Abstract
Multicenter trials have demonstrated that in patients with sinus rhythm ivabradine is effective in the therapy of ischemic heart disease and of impaired left ventricular systolic function. Ivabradine is ineffective in atrial fibrillation. Many patients with symptomatic heart failure have diastolic dysfunction with preserved left ventricular systolic function, and many have asymptomatic paroxysmal atrial fibrillation. Ivabradine is not indicated in these conditions, but it happens that it is erroneously used. Digoxin is now considered an outdated and potentially dangerous drug and while effective in the mentioned conditions, is rarely used. The aim of the study was to compare the therapeutic effects of ivabradine in diastolic heart failure with preserved left ventricular systolic function. Patients were assigned to ivabradine or digoxin according to a randomization cross-over design. Data were single-blind analyzed. The analysis was performed using an intention-to-treat method. Forty-two coronary patients were selected. In spite of maximally tolerated therapy with renin-antagonists, diuretics and β-blockers, they had congestive diastolic heart failure with preserved systolic function. Both ivabradine and digoxin had positive effects on dyspnea, Nterminal natriuretic peptide, heart rate, duration of 6-min. walk-test and signs of diastolic dysfunction, but digoxin was high-statistically more effective. Side-effects were irrelevant. Data were obtained in a single-center and from 42 patients with ischemic etiology of heart failure. The number of patients is small and does not allow assessing mortality. In coronary patients with symptomatic diastolic heart failure with preserved systolic function low-dose digoxin was significantly more effective than ivabradine and is much cheaper. One should be more critical about ivabradine and low-dose digoxin in diastolic heart failure. To avoid possible negative effects on the cardiac function and a severe reduction of the cardiac output the resting heart rate should not be decreased to <65 beats/min.
Key words: ivabradine, digoxin, diastolic heart failure
Introduction
Ivabradine lowers heart rate by selective inhibition of the sinus node If-channels; it is ineffective in atrial fibrillation; it was developed as antianginal drug and is mainly used in combination with β-blockers.1 Ivabradine is also used to reduce symptoms and mortality in patients with sinus rhythm (SR) who suffer from heart failure with left ventricular (LV) systolic dysfunction.2 It is claimed that ivabradine may be important for the improvement of the clinical outcome in patients with LV systolic dysfunction and heart rate ≥70 beats/min.3 These papers, authored from cardiologists who occupy top positions in the European and American Societies of Cardiology, were published in medical journals with a high scientific impact and the data (usually from the same group of authors) have been added to the recent Guidelines of the European Society of Cardiology. These claims had positive influence, and ivabradine is considered an important drug in the therapy of angina pectoris and of heart failure with LV systolic dysfunction.
Digoxin was used worldwide for centuries to treat heart failure and may be used without β-blockers. Studies with large number of patients proved the efficacy and safety of lowdose digoxin in heart failure and atrial fibrillation; it was also reported that at low dose, i.e. a serum concentration (SDC) between 0.5 and 0.9 ng/mL, digoxin, reduces mortality and hospitalization in patients with heart failure, including those in SR and with preserved LV systolic function.4-9 However, trials showing impressive benefit with renin-inhibitors and β-blockers across the whole spectrum of heart failure, and studies showing the benefit of spironolactone in patients with severe heart failure eclipsed the use of digoxin. Also, guidelines put in doubt digoxin’s use in heart failure10 and in atrial fibrillation.11 Furthermore, recent studies12,13 have reported that digoxin increases deaths in elderly patients with systolic failure and atrial fibrillation. However, in these studies12,13 digoxin was not given at a low-dose and information about renal function, serum electrolytes and concomitant medications is scarce. Indeed, a recent editorial14 states that digoxin use in a trial is not the same as digoxin use in another trial and that it is possible that the mortality was driven by the development of new heart failure and not by digoxin itself, which was only used in response to the development of heart failure. It should be realized that withdrawing digoxin in patients with heart failure had consequences.15,16 As estimated from hospital and Medicare data, more than 20 years ago in USA continuation of digoxin therapy in patients with heart failure would have saved 185,000 office visits and 137,000 hospital admissions, with a net annual saving of 406 million $ (90% CI, 106 to 822 millions).
Ivabradine is often used to treat LV systolic dysfunction and ischemic heart disease, but little is known about its effects in LV ‘diastolic’ dysfunction. However, heart failure with diastolic dysfunction and preserved LV systolic function (HFPEF) has become epidemic and is accompanied by high morbidity and mortality rates.17 Furthermore, with advancing age, duration and severity of cardiac pathology and comorbidities, asymptomatic paroxysmal atrial fibrillation (apAF) is frequent and has a negative impact on morbidity and mortality.18-20 It happens that patients with diastolic dysfunction are erroneously treated with ivabradine because the type of heart failure is misinterpreted. It also happens that patients are treated with ivabradine because the occurrence of apAF is undetected, but unfortunately, ivabradine is ineffective during paroxysmal atrial fibrillation. Thus, ivabradine is used in some types of heart failure with unknown or lacking benefits.
