Abstract
The present study evaluates the incidence of early and late seizures after head injury in patients under 18 years old. Factors correlating with a high risk of developing posttraumatic seizures were identified. Such risk factors were the severity of the head trauma and a Glasgow Coma Scale of 3–8. In contrast to many studies, we observed that the incidence of posttraumatic seizures was significantly higher in patients older than 12 years old (12–16 and 12–18). Most of the late seizures were paroxysmal electroencephalography (EEG) discharges diagnosed on a snapshot-EEG during the follow-up examination of the patients without clinical symptoms. We suppose that EEG-examination in head injured children is important to identify patients with epileptic potentials without clinical symptoms. Epileptic patterns of the EEG could worsen the diagnosis and clinical outcome of the children in accordance to studies performed in the adult population.
Key words: posttraumatic seizures, early seizures, late seizures, anticonvulsive therapy.
Introduction
It is a major goal to prevent children with head traumas from secondary brain injury like posttraumatic seizures. Posttraumatic seizures occur in as many as 15% of children with head trauma.1,2 In comparison to studies of posttraumatic seizures in adults there are few studies that focus on children. Trying to understand if the children that have sustained a traumatic brain injury (TBI) would be in higher risk of epileptic seizures in the adolescence or mature age, there have also been made experiments in rats. These showed that TBI lowered minimal clonic seizure thresholds at maturity but not during adolescence. Furthermore, the incidence of posttraumatic seizures was low despite electroencephalography (EEG) spiking. Because of children's more vulnerable brain it is not possible to adapt to them the results of the studies performed in adults. It is necessary to recognize patients at risk of developing seizures after brain injury because convulsions aggravate secondary damage to the brain by increasing the intracranial pressure and hypoxia and make monitoring of head injured children difficult due to postictal neurological deterioration.2 Recognized prognostic factors that influence the outcome of brain injured patients are, hypoxemia, hypotension, severity of primary insult, sustained intracranial hypertension, reduced or hyperemic cerebral blood flow and frank ischemia.3–7 During the first few days after trauma the brain is in a vulnerable state and secondary insults like posttraumatic seizures may worsen the outcome.7 Etiologies of this state are neurochemical changes as altered glucose and oxidative metabolism. Seizures and other epileptiform activity are known to stimulate glycolysis and similar neurochemical changes and this neurochemical perturbation worsens the clinical outcome.7–10 Early seizures are viewed as a predictor of poor outcome from many authors. A seizure occurring within the first week after trauma is conventionally termed an early seizure, and any convulsion after the first week of trauma is considered as late seizure.11 One important question is if the patients at risk of developing posttraumatic seizures could be identified and what could be done to prevent seizures. Prophylactic anti-convulsive medication in children with severe head trauma [Glasgow Coma Scale (GCS) 3–8] reduces posttraumatic seizures.12 However, the efficacy of phenytoin in the prevention of posttraumatic seizures in adults is discussed controversially. One double-blind, placebo-controlled study of phenytoin to prevent early as well as late posttraumatic seizures in adults was unable to show any efficacy.13 In another study, seizures occurred despite initiation of prophylactic phenytoin on admission to the emergency room.7 On the other hand, there are studies that found phenytoin to be effective in preventing early posttraumatic seizures but ineffective in the prevention of late seizures.14 In a study using continuous EEG monitoring in head injured adults it could be seen that 52% of the patients suffered from paroxysmal EEG discharges, diagnosed on the base of EEG alone.7
The purpose of the present study is to indicate factors that identify head injured children and adolescents as risk patients to develop posttraumatic seizures. This is valuable for the emergency physician evaluating a child to predict the risk of posttraumatic seizures and make decisions about further observation of the child in the intensive care unit (i.e. EEG-Monitoring etc.).
