Abstract
Genotype 5 hepatitis C has been poorly studied despite its worldwide spread. We have analyzed the early kinetics of genotype 5 hepatitis C virus RNA during pegylated interferon/ribavirin treatment in a 59-year-old man with active liver necroinflammatory changes and advanced liver fibrosis. The patient had a high viral load but a small serum level of hepatitis C core antigen. On combination antiviral treatment with pegylated-interferon alpha 2a, 180 µg/week, and ribavirin, 1200 mg/day, the patient experienced an impressive reduction in serum HCV RNA as early as day 2 of treatment and eventually became a sustained virological responder. Our viral kinetics data support previous clinical studies showing HCV genotype 5 could be as intrinsically sensitive to interferon as HCV genotypes 2 and 3.
Key words: hepatitis C, chronic, pegylated interferon alpha-2a, ribavirin, treatment outcome.
Introduction
The hepatitis C virus (HCV) genotype is a major determinant of the susceptibility of chronic hepatitis C to pegylated interferon and ribavirin treatment.1 HCV genotype 5 is one of the most common subtypes in Southern Africa,2,3 and is thought to account for a considerable number of infected persons worldwide. However, since only sporadic cases have been reported and are found in Europe and North America,4–6 fewer data are available regarding the actual susceptibility of HCV genotype 5 to the current antiviral treatment.4,7 In a French multicenter, retrospective study, sustained virological responses in patients with HCV genotype 5 were higher than in genotype 1 and similar to patients with genotype 2 and 3.8 Antaki et al. reported data suggesting that a 24 week course of pegylated interferon and ribavirin could suffice for infected patients with HCV genotype 5.9 These data might suggest that HCV genotype 5 is intrinsically sensitive to the current antiviral treatment.
Mounting evidence supports the concept that early changes of serum HCV-RNA after treatment onset may provide prognostic information on treatment outcome as well as insights into the intrinsic viral sensitivity.10,11 We therefore investigated the early, on treatment dynamics of HCV-RNA, in a HCV genotype 5 infected subject. Our Institutional Review Board approved the anonymous report of this patient data as well as the studies performed to obtain viral kinetics data from control patients.
Case Report
A 59-year-old, Caucasian male, was referred to our unit because of elevated serum aminotransferase levels since 8 months. He had been previously diagnosed with hypertension and type 2 diabetes, and was on lisinopril and glibenclamide. On admission, he appeared overweight (weight 79 kg; body mass index 29.4; waist circumference 100 cm) and showed active HCV replication with a viral load of 820,000 IU/mL (Amplicor HCV 3.0, Roche, Basel, Switzerland). HCV genotyping was performed twice (InnoLiPA, Innogenetics, Ghent, Belgium) and consistently showed a HCV genotype 5 infection. No risk factors for HCV transmission were identified: the patient had not received transfusion of blood-derived products and had not undergone any surgical procedure; he denied prior use of intravenous illicit drugs or unprotected sexual intercourse with subjects at risk; no family member was positive for HCV antibodies.
Further blood tests were performed at baseline after an overnight fasting and showed the following results: alanine aminotransferase 24 IU/mL (upper limit of normal range [ULN]=40), aspartate aminotransferase 22 IU/mL (ULN 40), gamma-glutamyl-transpeptidase 24 IU/mL (ULN 30), bilirubin 1.5 mg/dL, albumin 4.1 g/dL, glucose 125 mg/dL, glycated haemoglobin 8.25%, white blood cells 7950/µL (neutrophils 4670), haemoglobin 15.9 gr/dL, platelets 177,000/µL . Serum levels of HCV core antigen were also measured (Architect HCV Ag, Abbott Park, IL, USA) and found to be overall very low at 148.2 fmol/L.
The patient underwent percutaneous liver biopsy, that showed presence of moderate chronic active hepatitis, with a necroinflammatory Knodell score of 9, a Scheuer fibrosis score of 3, and absence of steatosis or iron deposits.
Due to the presence of active hepatitis with bridging fibrosis, the patient was started on combination antiviral treatment with pegylated-interferon alpha-2a, 180 µg subcutaneously once a week, and oral ribavirin, 1200 mg daily. Serum HCV-RNA levels were measured on samples obtained 5 minutes before the first dose of drugs and subsequently after 1, 2, 14, 28, 90 and 180 days.
