Abstract
A 26-year-old woman with a recent flare-up of systemic lupus erythematosus presented with peripheral retinal hemorrhages at a routine check-up. She is on a tapering dose of immunosuppressive agents. Her visual acuity was good. Fluorescein angiogram revealed vasculitic changes with capillary non-perfusion areas. A few weeks later, she developed cerebral lupus with advanced lupus nephritis. Immunosuppressive therapy was restarted and panretinal photocoagulation was delivered. Her visual acuity remained stable, despite development of a cataract from prednisolone therapy.
Key words: systemic lupus erythematosus, vasculitis, fluorescein angiogram, panretinal photocoagulation.
Introduction
Systemic lupus erythematosus is a multisystem autoimmune disorder which may have ocular manifestations such as cotton wool spots, retinal hemorrhages and vasculitis. These changes may occur at posterior pole or peripherally, without affecting visual acuity. Active disease in the eye may precede the systemic symptoms or herald further complications such as cerebral lupus. Close monitoring of disease activity is imperative to ensure optimal control and prevent further complications of both the disease and side effects of treatment. Side effects from medications also need to be properly evaluated during routine follow up.
Case Report
A 26-year-old Malay woman with a known case of SLE of six years came for routine dilated eye examination. Her visual acuity was good (6/9, N8) bilaterally with presence of posterior subcapsular cataract. She did not notice any deterioration in her visual acuity. Fundus examinations revealed blot hemorrhages and collateral vessels without gross evidence of vasculitis (Figure 1). Fluorescein angiography showed an extensive area of capillary non-perfusion and fuzzy appearance of the vessels, indicating active vasculitis (Figure 2).
Figure 1.
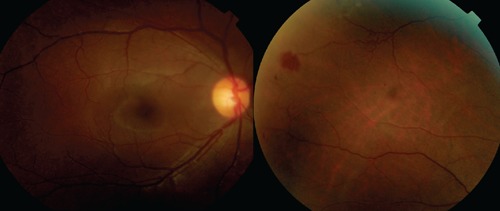
Colored photograph showing peripheral blotch hemorrhage with retina vessels tortuosity at periphery. Macula appearance is normal.
Figure 2.
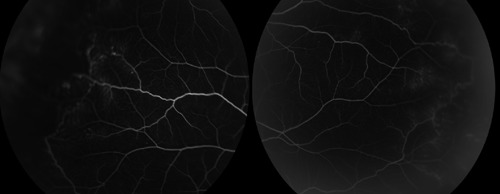
Fluorescein angiography showed extensive capillary non-perfusion and shunt vessels.
A repeat investigation showed elevated eryhtrocyte sedimentation rate (ESR) of 45 mm/h. Her complement factors were reduced at 6 mg/dL and 4 mg/dL for C3 and C4, respectively. The antinuclear antibody (ANA) titre was 1:640, however, anti-double stranded deoxyribonucleic acid (dsDNA) titer was not performed. Her creatinine level was also elevated at 237 umol/L.
She had had three relapses in the past, the most recent five months prior to presentation, which required admission for medical management. She first presented with lethargy, prolonged fever and rashes distributed mainly on her ankles. Blood tests were positive for both ANA and anti- dsDNA, and she had reduced complement factors. She was also diagnosed to have Evans syndrome, with antiphospholipid syndrome. At presentation, the patient was on a tapering dose of oral steroid (25 mg daily), and hydroxychloroquine (200 mg daily). Unfortunately, she had an epileptic attack four weeks after the recent ocular findings and was diagnosed to have cerebral lupus based on MRI findings and blood tests. She was also found to have lupus nephritis class III (International Society of Nephrology/ Renal Pathology Society 2003 Classification of Lupus Nephritis). Renal biopsy was performed when she was found to have proteinuria. Intravenous cyclophosphomide, rituximab and high-dose steroid (intravenous methylprednisolone 250 mg once, followed by 2 doses of 100 mg) was restarted. She completed 2 doses of both cyclophosphomide 400 mg and rituximab 500 mg. However, she developed pancytopenia which required both medications to be stopped. Oral prednisolone 1 mg/kg was started after 3 doses of intravenous methylprednisolone, with oral cyclosporine A 100 mg twice a day. Panretinal photocoagulation was eventually performed over the non-perfuse capillary area. Four months later, her ESR normalized to15 mmol/h, and fundus examination revealed resolution of hemorrhages without neovascularization or cotton wool spots. Her visual acuity remained good. She was transitioned on high-dose oral prednisolone, which was maintained at 10 mg daily, hydroxychloroquine 200 mg daily and cyclosporine A 25 mg twice daily, with no recurrences after six months follow up. The patient is currently on additional anti-hypertensive agents as she developed hypertension from the cyclosporine A treatment. Routine renal profile investigations remained stable.