Materials and Methods
This study is based on a retrospective review of 238 children and adolescents admitted to our hospital between a 6-year period. Data were recorded regarding the patients' age, sex, mechanism of injury, diagnosis (concussion, contusion trauma, others like fractures, haematomas etc.), occurrence of loss of consciousness, vital signs, GCS score and pediatric GCS score for children younger than 4 years old, development of early and late seizures, cranial computed tomography (CT) if necessary, EEG examination (in the follow up period and during the clinical stay a snapshot-EEG was taken but not continuous EEG-monitoring), conservative or operative treatment.
Patients were divided into two main categories based on their age (birth-4 year, 4–12 year, 12–18 year) and on ranges of the GCS (3–4, 5–8, 9–11, 12–15). The diagnoses were divided into 3 categories (concussion, contusions, others). Those patients who suffered from posttraumatic seizures were divided into 2 major groups of early (trauma event-7 days) and late (after 1 week period) seizures and each of these groups into 3 subgroups of generalized, focal and other (non-convulsive) seizures. The significance test used was the chi-square test. An exclusion criterion was a history of seizures.
Patient database
The age range is from birth to 18 years old. Sixty-three patients (63/238; 26.5%) were 0–4 year, 89 4–12 year (89/238; 37.4%) and 86 were 12–18 year (86/238; 36.2%). The male/female ratio was 132/106. The diagnosis of head traumas was 172/238 (72.2%) concussion, 50/238 (21%) contusions, 25/238 (10.5%) had other traumas like fractures, epidural and subdural haematomas. Twenty nine children (29/238; 12,1%) had a surgical therapy. The GCS was 3–4 in 3 cases (3/238; 1.2%), 5–8 in 10 and 9–11 in 10 cases (10/238; 4.2%), 12–15 in 215 children (215/238; 90.3%). The examination of the trauma patients included physical and neurological examination with control of consciousness, vital signs and GCS, if necessary EEG and in most cases with cranial CT. None of the patients was treated with anticonvulsants. None of the patients with early seizures developed late seizures in the year's follow-up (Supplementary Table 1).
Early seizures
We found out that 10.9% (26/238) of our patients with cranial traumas suffered from seizures. In the age group 0–4 years there were 4.7% (3/63), in the age group 4–12 8.9% (8/89) and in 12–18 17.4% (15/86). Eighteen percent (9/50) of the patients with seizures were diagnosed as contusio cerebri (with and without further diagnosis of haematomas and fractures), 32% (8/25) had isolated haematomas and haematomas with additional fractures and 8.1% (14/172) had concussion (Supplementary Tables 1–3).
Forty two percent (11/26) of the seizures were observed in the first 24 h until 7 days after the trauma (early seizures) and 58% after the first week (late seizures). In the group of the early seizures, 54.5% (6/11) were generalized, 27.2% (3/11) focal and 18.1% (2/11) paroxysmal EEG discharges (i.e. there were pathological changes in the EEG without clinical findings). We diverted this group into 3 subgroups (generalized, focal, others) and compared the distribution of seizures with the age, GCS and the diagnosis. Thirty three percent (2/6) of the patients with generalized early convulsions were between 0 and 4 years old, 16.6% (1/6) were between 4 and 12 years and 50% (3/6) between 12 and 18. Thirty three percent (2/6) had a GCS of 5–8, 16.6% (1/6) of 9–11 and 50% (3/6) of 12–15. Sixteen percent (1/6) were diagnosed with commotio cerebri and 83.3% (5/6) diagnosed with concussion, whereas 3 of them suffered additionally from an epidural haematoma (EDH), a sub-dural haematoma (SDH) and a fracture. The therapeutic procedure in 2 patients was an operative one (33%) and in 4 patients (66.6%) conservative (i.e. observation on ward). Only 3 patients (3/11) suffered from early focal convulsions. One in the 0-4-year-old age group, 1 in the 4–12 and 1 in the 12–18 (33.3%). One (33.3%) had a GCS of 3–4 and 2 children (66.6%) of 12–15. One diagnosed with a commotion, 1 with an EDH and 1 with a generalized edema. In the latter we performed a decompression craniectomy and the two others were observed for a certain time.