Figure 1 shows the kinetics of HCV-RNA observed. There was a very rapid decline of the viral load with a 2.2 log10 reduction after 48 hours of treatment followed by complete viral clearance within the fourth week of treatment. Mirroring the reduction in HCV-RNA, HCV core antigen serum levels also significantly declined from 148.2 to <3 fmol/L at day 2 of treatment, but remained still detectable below the lower limit of quantitation at week 4.
Figure 1.
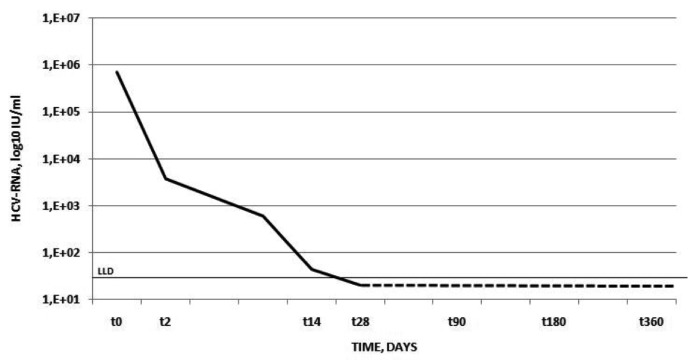
Kinetics of serum HCV-RNA in the case patient. On the X-axis is the time in days. On the Y-axis is the HCV-RNA serum concentration in log10 IU/mL. The continuous black line depicts the lower limit of detection of the assay.
We compared the 2.2 log10 decline of HCV RNA observed in this patient during the initial 48 hours of treatment with that observed in 61 patients with genotype 1, 44 patients with genotype 2 and 14 patients with genotype 3. We found that this patient with genotype 5 first phase kinetics of HCV RNA was higher than that of patients with genotype 1 (1.29±0.8 log10) but similar to patients with genotype 2 (2.38±0.9 log10) or genotype 3 (2.17±0.6 log10).
During treatment, the patient experienced a significant hemoglobin drop, which required ribavirin dose reduction to 800 mg per day. He went on anyway to complete a full 6-month combination treatment course. At the last follow-up, 48 months after treatment completion, the patient was still HCV-RNA negative and had persistently normal aminotransferase levels.
Discussion
Although HCV genotype 5 is responsible for a substantial number of hepatitis C cases worldwide,7 little knowledge is available regarding its sensitivity to the current antiviral treatment. The limited evidence available seems to suggest that the response rate to combination treatment with standard or pegylated interferon plus ribavirin is comparable to that observed in genotype 2 and 3 subjects12,13 even if the optimal duration of treatment is unknown. More recent data, however, did not confirm the higher intrinsic sensitivity of HCV-5 to the antiviral treatment, showing sustained virological response rates of these patients overlapping with those of difficult to treat genotype 1.14
We studied the early on treatment HCV-RNA kinetics in a case of genotype 5 HCV infection. The viral response to combination antiviral therapy was very rapid, with a steep first phase of viral decline (>2 log10 drop within 2 days) despite the presence of advanced fibrosis and significant necro-inflammation in the pre-treatment histology. Moreover, the patient showed a rapid virological response with HCV-RNA clearance at week four of therapy, that eventually translated into a long term, sustained virological response. The rapid response observed in this patient could also be affected by a favorable IL28B genotype. Unfortunately, we were unable to obtain this patient’s genetic data.
Our results appear to complement those from the early retrospective clinical series8, 12,13 and further suggest that HCV genotype 5 could be as intrinsically sensitive to interferon as genotypes 2 and 3. This is also in line with recent phylogenetic studies showing genotype 5 is ancestrally related to the ‘interferon sensitive’ genotype 3 of HCV.15 Nonetheless, this hypothesis has been challenged by a recent retrospective study from Europe, showing that HCV-5 could be as resistant to interferon-based treatment as genotype 1. Until further definitive data from randomized, controlled studies become available, patients with genotype 5 chronic hepatitis C should be treated with the same schedule currently used for patients with genotype 1.14 However, we believe that viral kinetics studies could be a useful tool to define the optimal duration of treatment with pegylated interferon and ribavirin in individual genotype 5 HCV infected patients.
