission by altering patterns of partnership formation and risky sexual behaviors such as high partner turnover and unprotected sex with outside/concurrent partners [8–13]. Given the much higher rate of separation than HIV-1 transmission among these couples, programs seeking to maintain stability in these relationships could be important in prevention efforts. Relationship dissolution may also result in HIV-1 infected partners having worse engagement with HIV-1 treatment and care. For example, previous studies suggest that partner stress is linked to worse medication adherence [14] while social support and couple-focused interventions are associated with improved participation in HIV-1 counseling and testing [15–18], adherence to antiretroviral therapy (ART) [19, 20], and other health-seeking behaviors in East Africa [21–23]. Given the clear importance of early ART initiation and adherence and reducing viral load to HIV-1 transmission risk and disease progression [24, 25], promoting relationship stability and involving both partners in treatment may have significant ramifications for HIV-1 prevention and health of infected partners.
Longitudinal stability of relationships is also relevant to analyzing data from cohorts of HIV-1 serodiscordant couples in that separation likely leads to interruption of sexual activity with the study partner, thereby reducing the number of observed HIV-1 transmissions and decreasing statistical power to detect associations. Furthermore, when evaluating correlates of HIV-1 transmission in observational studies, not controlling for stability of relationships could lead to confounding. Thus, it may be worthwhile to adjust statistical models for relationship stability or to consider censoring participants from an analysis when separation is reported in observational studies. Furthermore, observational and clinical studies could use correlates of relationship stability as a screening tool when recruiting participants in order to select couples with greater probabilities of HIV-1 transmission. It should be noted, however, that selectively excluding some couples from study participation would limit generalizability of study results.
A strength of this analysis is that HIV-1 serodiscordant couples were frequently asked about changes in relationship status and that we were able to ask couples reporting separation if HIV-1 discordance was the reason for separation. When combined with in-depth demographic and clinical data, this allowed evaluation of relationship dissolution that has not been possible in other cohorts of serodiscordant couples. While our findings that unmarried couples and those with discrepant reporting of marital status are important, it should be noted that there were few couples in either of these categories, which could result in unstable estimates of incidence.
To our knowledge, this is the first report of relationship dissolution rates in a cohort study of HIV-1 serodiscordant couples from sub-Saharan Africa, demonstrating that separation is common among these couples and was often due to HIV-1 discordance. F+M– couples without income had the highest separation incidence, adding to literature suggesting that economically disadvantaged African women are at the highest risk for poor HIV-related outcomes [26]. These observations could be an important consideration when designing programs and interventions, and may identify couples needing additional support such as extra counseling.
Acknowledgments
This research was funded by US National Institutes of Health (NIH) Grant AI068431. R. Mackelprang was supported by the Institute for Translational Health Science (TL1), a Roadmap Initiative from the National Institutes of Health/National Center for Research Resources (5TL1RR025016), P. Mitchell & S. Marshall, Program Directors. R. Choi was supported by the International Research Scientist Development Award (K01TW008406). R. Choi, A. Rositch and A Gatuguta were supported by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood and Lung Institute, National Institute of Dental and Craniofacial Research, National Institute on Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases Health, through the International Clinical Research Fellows Program at Vanderbilt (R24 TW007988). This research was supported by the University of Washington Center for AIDS Research (CFAR), an NIH funded program (P30 AI027757) which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA). C. Farquhar received support from NIH Grant K24 AI087399.
Footnotes
These data were presented in part at the XVIII International AIDS conference in Vienna (2010).
Contributor Information
Romel D. Mackelprang, Email: romelm@u.washington.edu, Department of Epidemiology, University of Washington, 325 Ninth Avenue, Seattle, WA 98104, USA; Department of Global Health, University of Washington, 325 Ninth Avenue, Seattle, WA 98104, USA.
Rose Bosire, Centre for Public Health Research, Kenya Medical Research Institute, Nairobi, Kenya.
Brandon L. Guthrie, Department of Epidemiology, University of Washington, 325 Ninth Avenue, Seattle, WA 98104, USA
Robert Y. Choi, Department of Medicine, University of Washington, 325 Ninth Avenue, Seattle, WA 98104, USA
Amy Liu, Department of Epidemiology, University of Washington, 325 Ninth Avenue, Seattle, WA 98104, USA.
Anne Gatuguta, Department of Community Health, University of Nairobi, Nairobi, Kenya.
Anne F. Rositch, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
James N. Kiarie, Department of Obstetrics and Gynaecology, University of Nairobi, Nairobi, Kenya
Carey Farquhar, Department of Epidemiology, University of Washington, 325 Ninth Avenue, Seattle, WA 98104, USA; Department of Global Health, University of Washington, 325 Ninth Avenue, Seattle, WA 98104, USA; Department of Medicine, University of Washington, 325 Ninth Avenue, Seattle, WA 98104, USA.