Two children (18.8%) suffered from early other non-convulsatory symptoms. One (50%) showed a convalsionary cry and the second child had a pathological EEG (general changes). None of them needed an operation because of the diagnosis of concussion in both cases. In the 0-4-year-old group, children with early seizures 1 (1/3) had a GCS of 5–8, and 2 (2/3) of 12–15. In the 4–12-year-old group, one (1/3) had a GCS of 3–4, 1 of 5–8 and 1 of 12–15. One (1/5) of the 12–18 year old children had a GCS of 5–8, another one 9–11 and 3 (3/5) 12–15.
Late seizures
The second group of patients that was examined in this study was the group of children who suffered from seizures one week after the trauma (Supplementary Tables 4 and 5). This was the group of the late seizures (15/26; 57.6%). Five patients belonged to the age group 4–12 (5/15; 33.3%), and 10 (10/15; 66.6%) in the 12–18-year-old group. Ten had a concussion (10/15), 4 had a contusion (4/15), 3 had an EDH, SDH respectively and one (1/15) a contusion with an additional fracture. One of the children (1/15; 6.6%) suffered from generalized convulsion after concussion, was in the age group between 12 and 18 and had a GCS of 12–15. The therapy was observation. Focal convulsions were shown in one child (1/15; 6.6%), belonging to the age group 12–18 and had a GCS of 12–15, and the diagnosis of a contusion trauma at the frontal lobe with skull base fracture. The therapy was a frontobasal revision. Most of the patients in this group suffered from paroxysmal EEG discharges without other clinical evidence of convulsions (13/15; 86.6%). Five (5/13; 38.4%) were in the age group 4–12 and 8 (8/13; 61.5%) in the group between 12–18. One (1/13; 7.6%) had a GCS of 5–8 and 12 (12/13; 92.3%) a GCS of 12–15. Nine of the children had a concussion (9/13; 69.2%), 3 a contusion trauma (3/13; 23%), and one had a fracture, one a SDH and another one an EDH. Two were operated (2/13; 15, 3%) and 11 (11/13; 84.6%) were observed on ward. The age distribution of this patient group compared to the GCS is shown in Supplementary Table 5. No patients were in the age group of 0–4 year. The 5 patients (5/5; 100%) of the 4–12 year had all a GCS of 12–15. In the group of 12–18 year, one had a GCS of 5–8 (1/10; 10%), and 9 of 12–15 (9/10; 90%). Only one of all the patients with posttraumatic seizures developed a seizure disorder and needed an anticonvulsive therapy (1/25; 4%). All the others had only a unique convulsion and did not need drug therapy.
Results
Age
In order to evaluate age as a risk of post-traumatic seizure development, patients were grouped according to their age and incidence of seizures was studied. There was a significant higher incidence of seizures in patients between 12 and 18 years old (15/86; 17.44%) of seizures than in the 0–4 year old group (3/63; 4.7%) (P<0.05). Between the age groups 0–4 (3/63; 4.7%) and 4–12 (8/89; 8.9%), there was not significant difference of seizures (Figure 1). The risk of seizure development is higher in adolescents (12–18) than in children (0–12).
Figure 1.
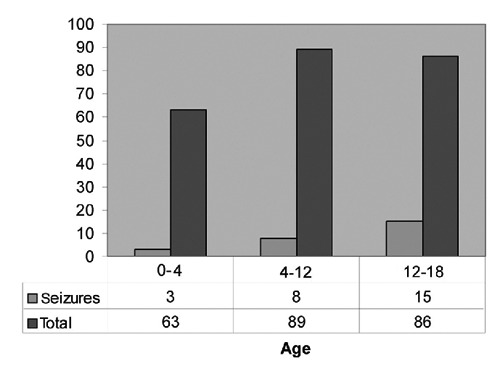
Correlation between age and seizure incidence. The distribution of seizures compared with the total number of head traumas between the age groups 0–4, 4–12 and 12–18 years is shown in a diagram. In the age group 12–18, there is a significant increase in seizure incidence. Under the diagram are the absolute numbers of patients observed.