Even if only used in patients with SR, 1-year therapy with ivabradine costs more than 1300 $, while 1-year therapy with digoxin would cost approximately 100 $. 2010 in USA there were 5.8 million people with heart failure16 and the different cost between ivabradine and digoxin is important. Nonetheless, at present the use of digoxin is considered reminiscence of old times and also dangerous.
We were interested to compare the therapeutic effects of ivabradine and low-dose digoxin in HFPEF.
Materials and Methods
Aim of the study
The aim of the study was to compare the therapeutic effects of ivabradine and low-dosedigoxin in patients with ischemic heart disease HFPEF.
Protocol and study design
This is an investigator-started study; the authors have no conflict of interest and there was no financial sponsoring. The study was planned according to the Good Clinical Quality standards. Selected patients gave their written informed consent and their physicians agreed. The protocol was approved from the Ethics Committee and is registered (NCT01796093). Concomitant medication remained unchanged during the study. Patients were assigned to ivabradine or digoxin according to a randomization cross-over design with two arms. Patients received either ivabradine or digoxin for 3 months and then the drugs were switched-off. Data were single-blind analyzed (ignoring the used drug and time of collection).
Tested drugs
Commercial brands of ivabradine and digoxin were used. Ivabradine was given at a dose of 7.5 mg bid. Digoxin was given at a low-dose (mostly 0.125 mg/day 6 times per weeks) aimed to reach the prefixed SDC range of 0.5-0.9 ng/mL.
Inclusion criteria
Ivabradine is frequently used in the therapy of ischemic heart disease. To get a homogeneous group with cardiac ischemia we selected patients with stable coronary heart disease and previous revasculization (percutaneous dilatation, stenting, or aortocoronary bypass). In spite of treatment with the maximally tolerated doses of β-blockers, renin-blocking drugs, and diuretics, patients had dyspnea class 3 of the New York Heart Association (NYHA) and HFPEF.
Exclusion criteria
Exclusion criteria comprised non-ischemic etiology of heart failure, reduced LV systolic cardiac function (LVEF <50%) or normal diastolic function, unstable angina pectoris, pacemaker rhythm, diabetes requiring insulin, moderate or severe renal or hepatic dysfunction. Patients with sleep apnea, hypertension, with renal insufficiency and with severe diabetes mellitus represent a substantial proportion of cases with HFPEF. However, many cardiologists objected that ivabradine is not indicated in these pathologies and therefore, we selected patients with heart failure of ischemic etiology.
Definition of diastolic heart failure with preserved left ventricular systolic function
The guidelines of the European Heart Society21 state that the diagnosis of HFPEF requires the following conditions to be satisfied: i) signs or symptoms of heart failure; ii) normal or mildly abnormal systolic LV function; iii) evidence of diastolic LV dysfunction. Normal or mildly abnormal systolic LV function implies both an LVEF >50% and an LV enddiastolic volume index (LVEDVI) <97 mL/m2. Diagnostic evidence of diastolic LV dysfunction can be obtained invasively (LV end-diastolic pressure >16 mmHg or mean pulmonary capillary wedge pressure >12 mmHg) or non-invasively by tissue Doppler (TD) (E/E1 >15). If TD yields an E/E ratio suggestive of diastolic LV dysfunction (15>E/E1 >8), additional noninvasive investigations are required for diagnostic evidence of diastolic LV dysfunction. These can consist of blood flow Doppler of mitral valve or pulmonary veins, echo measures of LV mass index or left atrial volume index (LAVi), electrocardiographic evidence of atrial fibrillation, or plasma levels of natriuretic peptides. If plasma levels of natriuretic peptides are elevated, diagnostic evidence of diastolic LV dysfunction also requires additional non-invasive investigations such as TD, blood flow Doppler of mitral valve or pulmonary veins, echo measures of LV mass index or LAVi, or electrocardiographic evidence of atrial fibrillation. We used several parameters: signs or symptoms of heart failure; pathologic NBproBNP; presence of apAF; and echocardiographic evidence of normal systolic LV function and diastolic LV dysfunction.
