Abstract
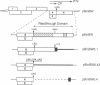
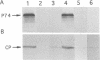
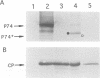
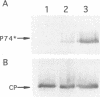
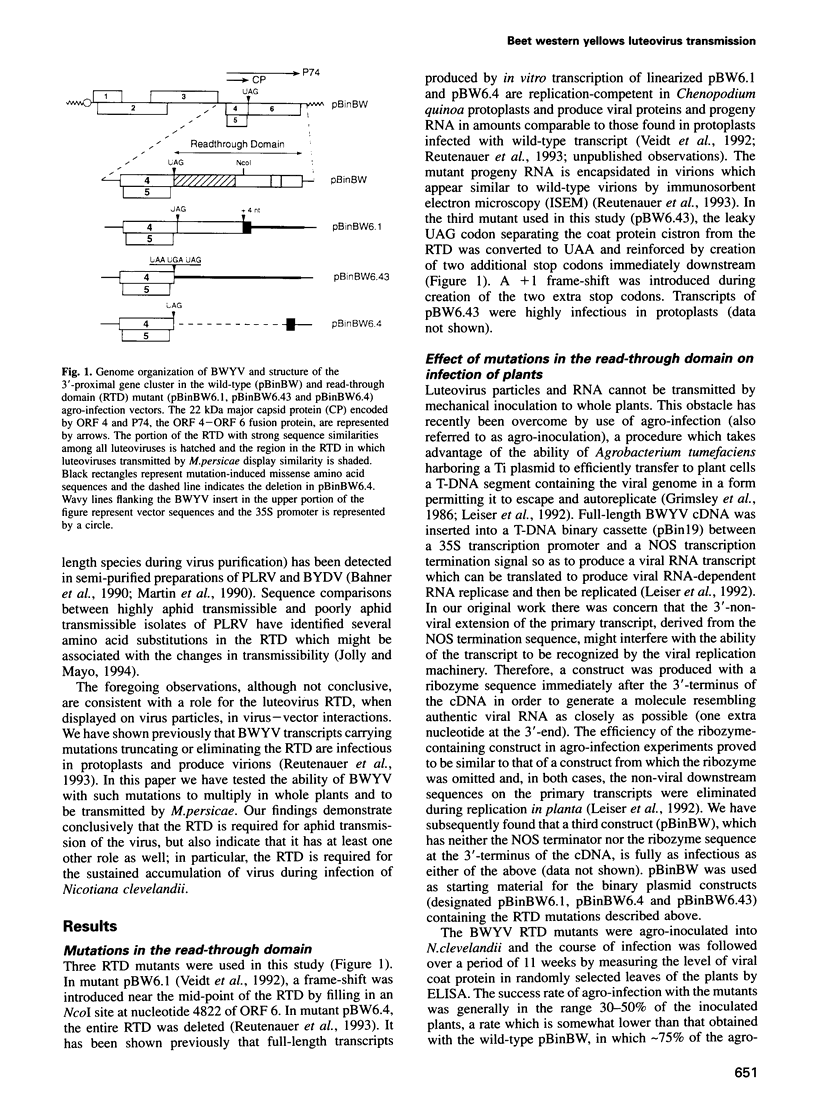
Beet western yellows luteovirus is obligately transmitted by the aphid Myzus persicae in a circulative, non-propagative fashion. Virus movement across the epithelial cells of the digestive tube into the hemocoel and from the hemocoel into the accessory salivary glands is believed to occur by receptor-mediated endocytosis and exocytosis. Virions contain two types of protein; the major 22 kDa capsid protein and the minor read-through protein, P74, which is composed of the major capsid protein fused by translational read-through to a long C-terminal extension called the read-through domain. Beet western yellows virus carrying various mutations in the read-through domain was tested for its ability to be transmitted to test plants by aphids fed on agro-infected plants and semi-purified or purified virus preparations. The results establish that the read-through domain carries determinants that are essential for aphid transmission. The findings also reveal that the read-through domain is important for accumulation of the virus in agro-infected plants.