Trauma type and Glasgow Coma Scale
A factor, which plays a crucial role in the development of seizures, is the trauma type and the GCS. It was aim of the study to evaluate if there are differences in seizure incidence and contusion or concussion or in other types of trauma. Patients with contusion traumas (9/50; 18% contusions) had a statistically significant higher incidence of seizures compared to concussion (14/172; 8.1%) (P<0.05). Additionally, there was a statistically highly significant increase of seizure incidence in the patient group with other diagnoses, i.e. fractures and haematomas (8/25; 32%) than those with concussion (14/172; 8.1%)(P<0.001). Between contusion traumas and the group of other diagnoses (fractures, EDH, SDH) a statistical significant difference in seizure incidence could not be seen (Figure 2). There were fourfold higher seizure-incidence in patients with GCS of 3–8 (5/13; 38.5%) in comparison to patients with a GCS of 9–15 (21/225; 9.3%). This difference is statistically highly significant (P<0.001).
Figure 2.
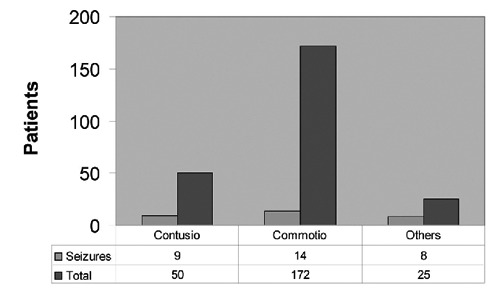
Trauma type and seizure incidence. The percentage of seizures in the groups of different diagnosis can be seen this diagram. Eighteen percent of the contusional traumas, 8.1% of children with commotion and 32% with other diagnoses [epidural haematoma (EDH), subdural haematoma (SDH) fractures], showed seizures. Patients with other diagnosis i.e. EDH, SDH and fractures had a significant increased risk of developing posttraumatic seizures.
Seizure type
It was also a point of interest to investigate the type of seizures developed in early post-traumatic and late posttraumatic seizure disorder. When seizures developed early after the trauma than most of the times they were generalized (6/9; 66.6%), whereas in late post-traumatic seizures mostly of the cases developed non-convulsive seizures (13/15; 86.6% P<0.05) (Figure 3).
Figure 3.
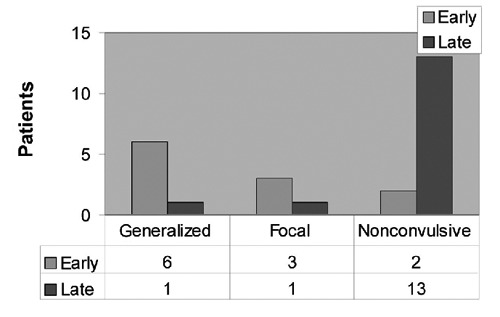
Difference in seizure types in early and late posttraumatic seizures. The difference of the seizure types between early and late seizures is shown. Most of the early seizures 54.5% are generalized convulsions, whereas 86.6% of late seizures are non-convulsive. This difference is statistical significant.
Discussion
The present study evaluates the incidence of seizures (early and late) after head trauma in the pediatric and adolescent patients admitted in our hospital for a 6-year period. We were able to find statistical significant factors correlating with the development of posttraumatic seizures.