Screening, selection and collection of data
A total of 102 patients with dyspnea were screened; 20 (19.6%) had no heart failure, 46 (45%) had reduced systolic function (LVEF<50%). Forty-two (41%) had HFPEF and were selected. A dynamic 24-36-h electrocardiogram (ECG) was performed at selection. The following data were collected at selection and at the end of each treatment: i) medical history and concomitant medications; ii) physical examination; iii) query about side-effects; iv) a standardized22 6-MWT; v) laboratory analysis, including NB-proBNP; vi) SDC, at least twice during the 3-month therapy with digoxin; vii) an ECG; and viii) echocardiography. NB-proBNP values were immediately analyzed with a Roche Cardiac Reader (Nutley, NJ, USA); the analytical range was between 60 pg/mL and 3000 pg/mL. SDC was measured in a single Swiss qualified external laboratory. Echocardiographic data were collected from an experienced cardiologist using 3-D Philips iE33 Matrix equipment, according to general standards23,24: i) Doppler E/A ratio; ii) color tissue Doppler E/E1 ratio (septal E/E1, i.e. septal values combination of E1 with peak E velocity), which is a valuable non-invasive surrogate for determining LV diastolic pressures and diagnosing heart failure with preserved LV systolic function;17,23-25; iii) LAVi (i.e. the ratio of left atrial volume divided by body surface area); and iv) LV end-diastolic dimension (LVDd). LAVi and LVDd have been found to be predictive of mortality independently from preserved LVEF.26
Statistical analysis
Statistical analysis was performed with Statgraphics software. The analysis was performed using an intention-to-treat method. Data are expressed as mean ± 1 standard deviation. The within-group difference between ivabradine and digoxin was compared. It is known that patients with apAF differ from those with SR17-19 and the within-group difference between these two cardiac rhythms was also compared. Absolute values and percentual changes in relation to baseline measurements were analyzed. The 2 hypotheses tested were: null hypothesis: mu1-mu2=0.0, and alternative hypothesis: mu1-mu2>>0.0. Comparisons within groups were made using paired t tests or the nonparametric Wilcoxon signed-rank test, where appropriate. Between-group comparisons were performed by unpaired t tests or the nonparametric Mann-Whitney U test, respectively. Chi-square test or Kruskal-Wallis test were used to compare continuous normally or not normally distributed and qualitative variables, where appropriate. Multivariate analysis of variance was performed. A P value of <0.05 was considered statistically significant.
Results
General data
Collected data are summarized in Tables 1-3. All patients (20 males and 22 females) completed the study. With the dynamic ECG recording we found that 22/42 patients (52%) had apAF. Since the arrhythmia was paroxysmal, it cannot be excluded that we have missed some episodes.
Table 1.
Gender, cardiac rhythm and concomitant medications.
| SR (no.) |
apAF (no.) |
|
|---|---|---|
| Gender | ||
| Males | 10 | 10 |
| Females | 10 | 12 |
| Phenprocoumon (INR 2-3) | 0 | 22 |
| Aspirin 100 mg | 20 | 22 |
| β-blocker | 20 | 22 |
| ACE-Inhibitor | 10 | 12 |
| A2-Antagonist | 10 | 10 |
| Hydrochlorothiazide | 11 | 12 |
| Loop diuretics | 9 | 10 |
| Spironolactone | 15 | 18 |
| Amlodipine | 7 | 7 |
| Gliclazide | 4 | 7 |
| Metformin | 7 | 7 |
apAF, asymptomatic paroxysmal atrial fibrillation; SR, sinus rhythm; INR, International New Ratio.
Table 2.
Effects of ivabradine and digoxin/all patients.
| Before mean±SD |
Ivabradine mean±SD |
P | Digoxin mean±SD |
P | DIGvsIVA P |
|
|---|---|---|---|---|---|---|
| NYHA | 3 | 2.6±0.5 | # | 2.2±0.4 | # | § |
| NB-proBNP | 953±385 | 815±294 | NS | 655±193 | # | § |
| HR | 85±5 | 81±5 | NS | 76±4 | # | NS |
| SBP | 136±6 | 135±5 | NS | 135±5 | NS | NS |
| DBP | 77±4 | 76±3 | NS | 77±4 | NS | NS |
| 6MWT | 334±66 | 361±66 | ° | 403±62 | # | § |
| maxHR | 160±9 | 149±8 | # | 145±7 | # | § |
| LVEF | 64±5 | 63±5 | NS | 68±4 | # | § |
| E/A | 06±0.1 | 07±0.1 | NS | 0.9±0.1 | # | § |
| E/E1 | 14.4±0.3 | 14.1±0.4 | ° | 13.0±0.4 | # | § |
| LAVi | 27±3 | 25±3 | # | 23±3 | # | § |
| LVDd | 52±3 | 51±3 | NS | 48±2 | # | § |
NYHA, dyspnea NYHA class; NB-proBNP, N-terminal natriuretic peptide (pg/mL); HR, heart rate (beats/min); SBP, systolic blood pressure (mmHg); DBP, diastolic blood pressure (mmHg); 6MWT, 6-min walk-test (m); maxHR, maximal heart rate (beats/min); LVEF, left ventricular ejection fraction (%); E/A, ratio of Doppler-E and -A wave; E/E1, color tissue Doppler septal E/E1 ratio, i.e. septal values combination of E’ with peak E velocity; LAVi, index of left atrium (mL/m2); LVDd, end-diastolic left ventricular dimension (mm); SD, 1 standard deviation; NS, P value baseline versus therapy, non-significant. *P value baseline versus therapy, P<0.05;
° P value baseline versus therapy, P<0.005;
# P value baseline versus therapy , P<0.001;
§ P value within groups (ivabradine-digoxin), P<0.001.
Table 3.