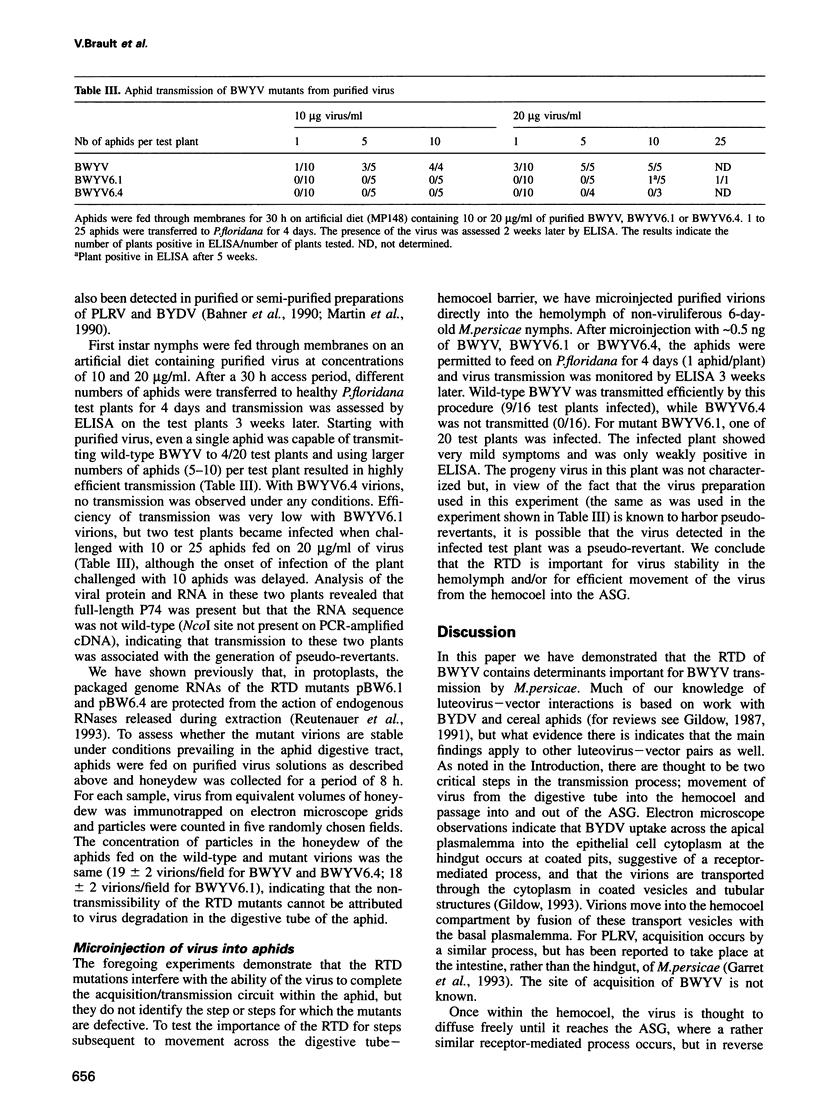
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahner I., Lamb J., Mayo M. A., Hay R. T. Expression of the genome of potato leafroll virus: readthrough of the coat protein termination codon in vivo. J Gen Virol. 1990 Oct;71(Pt 10):2251–2256. doi: 10.1099/0022-1317-71-10-2251. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh-Kumar S. P., Brault V., Miller W. A. Precise mapping and in vitro translation of a trifunctional subgenomic RNA of barley yellow dwarf virus. Virology. 1992 Apr;187(2):711–722. doi: 10.1016/0042-6822(92)90474-4. [DOI] [PubMed] [Google Scholar]
- Esau K., Hoefert L. L. Development of infection with beet western yellows virus in the sugarbeet. Virology. 1972 Jun;48(3):724–738. doi: 10.1016/0042-6822(72)90156-0. [DOI] [PubMed] [Google Scholar]
- Garret A., Kerlan C., Thomas D. The intestine is a site of passage for potato leafroll virus from the gut lumen into the haemocoel in the aphid vector, Myzus persicae Sulz. Arch Virol. 1993;131(3-4):377–392. doi: 10.1007/BF01378639. [DOI] [PubMed] [Google Scholar]
- Grimsley N., Hohn B., Hohn T., Walden R. "Agroinfection," an alternative route for viral infection of plants by using the Ti plasmid. Proc Natl Acad Sci U S A. 1986 May;83(10):3282–3286. doi: 10.1073/pnas.83.10.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Wipf-Scheibel C., Richards K., Lecoq H., Jonard G. Nucleotide sequence of cucurbit aphid-borne yellows luteovirus. Virology. 1994 Aug 1;202(2):1012–1017. doi: 10.1006/viro.1994.1429. [DOI] [PubMed] [Google Scholar]
- Habili N., Symons R. H. Evolutionary relationship between luteoviruses and other RNA plant viruses based on sequence motifs in their putative RNA polymerases and nucleic acid helicases. Nucleic Acids Res. 1989 Dec 11;17(23):9543–9555. doi: 10.1093/nar/17.23.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood A. M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994 Jan;68(1):1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C. A., Mayo M. A. Changes in the amino acid sequence of the coat protein readthrough domain of potato leafroll luteovirus affect the formation of an epitope and aphid transmission. Virology. 1994 May 15;201(1):182–185. doi: 10.1006/viro.1994.1283. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leiser R. M., Ziegler-Graff V., Reutenauer A., Herrbach E., Lemaire O., Guilley H., Richards K., Jonard G. Agroinfection as an alternative to insects for infecting plants with beet western yellows luteovirus. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9136–9140. doi: 10.1073/pnas.89.19.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisner S. M., Turgeon R., Howell S. H. Effects of host plant development and genetic determinants on the long-distance movement of cauliflower mosaic virus in Arabidopsis. Plant Cell. 1993 Feb;5(2):191–202. doi: 10.1105/tpc.5.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisner S. M., Turgeon R. Movement of virus and photoassimilate in the phloem: a comparative analysis. Bioessays. 1993 Nov;15(11):741–748. doi: 10.1002/bies.950151107. [DOI] [PubMed] [Google Scholar]