Discussion
SLE is a chronic, inflammatory autoimmune disorder with systemic and ocular manifestations. The underlying pathophysiology is thought to be due to immune complex depositions which lead to vasculitis and thrombosis. Briefly, SLE-related retinopathy may either be in the classic form of cotton wool spots with or without hemorrhages, or occlusion of the retinal vascular tree.1 Ocular manifestation is one of the known complications in SLE. Although not part of diagnostic criteria for SLE according to the American Rheumatology Association, ocular manifestation is still an important clue to disease activity as it provides direct visualization of vascular changes. The ophthalmologist should be alert to the possibility that active systemic disease is likely in the presence of active ocular manifestations.2 This case amply illustrates that despite improving systemic symptoms, the disease is still active as evidenced by the clinical manifestation in the eye. It is, therefore, very important for physicians to be aware of active ocular SLE, thereby providing a lower threshold to re-start previous treatment in the events of early flare-up of disease activity. Although SLE may relapse during treatment with tapering doses of immunosuppressive, therapy, this singular case highlights the fact that asymptomatic patients still need to be carefully evaluated every six months for both clinical and ocular manifestations, as well as for the development of side effects from medications such as cataract, glaucoma and maculopathy. Assessment of disease activity in SLE is problematic, and evaluation of cerebral lupus may be a diagnostic challenge. Although there is no clear association between retinal vasculitis and cerebral lupus, the presence of retinopathy in general is a feature of more active disease.3 It is important to bear in mind the possibility of more serious complications of SLE such as cerebral lupus and lupus nephritis. We present a rare case which highlights the need for careful evaluation of fundus appearance to look for signs of retinopathy and vasculopathy, even in patients with good visual acuity and normal macula appearance, or the absence of cotton wool spots, such as in this patient. It is important to realize that SLE-related vasculitis and a capillary non-perfused area may also occur at periphery, thus masking the seriousness of the underlying disease activity and the need for urgent management if appropriate clinical assessment is not made.