Effects of ivabradine and digoxin/patients with sinus rhythm.
| Before mean±SD |
Ivabradine mean±SD |
P | Digoxin mean±SD |
P | DIGvsIVA P |
|
|---|---|---|---|---|---|---|
| NYHA | 3 | 2.7±0.5 | # | 2.1±0.3 | # | § |
| NB-proBNP | 735±125 | 665±121 | ° | 582±137 | # | § |
| HR | 81±3 | 77±8 | NS | 74±3 | # | § |
| 6MWT | 392±23 | 420±28 | ° | 455±28 | # | § |
| maxHR | 153±5 | 143±4 | # | 133±5 | # | § |
| LVEF | 66±5 | 65±4 | NS | 69±3 | # | § |
| E/A | 06±0.2 | 07±0.2 | ° | 0.9±0.2 | # | § |
| E/E1 | 14.1±0.3 | 13.9±0.3 | ° | 13.3±0.4 | # | § |
| LAVi | 25±2 | 23±2 | # | 20±2 | # | § |
| LVDd | 50±1 | 49±1 | * | 46±1 | # | § |
NYHA, dyspnea NYHA class; NB-proBNP, N-terminal natriuretic peptide (pg/mL); HR, heart rate (beats/min); 6MWT, 6-min walk-test (m); maxHR, maximal heart rate (beats/min); LVEF, left ventricular ejection fraction, (%); E/A, ratio of Doppler-E and -A wave; E/E1, color tissue Doppler septal E/E1 ratio, i.e. septal values combination of E’ with peak E velocity; LAVi, index of left atrium (mL/m2); LVDd, end-diastolic left ventricular dimension (mm); SD, 1 standard deviation; NS, P value baseline versus therapy, non-significant.
* P value baseline versus therapy, P<0.05;
° P value baseline versus therapy, P<0.005;
# P value baseline versus therapy , P<0.001;
§ P value within groups (ivabradine-digoxin), P<0.001.
Age (years) was 61.8±4.4 in all patients, 58.9±2.3 in patients with SR, and 64.4±4.2 in patients with apAF. The within-group (SR and apAF) analysis shows that patients with apAF were significantly (P<0.005) older.
Pretreatment body weight (kg) was 88.2±8.2. It and changed to 88.7±8.7 with ivabradine, and to 85.9±7.8 with digoxin. The changes were non-significant.
Laboratory
The fluctuations were minor; the hepatic, renal and electrolyte values did not change and no untoward-effects were detected. The SDC (ng/mL) was 0.6±0.4.
Dyspnea (NYHA class)
The class of pretreatment dyspnea (Table 3) was 3. It decreased to 2.6±0.5 with ivabradine, and to 2.2±0.4 with digoxin (both changes P<0.0001). In patients with SR (Table 3) it decreased to 2.7±0.5 with ivabradine, and to 2.1±0.3 with digoxin (both changes P<0.0001). In patients with apAF (Table 4) it decreased to 2.8±0.4 with ivabradine (P<0.05), and to 2.3±0.5 with digoxin (P<0.0001). The within-group (ivabradine and digoxin) analysis shows that in all groups the decrease was high-significantly (P<0.0001, CI 95 %) greater with digoxin.
Table 4.
Effects of ivabradine and digoxin/patients with asymptomatic paroxysmal atrial fibrillation.
| Before mean±SD |
Ivabradine mean±SD |
P | Digoxin mean±SD |
P | DIGvsIVA P |
|
|---|---|---|---|---|---|---|
| NYHA | 3 | 2.8±0.5 | # | 2.2±0.4 | # | § |
| NB-proBNP | 953±125 | 815±294 | NS | 655±193 | # | § |
| HR | 88±4 | 85±3 | NS | 78±3 | # | § |
| 6MWT | 281±45 | 307±88 | ° | 355±42 | # | § |
| maxHR | 167±6 | 149±8 | # | 145±7 | # | § |
| LVEF | 63±5 | 65±5 | NS | 67±5 | # | § |
| E/A | 05±0.4 | 06±0.3 | * | 0.9±0.2 | # | § |
| E/E1 | 14.5±0.2 | 14.3±0.4 | * | 13.0±2.1.4 | # | § |
| LAVi | 29±2 | 27±2 | # | 25±2 | # | § |
| LVDd | 54±3 | 53±3 | NS | 48±2 | # | § |
NYHA, dyspnea NYHA class; NB-proBNP, N-terminal natriuretic peptide (pg/mL); HR, heart rate (beats/min); 6MWT, 6-min walk-test (m); maxHR, maximal heart rate (beats/min); LVEF, left ventricular ejection fraction, (%); E/A, ratio of Doppler-E and -A wave; E/E1, color tissue Doppler septal E/E1 ratio, i.e. septal values combination of E’ with peak E velocity; LAVi, index of left atrium (mL/m2); LVDd, end-diastolic left ventricular dimension (mm); SD, 1 standard deviation; NS, P value baseline versus therapy, non-significant.
* P value baseline versus therapy, P<0.05;
° P value baseline versus therapy, P<0.005;
# P value baseline versus therapy , P<0.001;
§ P value within groups (ivabradine-digoxin), P<0.001.
NB-proBNP (pg/mL)
In all patients (Table 2) pretreatment average NB-proBNP was 953±385. It decreased to 815±294 (NS) with ivabradine, and to 655±193 with digoxin (P<0.0001). In patients with SR (Table 3) pretreatment average NBproBNP was 735±125. It decreased to 665±121 with ivabradine (P<0.005), and to 582±137 with digoxin (P=0.0009). In patients with apAF (Table 4) pretreatment average NB-proBNP was 1151±434. It decreased to 952±338 (NS) with ivabradine, and to 721±214 with digoxin P<0.0001). The within-group (SR and apAF) analysis shows that in patients with apAF pretreatment average NB-proBNP was significantly (P<0.005) higher (more heart failure). The within-group (ivabradine and digoxin) analysis shows that in all groups the decrease was high-significantly (P<0.009, CI 95%) greater with digoxin.