References
- 1.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guthrie BL, de Bruyn G, Farquhar C. HIV-1-discordant couples in sub-Saharan Africa: explanations and implications for high rates of discordancy. Curr HIV Res. 2007;5:416–429. doi: 10.2174/157016207781023992. [DOI] [PubMed] [Google Scholar]
- 3.Floyd S, Crampin A. The long-term social and economic impact of HIV on the spouses of infected individuals in northern Malawi. Trop Med Int Health. 2008;13:520–531. doi: 10.1111/j.1365-3156.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- 4.Grinstead O, Gregorich S. Positive and negative life events after counselling and testing: the Voluntary HIV-1 Counselling and Testing efficacy study. AIDS. 2001;15:1045–1052. doi: 10.1097/00002030-200105250-00013. [DOI] [PubMed] [Google Scholar]
- 5.Porter L, Hao L, Bishai D, Serwadda D, Wawer MJ, et al. HIV status and union dissolution in sub-Saharan Africa: the case of Rakai, Uganda. Demography. 2004;41:465–482. doi: 10.1353/dem.2004.0025. [DOI] [PubMed] [Google Scholar]
- 6.Mbonu NC, Van den Borne B, De Vries NK. Gender-related power differences, beliefs and reactions towards people living with HIV/AIDS: an urban study in Nigeria. BMC public health. 2010;10:334. doi: 10.1186/1471-2458-10-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell R. Gender and power: society, the person, and sexual politics. Palo Alto: Stanford Univ Press; 1987. p. 334. [Google Scholar]
- 8.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11:641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Ndase P, Celum C, Thomas K, Donnell D, Fife KH, et al. Outside sexual partnerships and Risk of HIV Acquisition for HIV uninfected partners in African HIV serodiscordant partnerships. J Acquir Immune Defic Syndr. 2012;59:65–71. doi: 10.1097/QAI.0b013e318237b864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton A, van Der Straten A. Concurrent sexual partnerships among individuals in HIV sero-discordant heterosexual couples. Int J STD AIDS. 2009;20:679–682. doi: 10.1258/ijsa.2009.009158. [DOI] [PubMed] [Google Scholar]
- 11.Mah TL, Halperin DT. Concurrent sexual partnerships and the HIV epidemics in Africa: evidence to move forward. AIDS Behav. 2010;14:11–16. doi: 10.1007/s10461-008-9433-x. dicussion 34–37. [DOI] [PubMed] [Google Scholar]
- 12.Sandøy IF, Dzekedzeke K, Fylkesnes K. Prevalence and correlates of concurrent sexual partnerships in Zambia. AIDS Behav. 2010;14:59–71. doi: 10.1007/s10461-008-9472-3. [DOI] [PubMed] [Google Scholar]
- 13.Boerma JT, Urassa M, Nnko S, Ng’weshemi J, Isingo R, et al. Sociodemographic context of the AIDS epidemic in a rural area in Tanzania with a focus on people’s mobility and marriage. Sex Transm Infect. 2002;78(Suppl 1):i97–i105. doi: 10.1136/sti.78.suppl_1.i97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molloy GJ, Perkins-Porras L, Strike PC, Steptoe A. Social networks and partner stress as predictors of adherence to medication, rehabilitation attendance, and quality of life following acute coronary syndrome. Health Psychol. 2008;27:52–58. doi: 10.1037/0278-6133.27.1.52. [DOI] [PubMed] [Google Scholar]
- 15.El-Bassel N, Gilbert L, Witte S, Wu E, Hunt T, et al. Couplebased HIV prevention in the United States: advantages, gaps, and future directions. J Acquir Immune Defic Syndr. 2010;55(Suppl 2):S98–S101. doi: 10.1097/QAI.0b013e3181fbf407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweat M, Gregorich S, Sangiwa G, Furlonge C, Balmer D, et al. Cost-effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. Lancet. 2000;356:113–121. doi: 10.1016/S0140-6736(00)02447-8. [DOI] [PubMed] [Google Scholar]
- 17.Burton J, Darbes LA, Operario D. Couples-focused behavioral interventions for prevention of HIV: systematic review of the state of evidence. AIDS Behav. 2010;14:1–10. doi: 10.1007/s10461-008-9471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desgreés-du-Loû A, Orne-Gliemann J. Couple-centred testing and counselling for HIV serodiscordant heterosexual couples in sub-Saharan Africa. Reprod Health Matters. 2008;16:151–161. doi: 10.1016/S0968-8080(08)32407-0. [DOI] [PubMed] [Google Scholar]
- 19.Remien RH, Stirratt MJ, Dolezal C, Dognin JS, Wagner GJ, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19:807–814. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 20.Simoni JM, Frick PA, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychol. 2006;25:74–81. doi: 10.1037/0278-6133.25.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiarie JN, Kreiss JK, Richardson BA, John-Stewart GC. Compliance with antiretroviral regimens to prevent perinatal HIV-1 transmission in Kenya. AIDS. 2003;17:65–71. doi: 10.1097/01.aids.0000042938.55529.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farquhar C, Kiarie JN, Richardson BA, Kabura MN, John FN, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37:1620–1626. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byamugisha R, Astrøm AN, Ndeezi G, Karamagi CA, Tylleskär T, et al. Male partner antenatal attendance and HIV testing in eastern Uganda: a randomized facility-based intervention trial. J Int AIDS Soc. 2011;14:43. doi: 10.1186/1758-2652-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Pronyk P. Exploring the role of economic empowerment in HIV prevention. AIDS. 2008;22:S57–S71. doi: 10.1097/01.aids.0000341777.78876.40. [DOI] [PubMed] [Google Scholar]