Retinal occlusive vasculitis in SLE may be a result of immune complex deposition in the vessel wall. Ocular deposition of immune complexes at choroidal capillaries has been demonstrated using immunofluorescent techniques at autopsy.3 Immune complex deposition has also been identified at the vascular walls of sclera, retina, ciliary vessels as well as basement membranes of ciliary body and cornea.4 Adhesion molecule upregulation involving small vessels leads to vascular inflammation,5 and may be a compounding factor in this case, explaining the involvement of the peripheral retinal vessels. In cases of vasculitis secondary to SLE, anti- neutrophil cytoplasmic antibody is not associated to its development,6 unlike in the other pauci- immune-small vessel vascilitides. Recognizing the association of SLE and antiphospholipid antibody (APS) syndrome is important as APS may lead to the development of vasculopathy.7 In our case, there was consecutive development of cerebral lupus and lupus nephritis following retinal manifestations of SLE, thus suggesting active relapse of the underlying vascular inflammation. Fluorescein angiography is invaluable in determining the degree of active vasculopathy. Capillary non-perfused area, as seen in angiography, is indicative of retinal ischemia secondary to active vasculitis, thus may require laser photocoagulation. Additionally, fluorescein leakage indicates abnormal vessel permeability in active disease,8 even with clinically normal vessel appearance and without visual complaints. The management of SLE with ocular involvement should be directed towards controlling systemic inflammation and minimizing ocular complications. Systemic immunosuppressive agents with monitoring of inflammatory markers are essential to optimize the control of SLE. Although there is no evidence of neovascularization, laser photocoagulation should be performed over areas of extensive capillary non-perfusion to prevent further ischemia, as in our case. The attending rheumatologist should always enquire about visual symptoms or diminished visual acuity.9 Serological evidence of remission of the systemic lupus erythematosus may not reflect ocular retinal vasculitis secondary to the underlying problem. A study reported that an SLE patient on remission as evidenced by serological tests was found to have severe occlusive vasculitis.10 SLE-related retinopathy has also been associated with other systemic organ involvements such as cerebral lupus and lupus nephropathy. Although there is no clear evidence of direct associations between systemic organ involvement and ocular manifestations of SLE, the underlying pathology is related to vasculopathy secondary to the disease process. Therefore, patients with evidence of retinal vasculitis may reflect the systemic vascular damage and need to be monitored closer. An important learning point is that our patient was already being treated with immunosuppressive agents, and a tapering dose of steroid was given very gradually when she developed retinal vasculitis. Despite that, she went on to develop cerebral vasculitis, proving that clinical symptoms and serological evidence may not be adequate evaluation parameters. Ophthalmologists and rheumatologist should work together in assessing the disease activity of SLE patients.
References
- 1.Arevelo JF, Lowder CY, Muci-Mendoza R. Ocular manifestations of systemic lupus erythematosus. Curr Opin Ophthalmol. 2002;13:404–10. doi: 10.1097/00055735-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Giorgi D, Pace F, Giorgi A, et al. Retinopathy in systemic lupus erythematosus: pathogenesis and approach to therapy. Hum Immunol. 1999;60:688–96. doi: 10.1016/s0198-8859(99)00035-x. [DOI] [PubMed] [Google Scholar]
- 3.Aronson AJ, Ordonez NG, Diddie KR, et al. Immune-complex deposition in the eye in systemic lupus erythematosus. Arch Intern Med. 1979;139:1312–3. [PubMed] [Google Scholar]
- 4.Karpuk AG, Schwartz MM, Dickey LE, et al. Ocular immune reactants in patients dying with systemic lupus erythematosus. Clin Immunol Immunopathol. 1985;35:295–312. doi: 10.1016/0090-1229(85)90091-1. [DOI] [PubMed] [Google Scholar]
- 5.Cid MC. Endothelial cell biology, perivascular inflammation and vasculitis. Cleve Clin J Med. 2002;69(Suppl 2):S1145–9. doi: 10.3949/ccjm.69.suppl_2.sii45. [DOI] [PubMed] [Google Scholar]
- 6.Kalamia KT, Balabanova M. Vasculitis in systemic lupus erythematosus. Clin Dermatol. 2004;22:148–56. doi: 10.1016/j.clindermatol.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non- SLE disorder. Prevalence and clinical significance. Ann Intern Med. 1990;112:682–98. doi: 10.7326/0003-4819-112-9-682. [DOI] [PubMed] [Google Scholar]
- 8.Lanham JG, Barrie T, Kohner EM, et al. SLE retinopathy: evaluation by fluorescein angiography. Ann Rheum Dis. 1982;41:473–8. doi: 10.1136/ard.41.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley R, Graham C, Jones S, et al. Ocular toxicity and hydroxychloroquine: guideline for screening 2004. Available from: http://www.rcophth.ac.uk/docs/publications/oculartoxicity2004.pdf.
- 10.Vine AK, Bar CC. Proliferative lupus retinopathy. Arch Ophthalmol. 1984;102:852–4. doi: 10.1001/archopht.1984.01040030672015. [DOI] [PubMed] [Google Scholar]