Heart rate
Heart rate (beats/min) was measured in the sitting position and is shown in Figure 1. In all patients (Table 2) pretreatment heart rate was 85±5. It decreased to 81±5 with ivabradine (NS) and to 76±4 with digoxin (P<0.0001). In patients with SR (Table 3) pretreatment heart rate was 81±3. It decreased to 77±2 with ivabradine (NS), and to 74±3 with digoxin (P<0.001). In patients with apAF (Table 4) pretreatment heart rate was 88±4. It decreased to 85±3 with ivabradine (NS), and to 78±3 with digoxin (P<0.0001). The within-group (ivabradine and digoxin) analysis shows that in all groups the decrease was high-significantly (P<0.003, CI 95%) greater with digoxin.
Figure 1.
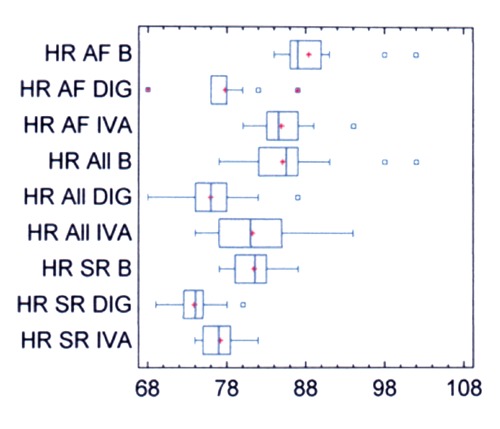
Heart rate before and after therapy. HR, heart rate (beats/min); B, before therapy with test drugs; DIG, digoxin; IVA, ivabradine; All, all patients; AF, patients with asymptomatic paroxysmal atrial fibrillation; SR, patients with sinus rhythm.
Blood pressure
Blood pressure (mmHg) was measured in the sitting position. Pretreatment systolic blood pressure (Table 2) was 136.6±6. It changed to 135±5 with ivabradine, and to 135±5 with digoxin. Pretreatment diastolic blood pressure (Table 2) was 77±4. It changed to 76±3 with ivabradine, and to 77±4 with digoxin. All changes were non-significant.
6-MWT (m)
In all patients (Table 2) pretreatment average length was 334±66. It increased to 361±66 with ivabradine, and to 403±62 with digoxin (both changes P<0.0001). In patients with SR (Table 3) pretreatment average length was 392±23. It increased to 420±28 with ivabradine (P=0.005), and to 455±28 with digoxin (P<0.0001). In patients with apAF (Table 4) pretreatment average length was 281±45. It increased to 307±38 with ivabradine (P=0.0002), and to 355±42 with digoxin (P<0.0001). The within-group (SR and apAF) analysis shows that in patients with apAF pretreatment length was high-significantly (P<0.0001) shorter. The within-group (ivabradine and digoxin) analysis shows that the effect was statistically high-significantly (P<0.0001, CI 95%) greater with digoxin.
Maximal heart rate
Maximal heart rate (beats/min) was measured during the 6-MWT and is shown in Figure 2. In all patients (Table 2) pretreatment maximal heart rate was 160±9. It decreased to 149±8 with ivabradine, and to 139±8 with digoxin (both P<0.0001). In patients with SR (Table 3) pretreatment maximal heart rate was 153±5. It decreased 143±4 with ivabradine, and to 133±5 with digoxin (both changes P<0.001). In patients with apAF (Table 4) pretreatment maximal heart rate was 167±6. It decreased to 149±8 with ivabradine, and to 145±7 with digoxin (both P<0.0001). The within-group (SR and apAF) analysis shows that in patients with apAF pretreatment maximal heart rate was high-significantly (P<0.0001) higher. The within-group (ivabradine and digoxin) analysis shows that the decrease was statistically high-significantly (P<0.0001, CI 95%) greater with digoxin.
Figure 2.
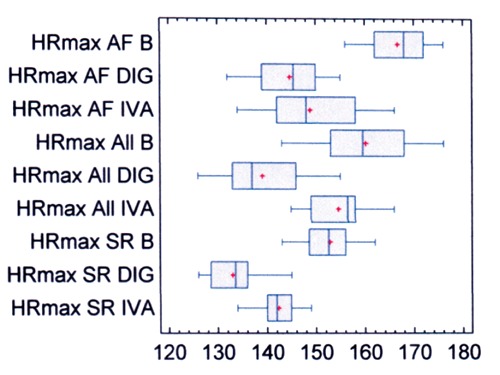
Maximal heart rate during the 6-min walk test, before and after therapy. HR, heart rate (beats/min); B, before therapy with test drugs; DIG, digoxin; IVA, ivabradine; All, all patients; AF, patients with asymptomatic paroxysmal atrial fibrillation; SR, patients with sinus rhythm.