Acknowledgments:
this work was supported by A.O.R.N. V. Monaldi, Naples, Italy. DI received support from the PhD program in Clinical and Experimental Microbiology, Second University of Naples.
References
- 1.Trepo C. Genotype and viral load as prognostic indicators in the treatment of hepatitis C. J Viral Hepat. 2000;7:250–7. doi: 10.1046/j.1365-2893.2000.00233.x. [DOI] [PubMed] [Google Scholar]
- 2.Simmonds P. Variability of hepatitis C virus. Hepatology. 1995;21:570–83. doi: 10.1002/hep.1840210243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smuts HE, Kannemeyer J. Genotyping of hepatitis C virus in South Africa. J Clin Microbiol. 1995;33:1679–81. doi: 10.1128/jcm.33.6.1679-1681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verbeeck J, Peigue-Lafeuille H, Ross RS, et al. HCV genotype 5: epidemiology and spread of an uncommon genotype. J Clin Virol. 2008;41:170–1. doi: 10.1016/j.jcv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Levi JE, Takaoka DT, Garrini RH, et al. Three cases of infection with hepatitis C virus genotype 5 among Brazilian hepatitis patients. J Clin Microbiol. 2002;40:2645–7. doi: 10.1128/JCM.40.7.2645-2647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbeeck J, Maes P, Lemey P, et al. Investigating the origin and spread of hepatitis C virus genotype 5a. J Virol. 2006;80:4220–6. doi: 10.1128/JVI.80.9.4220-4226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen MH, Keeffe EB. Prevalence and treatment of hepatitis C virus genotypes 4, 5, and 6. Clin Gastroenterol Hepatol. 2005;3:S97–S101. doi: 10.1016/s1542-3565(05)00711-1. [DOI] [PubMed] [Google Scholar]
- 8.Bonny C, Fontaine H, Poynard T, et al. Effectiveness of interferon plus ribavirin combination in the treatment of naive patients with hepatitis C virus type 5. A French multicentre retrospective study. Aliment Pharmacol Ther. 2006;24:593–600. doi: 10.1111/j.1365-2036.2006.03018.x. [DOI] [PubMed] [Google Scholar]
- 9.Antaki N, Hermes A, Hadad M, et al. Efficacy of interferon plus ribavirin in the treatment of hepatitis C virus genotype 5. J Viral Hepat. 2008;15:383–6. doi: 10.1111/j.1365-2893.2007.00946.x. [DOI] [PubMed] [Google Scholar]
- 10.Layden JE, Layden TJ, Reddy KR, et al. First phase viral kinetic parameters as predictors of treatment response and their influence on the second phase viral decline. J Viral Hepat. 2002;9:340–5. doi: 10.1046/j.1365-2893.2002.00377.x. [DOI] [PubMed] [Google Scholar]
- 11.Durante-Mangoni E, Zampino R, Portella G, et al. Correlates and prognostic value of the first-phase hepatitis C virus RNA kinetic during treatment. Clin Infect Dis. 2009;49:498–506. doi: 10.1086/600887. [DOI] [PubMed] [Google Scholar]
- 12.Delwaide J, Gerard C, Reenaers C, et al. Hepatitis C virus genotype 5 in southern Belgium: epidemiological characteristics and response to therapy. Dig Dis Sci. 2005;50:2348–51. doi: 10.1007/s10620-005-3060-4. [DOI] [PubMed] [Google Scholar]
- 13.Legrand-Abravanel F, Sandres-Sauné K, Barange K, et al. Hepatitis C virus genotype 5: epidemiological characteristics and sensitivity to combination therapy with interferon-alpha plus ribavirin. J Infect Dis. 2004;189:1397–400. doi: 10.1086/382544. [DOI] [PubMed] [Google Scholar]
- 14.D’Heygere F, George C, Van Vlierberghe H, et al. Efficacy of interferon-based antiviral therapy in patients with chronic hepatitis C infected with genotype 5: A meta-analysis of two large prospective clinical trials. J Med Virol. 2011;83:815–9. doi: 10.1002/jmv.22049. [DOI] [PubMed] [Google Scholar]
- 15.Pang PS, Planet PJ, Glenn JS. The evolution of the major hepatitis C genotypes correlates with clinical response to interferon therapy. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006579.e6579 [DOI] [PMC free article] [PubMed] [Google Scholar]