- Lentz T. L. The recognition event between virus and host cell receptor: a target for antiviral agents. J Gen Virol. 1990 Apr;71(Pt 4):751–766. doi: 10.1099/0022-1317-71-4-751. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Mayo M. A. The location of the 5' end of the potato leafroll luteovirus subgenomic coat protein mRNA. J Gen Virol. 1991 Nov;72(Pt 11):2633–2638. doi: 10.1099/0022-1317-72-11-2633. [DOI] [PubMed] [Google Scholar]
- Mozo T., Hooykaas P. J. Electroporation of megaplasmids into Agrobacterium. Plant Mol Biol. 1991 May;16(5):917–918. doi: 10.1007/BF00015085. [DOI] [PubMed] [Google Scholar]
- Nathanson S. D., Nelson L. T., Lee M. A spontaneous subcutaneous tumor in C57BL/6 mice that metastasizes to the liver. Clin Exp Metastasis. 1993 Jan;11(1):45–54. doi: 10.1007/BF00880065. [DOI] [PubMed] [Google Scholar]
- Niesbach-Klösgen U., Guilley H., Jonard G., Richards K. Immunodetection in vivo of beet necrotic yellow vein virus-encoded proteins. Virology. 1990 Sep;178(1):52–61. doi: 10.1016/0042-6822(90)90378-5. [DOI] [PubMed] [Google Scholar]
- Reutenauer A., Ziegler-Graff V., Lot H., Scheidecker D., Guilley H., Richards K., Jonard G. Identification of beet western yellows luteovirus genes implicated in viral replication and particle morphogenesis. Virology. 1993 Aug;195(2):692–699. doi: 10.1006/viro.1993.1420. [DOI] [PubMed] [Google Scholar]
- Tacke E., Prüfer D., Salamini F., Rohde W. Characterization of a potato leafroll luteovirus subgenomic RNA: differential expression by internal translation initiation and UAG suppression. J Gen Virol. 1990 Oct;71(Pt 10):2265–2272. doi: 10.1099/0022-1317-71-10-2265. [DOI] [PubMed] [Google Scholar]
- Veidt I., Bouzoubaa S. E., Leiser R. M., Ziegler-Graff V., Guilley H., Richards K., Jonard G. Synthesis of full-length transcripts of beet western yellows virus RNA: messenger properties and biological activity in protoplasts. Virology. 1992 Jan;186(1):192–200. doi: 10.1016/0042-6822(92)90073-x. [DOI] [PubMed] [Google Scholar]
- Veidt I., Lot H., Leiser M., Scheidecker D., Guilley H., Richards K., Jonard G. Nucleotide sequence of beet western yellows virus RNA. Nucleic Acids Res. 1988 Nov 11;16(21):9917–9932. doi: 10.1093/nar/16.21.9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., van den Heuvel S., Helin K., Fattaey A., Ewen M., Livingston D., Dyson N., Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993 Jul;7(7A):1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]