Echocardiographic data
In all patients (Table 2) pretreatment LVEF was 64±5. It changed to 63±5 with ivabradine (NS), and to 68±4 with digoxin (P<0.0001). In patients with SR (Table 3) pretreatment LVEF was 66±5. It changed to 65±4 with ivabradine (NS), and to 69±3 with digoxin (P<0.0001). In patients with apAF (Table 4) pretreatment LVEF was 63±5. It did not change with ivabradine, and changed to 67±5 with digoxin (P<0.0001). The within-group (ivabradine and digoxin) analysis shows that in all groups the change was statistically high-significantly (P<0.0001, CI 95%) greater with digoxin.
In all patients (Table 2) pretreatment E/A was 0.6±0.1. It increased to 0.7±0.2 with ivabradine (P=0.002), and to 0.9±0.1 with digoxin (P<0.0001). In patients with SR (Table 3) pretreatment E/A was 06±0.2. It increased to 0.7±0.2 with ivabradine (P=0.002), and to 0.9±0.2 with digoxin (P<0.0001). In patients with apAF (Table 4) pretreatment E/A was 05±0.4. It increased to 0.6±0.3 with ivabradine (P=0.05), and to 0.9±0.2 with digoxin (P<0.0001). The within-group (SR and apAF) analysis shows that in patients with apAF pretreatment E/A was high-significantly (P<0.0001, CI 95%) lower. The within-group (ivabradine and digoxin) analysis shows that in all groups the effect was statistically high-significantly (P<0.0001, CI 95 %) greater with digoxin.
In all patients (Table 2) E/E1 was 14.4±0.3. It decreased to 14.1±0.4 with ivabradine (P=0.002), and to 13.1±1.6 with digoxin (P<0.00001). In patients with SR (Table 3) pretreatment E/E1 was 14.1±0.3. It decreased to 13.9±0.3 with ivabradine (P=0.0002), and to 13.3±0.4 with digoxin (P<0.0001). In patients with apAF (Table 4) pretreatment E/E1 was 14.5±0.2. It decreased to 14.3±0.4 with ivabradine (P=0.0004), and to 13.0±2.1 with digoxin (P<0.0001). The within-group (SR and apAF) analysis shows that in patients with apAF pretreatment E/E1 was high-significantly (P<0.0001, CI 95%) higher. The within-group (ivabradine and digoxin) analysis shows that in all groups the effect was statistically highsignificantly (P<0.0001, CI 95%) greater with digoxin.
LAVi (mL/m2) is shown in Figure 3. In all patients (Table 2) pretreatment LAVi was 27±3. It decreased to 25±3 with ivabradine, and to 23±3 with digoxin (both P<0.0001). In patients with SR (Table 3) pretreatment LAVi was 25±2. It decreased to 23±2 with ivabradine, and to 20±2 with digoxin (both changes P<0.00001). In patients with apAF (Table 4) pretreatment LAVi was 29±2. It decreased to 27±2 with ivabradine, and to 25± 2 with digoxin (both changes P<0.0001). The within-group (SR and apAF) analysis shows that in patients with apAF pretreatment LAVi was high-significantly (P<0.0001, CI 95%) greater. The withingroup (ivabradine and digoxin) analysis shows that in all groups the effect was statistically high-significantly (P<0.0001, CI 95%) greater with digoxin. In all patients (Table 2) pretreatment LVDd was 52±3. It decreased to 51±3 with ivabradine (NS), and to 48±2 with digoxin (P<0.0001). In patients with SR (Table 3) pretreatment LVDd was 50±1. It decreased to 49±1 with ivabradine (P<0.02), and to 46±1 with digoxin (P<0.0001). In patients with apAF (Table 4) pretreatment LVDd was 54±3. It decreased to 53±3 with ivabradine (NS), and to 50±2 with digoxin (P<0.0001). The withingroup (SR and apAF) analysis shows that in patients with apAF pretreatment LVDd was high-significantly (P<0.0001, CI 95%) greater. The within-group (ivabradine and digoxin) analysis shows that in all groups the effect was statistically high-significantly (P<0.0001, CI 95%) greater with digoxin.
Figure 3.
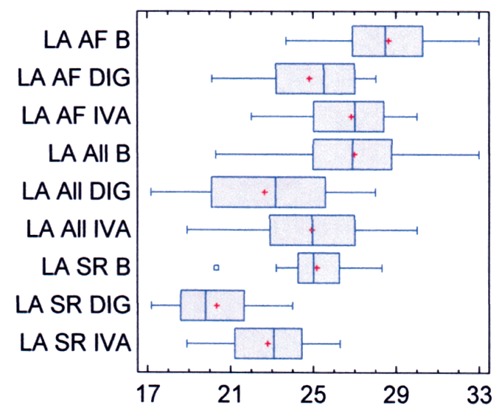
Left atrial index before and after therapy. LA, left atrial index (mL/m2); B, before therapy with test drugs; DIG, digoxin; IVA, ivabradine; All, all patients; AF, patients with asymptomatic paroxysmal atrial fibrillation; SR, patients with sinus rhythm.
