Abstract
Pure red cell aplasia is a rare cause of anemia, caused by an absence of red blood cell precursors in the bone marrow. It is usually a paraneoplastic syndrome, associated most commonly with large-cell granular lymphocyte leukemia but also thymoma. For patients who present both pure red cell aplasia and thymoma, thymectomy leads to an initial remission of the aplasia in 30% of cases. However, sustained remission may require the addition of medications such as corticosteroids, cyclosporine, or cyclophosphamide. We present a case of pure red cell aplasia associated with a thymoma in an otherwise healthy 80 year-old woman.
Key words: anemia, pure red cell aplasia, thymoma, paraneoplastic syndrome.
Introduction
Pure red cell aplasia is a rare cause of anemia, caused by an absence of red blood cell precursors in the bone marrow. It is often a paraneoplastic syndrome which may be associated with a thymoma.1,2 Thymectomy is usually indicated in these patients. Although surgery leads to an initial remission of the aplasia in 30% of cases, sustained remission may require the addition of medications such as corticosteroids, cyclosporine, or cyclophosphamide.1,3
Case Report
A previously healthy 80 year-old woman was referred to our institution for progressive fatigue and normocytic anemia. Her initial hemoglobin level was 58 g/L (N=120–140 g/L) and the reticulocyte count was zero. She was transfused two units of packed red blood cells every three weeks in order to maintain a hemoglobin level around 85 g/L. Bone marrow aspirate and biopsy revealed an abundance of myeloid cells and some megakaryocytes, but a complete absence of erythroid precursors (Figure 1A,B). This was consistent with the diagnosis of pure red cell aplasia. A thorough search for an associated disease included computed tomography of the chest, which showed an anterior mediastinal mass (Figure 2). The patient underwent elective transsternal thymectomy based on the clinical suspicion of thymoma. The diagnosis was confirmed by pathological analysis. The post-operative course was uncomplicated. The anemia resolved and remission was maintained for 6 months. However, the pure red cell aplasia subsequently recurred. Repeat imaging did not show any recurrent thymoma, and remission was achieved once more with the addition of cyclosporine.
Figure 1.
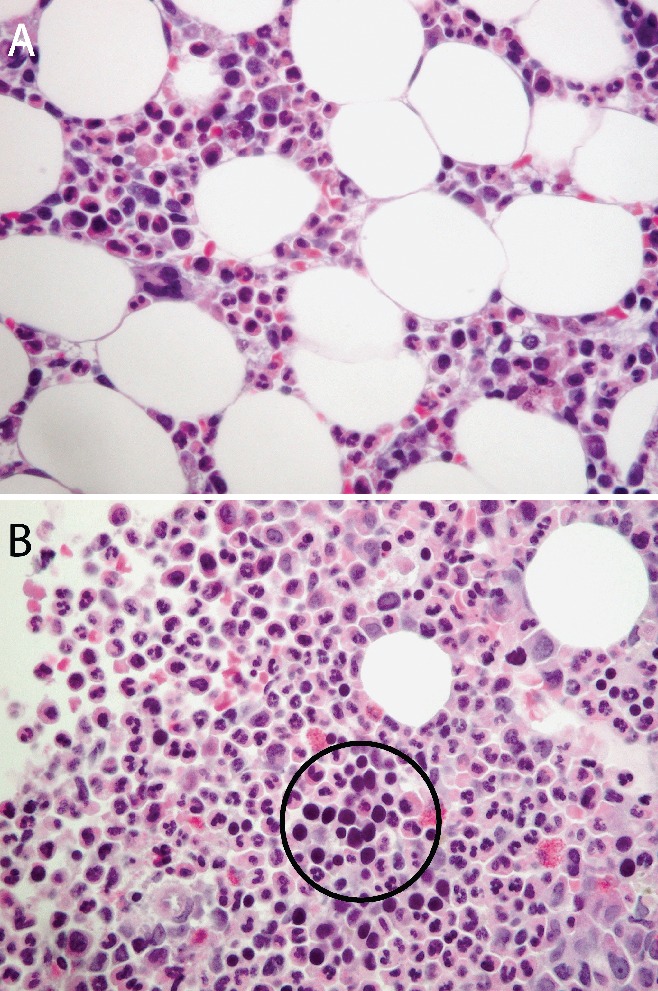
Bone marrow biopsy of a patient with pure red cell aplasia (A) showing an abundance of myeloid cells and some megakaryocytes. Erythroid precursors are absent. Contrast with normal marrow of another patient (B) showing numerous erythroid precursors (cells with dark, round nuclei; a cluster is seen within the encircled area) (hematoxylin and eosin, 40×).
Figure 2.

Computed tomography of the chest showing a mass in the anterior mediastinum (Ao, ascending aorta; Ma, mass).