Side effects
Three patients (7%) reported phosphenes in the first 2 weeks of therapy with ivabradine and 3 patients (7%) reported slight dizziness in the 1st week of therapy with digoxin. The side-effects disappeared spontaneously and did not require the discontinuation of therapy.
Discussion
In spite of previous coronary revascularization and maximally tolerated therapy with β-blockers, renin-antagonists and diuretics, selected patients had symptomatic congestive heart failure due to HFPEF. Pretreatment values for dyspnea, pathologic 6-MWT, NTproBNP, and echocardiographic changes confirm the severity of the heart failure and the diagnosis of HFPEF. As known from the literature18-20 patients with apAF were significantly older, had more severe failure and thus, a worse prognosis than patients with SR. Ivabradine and digoxin were both effective in the therapy of dyspnea, NB-proBNP, heart rate, duration of 6-MWT and maximal heart rate, but the improvement was high-statistically greater with digoxin. Average body weight: the changes did not reach statistical significance, but it remained unchanged with ivabradine and decreased by about 2 kg with digoxin. Since we selected patients with preserved LV systolic function pretreatment LVEF was normal. It increased slightly with digoxin and was unchanged with ivabradine. LVEF remained within the physiologic range and thus the increase with digoxin is clinically undetected and the medical impact of the increase is questionable. However, the different mechanisms of action of ivabradine and digoxin explain their effects on LVEF. Ivabradine has no direct positive inotropic effect.1,2 On the other hand, at pharmacologically effective doses digoxin binds with high affinity and specificity to the Na+K+-ATPase complex, reduces the conduction within the atrioventricular node and induces a positive inotropic effect.8,27
The average Doppler mitral E/A ratio was 0.6, indicating a stage 1 of diastolic dysfunction but the high color-Doppler E/E1 ratio indicates a marked LV diastolic dysfunction and suggests that many patients had elevated filling pressure with some restrictive component. Both drugs had positive therapeutic effects, but digoxin was high-statistically more effective. It should be stressed that we used lowdose digoxin and that the resting heart rate was not reduced to <74 beats/min. This is important because in patients with severe diastolic dysfunction and with a restrictive pattern (with an E/E1 >17 or pseudo normalization in the E/A ratio) bradycardia with prolongation of diastole may severely impair cardiac output by reducing the number of filling cycles per minute.
LAVi and LVDd were pathologically increased, especially in patients with apAF. Both drugs were effective, but digoxin was high-statistically more effective. The pathological value of LAVi, both before and after treatment, was at low level for patients with diastolic heart failure. In our patients the heart failure was due to ischemic heart disease. There are large individual variations, but in our experience in patients with heart failure the average LAVi is usually greater in patients with sleep apnea, hypertension (especially if there is a renal failure) and also in severe diabetes mellitus, than in patients with ischemic heart disease. In our patients ischemic heart disease had been treated by revascularization and drugs. The therapy seems to have contributed to relatively small elevation in LAVi values, however, still many patients had elevated filling pressure with some restrictive component and 52% had apAF. Thus there is dissociation between the dimension and the function of the left atrium, suggesting that the anti-ischemic therapy may have reduced the left atrial dimension without clearly reducing LV dysfunction and without a clear effect on the occurrence of apAF. It is known that the occurrence of apAF is not fully explained by the size of the left atrial and that other factors (e.g. atrial fibrosis) play a relevant role in the occurrence of this arrhythmia.11,12
Limitations of our study
Our study has important limitations. First, our results should not be extrapolated to LV dysfunction of non-ischemic etiology (especially sleep apnea and hypertension). Second, atrial fibrillation was paroxysmal and it is thus possible that we have missed some episodes in dynamic ECG recording and we may have erroneously considered some patients to be in SR. Many cardiologists assume that ivabradine is good and digoxin is obsolete and we could obtain data in only 42 patients. This small number does not allow assessing mortality. Some might also suggest that the statistical power is low, can lead to spurious results, and that ascertainment of end points can introduce bias even if when analysis of data itself was blinded. Thus, we are left for the moment to make decision under conditions of some uncertainty. However, our results indicate that ivabradine is marginally effective in the therapy of ischemic heart failure with HFPEF and that low-dose digoxin was effective.
Conclusions
Multicenter trials have demonstrated that in patients with SR ivabradine is effective in the therapy of ischemic heart disease and of LV systolic dysfunction. Ivabradine is surely ineffective in atrial fibrillation. Nonetheless, many patients with symptomatic heart failure have HFPEF, and some have undetected apAF. Ivabradine is not indicated in these conditions but it is nevertheless erroneously used. In these conditions, low-dose digoxin allowing a careful use without decreasing resting heart rate <65 beats/min was highly effective and is much cheaper than ivabradine. As stated in an editorial9 one should be more critical about ivabradine and low-dose digoxin in heart failure.
Acknowledgment
The authors would like to thank Mrs. J. Bugmann and Mrs. H. Brogli who have worked as medical assistants. Mrs. J. Bugmann wrote the manuscript.
References
- 1.Ferrari R. A step further with ivabradine: SIGNIfY (Study assessIngG the morbiditymortality beNefits of the If inhibitor ivabradine in patients with coronarY artery disease). Eur Heart J 2009;11:D19-27 [Google Scholar]
- 2.Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebocontrolled study. Lancet 2010;376:875-85 [DOI] [PubMed] [Google Scholar]
- 3.Fox K, Komajada M, Ford, et al. Effect of ivabradine in patients with left-ventricular systolic dysfunction: a polled analysis of individual patients: data from the BEAUTIFUL and SHIFT trials. Eur Heart J 2013; 34:2263-70 [DOI] [PubMed] [Google Scholar]
- 4.Arnold SB, Byrd RC, Meister W, et al. Longterm digitalis therapy improves left ventricular function in heart failure. N Engl J Med 1980;303:1443-8 [DOI] [PubMed] [Google Scholar]
- 5.Campbell TJ, MacDonald PS. Digoxin in heart failure and cardiac arrhythmias. Med J Aust 2003;179:98-102 [DOI] [PubMed] [Google Scholar]
- 6.Nikolaidou T, Channer KS. Rate control in permanent atrial fibrillation. BMJ 2007;335:1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brophy JM. Rehabilitating digoxin. Eur Heart J 2006;27:127-9 [DOI] [PubMed] [Google Scholar]
- 8.Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIGOXIN trial. Eur Heart J 2006;27:178-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castagno D, Petrie MC, Claggett B, McMurray J. Should we SHIFT our thinking about digoxin? Observations on ivabradine and heart rate reduction in heart failure. Eur Heart J 2012;33:1137-41 [DOI] [PubMed] [Google Scholar]
- 10.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Committee for Practice Guidelines (CPG). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008;10:933-89 [DOI] [PubMed] [Google Scholar]
- 11.Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation-executive summary. J Am Coll Cardiol 2006;48:854-906 [DOI] [PubMed] [Google Scholar]
- 12.Singh J, Subhashni D. Digoxin use increased mortality in patients with AF. AJN 2013;113:66 [Google Scholar]
- 13.Whitbeck MG, Charnigo RJ, Khairy P, et al. Increased mortality among patients taking digoxin: analysis from the AFFIRM study. Eur Heart J 2013. [Epub Ahead of Print]. [DOI] [PubMed] [Google Scholar]
- 14.Murpy SA. When ‘digoxin use’ is not the same as ‘digoxin use’: lesson from the AFFIRM trial. Eur Heart H 2013;34:1465-7 [DOI] [PubMed] [Google Scholar]
- 15.Packer M, Gheorghiade M, Young JB, et al. Withdrawal of digoxin therapy from patients treated with angiotensin-converting enzyme inhibitors. N Engl J Med 1993;329:1-7 [DOI] [PubMed] [Google Scholar]
- 16.Ward RE, Gheorghiade M, Young JB. Economic outcomes of withdrawal of digoxin therapy in adult patients with stable congestive heart failure. J Am Coll Cardiol 1995;26:93-101 [DOI] [PubMed] [Google Scholar]
- 17.Arques S, Roux E, Luccioni R. Current clinical applications of spectral tissue Doppler echocardiography (E/E’ ratio) as a noninvasive surrogate for left ventricular diastolic pressures in the diagnosis of heart failure with preserved left ventricular systolic function. Cardiovasc Ultrasound 2007;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics. 2010 update: a report from the American Heart Association. Circulation 2010;121:e46. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JL, Gilbert EM, Alpert BL, et al. Prevention of symptomatic recurrences of paroxysmal atrial fibrillation in patients initially tolerating antiarrhythmic therapy. A multicenter, double-blind, crossover study of flecainide and placebo with transtelephonic monitoring. Flecainide Supraventricular Tachycardia Study Group. Circulation. 1989;80:1557-70 [DOI] [PubMed] [Google Scholar]
- 20.Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 1995:98:476-84 [DOI] [PubMed] [Google Scholar]
- 21.Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure wit with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539-50 [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Sullivabradinen MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919-23 [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas PS, De Cara JM, Devereux RB, et al. Echocardiographic imaging in clinical trials: American Society of Echocardiography Standards for echocardiography core laboratories: endorsed by the American College of Cardiology Foundation. J Am Soc Echocardiogr 2009; 22:755-65 [DOI] [PubMed] [Google Scholar]
- 24.Al-Omari M, Finstuen J, Appleton CP, et al. Echocardiographic assessment of left ventricular diastolic function and filling pressure in atrial fibrillation. Am J Cardiol 2008;101:1759-65 [DOI] [PubMed] [Google Scholar]
- 25.Cocco G, Pandolfi S. Physical exercise with weight reduction lowers blood pressure and improves abnormal left ventricular relaxation in pharmacologically treated hypertensive patients. J Clin Hypertens 2011; 13:23-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel DA, Lavie CJ, Milani RV, Ventura HO. Left atrial volume index is predictive of mortality independent of left ventricular geometry in a large clinical cohort with preserved ejection fraction. Mayo Clin Proc 2011;8:730-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith TW. The basic mechanism of inotropic action of digoxin glycosides. J Pharmacol 1984;15:35-51 [PubMed] [Google Scholar]