Discussion
Pure red cell aplasia (PRCA) is a rare cause of anemia originally described in 1922.1 It is a normocytic, normochromic anemia due to an absence of red cell precursors in the bone marrow. The pathophysiology of PRCA is not well understood but appears related to abnormal T-cell function and the presence of IgG antibodies which target erythroblasts and ery-thropoietin.2 PRCA may sometimes be congenital but it is usually acquired and in this case is most frequently associated with hematologic disorders, especially large-cell granular lymphocyte leukemia (LGL)2 and other hematologic malignancies; it may also occur in the setting of viral infections (such as parvovirus B19 infection), autoimmune disorders, and the use of certain medications.3 PRCA has been associated with a thymoma in about 10% of cases in modern series; conversely, PRCA is very rare in patients with a known thymoma.2,3 Thymomas are unusual neoplasms of thymic epithelial tissue, which exhibit a spectrum of clinical behaviour ranging from small indolent tumors to aggressive thymic carcinomas.4 In addition, thymomas may be associated with a wide range of paraneoplastic syndromes, the prototype of which is myasthenia gravis. Both early stage and advanced thymomas may occur with PRCA, and the two disorders may even present metachronously, in some cases several years apart.3,4
The diagnosis of PRCA is confirmed by bone marrow aspirate and biopsy. A thorough workup is necessary in search of an associated disease, including a complete hematologic workup and virologic studies.1 Importantly, a CT scan of the chest is mandatory in every case of PRCA in order to rule out the presence of an associated thymoma or lymphoid malignancy.1 A suspected thymoma is usually an indication for thymectomy.1 A thymic mass in a patient with PRCA may be assumed to be a thymoma until proven otherwise; thus resection is indicated without preoperative histologic confirmation.1 Transsternal thymectomy has a low morbidity and mortality and, more recently, minimally invasive thoracoscopic approaches have been described as well.5 Several thymoma-associated syndromes may respond to thymectomy; unfortunately, the response of PRCA to thymectomy has been inconsistent and the initial remission rate is around 30%.1 In addition, in contrast to myasthenia gravis, which is known to respond to thymectomy even in the absence of a thymoma, in PRCA there is currently no role for thymectomy in the absence of an identifiable thymic mass.6 Only a few small and isolated case series of PRCA associated with thymoma have been published. The largest reported series involved 12 patients who underwent thymectomy;3 none of these patients achieved complete remission of their anemia after thymectomy alone; remission was only possible with the addition of an adjuvant drug regimen consisting of steroids, cyclosporine, or cyclophosphamide.3 Corticosteroids are often considered the first line of pharmacotherapy;1 cyclosporine and cyclophosphamide have also been used successfully, but serious potential side-effects of these drugs, including nephrotoxicity and immunosuppression, may limit their use.1
Median survival of PRCA patients is approximately 12 years, although the prognosis and response to treatment are significantly related to associated disorders.2 Most deaths result from an associated condition or from failure of PRCA to respond to therapy, leading to iron overload and end organ dysfunction.2 Patients with an underlying LGL leukemia tend to respond to treatment somewhat better than those with idiopathic PRCA.2 However, it has been reported that PRCA-associated thymoma has a worse prognosis stage for stage than non PRCA thymoma.4
Conclusion
In summary, the association of pure red cell aplasia with thymoma is important to recognize because thymectomy is not only necessary to treat the thymoma, but may have an impact on the course of the aplasia. Ultimately, a combined surgical and medical approach may be required in order to control the anemia in these patients.
Acknowledgements:
all authors have participated in, read and approved the final writing of the manuscript.
References
- 1.Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. Br J Haematol. 2008;142:505–14. doi: 10.1111/j.1365-2141.2008.07216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacy MQ, Kurtin PJ, Tefferi A. Pure red cell aplasia: association with large granular lymphocyte leukemia and the prognostic value of cytogenetic abnormalities. Blood. 1996;87:3000–6. [PubMed] [Google Scholar]
- 3.Thompson CA, Steensma DP. Pure red cell aplasia associated with thymoma: clinical insights from a 50-year single-institution experience. Br J Haematol. 2006;135:405–7. doi: 10.1111/j.1365-2141.2006.06295.x. [DOI] [PubMed] [Google Scholar]
- 4.Murakawa T, Nakajima J, Sato H, et al. Thymoma associated with pure red-cell aplasia: clinical features and prognosis. Asian Cardiovasc Thorac Ann. 2002;10:150–4. doi: 10.1177/021849230201000213. [DOI] [PubMed] [Google Scholar]
- 5.Meyer DM, Herbert MA, Sobhani NC, et al. Comparative clinical outcomes of thymectomy for myasthenia gravis performed by extended transsternal and minimally invasive approaches. Ann Thorac Surg. 2009;87:385–90. doi: 10.1016/j.athoracsur.2008.11.040. discussion 390–1. [DOI] [PubMed] [Google Scholar]
- 6.Zeok JV, Todd EP, Dillon M, et al. The role of thymectomy in red cell aplasia. Ann Thorac Surg. 1979;28:257–60. doi: 10.1016/s0003-4975(10)63116-5. [DOI] [PubMed] [Google Scholar]