We found out that 10.9% of children with head trauma had posttraumatic seizures. This results correlated with those of other studies where the incidence is 4.2% and 21%.2,15,16
There are studies showing that the majority of early seizures (1st week after trauma) developed during the first 24 h in 59%.11 In our study 100% of early seizures developed during the first 24 h after trauma. Only 22% of the seizures were early seizures but 81.1% of convulsions (clinical symptomatic seizures) developed in the early posttraumatic period. These results are in contrast to other studies that showed that the majority of the seizures occurred in the first week after head trauma.1,2 Other studies could show the same incidence for both early and late seizures.15 The high occurrence of late posttraumatic seizures in our study is explained in accordance to the high occurrence of non-convulsatory seizures without clinical evidence of seizure disorder but only diagnosed with EEG examination with generalized or focal spike-waves and generalized pathological changes. This finding correlates with the results in a study of posttraumatic seizures in adults where continuous EEG was performed, that found out that 52% of the patients with post-traumatic seizures had non-convulsive seizures and were diagnosed in the EEG basis alone.7 Another study using short-duration EEGs found similar results.17
The patients with contusion traumas and diagnoses like fractures with SDH, EDH and intracranial haematomas have a significant higher risk of developing seizures than patients with concussion. There was no significant difference between patients with contusions and other injuries like EDH, SDH and fractures. Similar results were observed in patients with a GCS score of 3–8 (4 times higher then in GCS 9–15), which correlates with worse traumas. Our results agree to those studies showing that there is a highly significant correlation with a GCS ⊆ 8 (until 7 times higher)1,18 and the development of posttraumatic seizures. However other study groups showed a higher seizure-incidence in patients with minor head injuries.9 We showed that a GCS score of 3–8 and a severe head trauma is a statistically highly significant risk factor for the development of post-traumatic seizures.
A number of studies showed a higher incidence for seizures in younger children than in older ones.19–23 Other study groups could not find a difference between children younger or older than 5 and 3 years.1,21 However we could show that in the age group 12–18 year there was a significantly higher incidence of seizures compared to children younger than 4 years. These results are in contrast to many other studies. The percentage of severe traumas as well as a GCS score of 3–8 was similar in all 3 age groups. Age >12 years as another risk factor for the development of seizures. One explanation is that in contrast to other studies where the age limit was 16 year, the age limit in our study was 18 year. Six of the 37 patients older than 16 years had seizures (16.2%) and 9/34 (26.4%) were between 12 and 16 years old. This difference is statistically not significant, but we also observed that the incidence of developing seizures in the age group 12–16 year in comparison with the group <4 year was also significantly higher (P<0.001) so that the higher age limit of 18 year did not explain our findings.
There was one child with a generalized edema, which developed early seizures, and the operative treatment with decompressive craniectomy was indicated. After the craniectomy a second convulsion did not occur. All our patients with early and late convulsions after head trauma did not develop a second one or non-convulsive status epileptics so that we do not recommend the anticonvulsive phenytoin administration if the patient only suffered from an early or a late convulsion. When continuous EEG-monitoring is performed and epileptic potentials are found, antiepileptic drugs could be used to stop these potentials. However, the prophylactic use of anticonvulsants in children who have the risk factors of developing seizures is recommended in other studies1 and was not subject of our study.
Conclusions
In this study we characterized factors correlating with a higher possibility of developing posttraumatic seizures in children after head trauma. These factors are severe head traumas like contusions, EDH, SDH, fractures and specially fractures with skull impression, a GCS of 3–8, and age of >12 years old. We found that more than a half of the patients developed late seizures but in contrast to the patients with early seizures most of them were paroxysmal EEG discharges found in EEG examinations, which was in most cases performed in the outpatient follow up. There is a need to take EEG examinations in children with head traumas, or if possible, continuous EEG monitoring, to observe epileptic potentials, which can worsen the prognosis of the head, injured children. No one of our patients was treated with anticonvulsive drugs. However, none of them having early seizures developed a second seizure although they were not treated with antiepileptic drugs like phenytoin.
Acknowledgments:
the authors would like to thank Dr. Michael Fritsch for his kind support.
References
- 1.Hahn YS, Fuchs S, Flannery AM, et al. Factors influencing posttraumatic seizures in children. Neurosurgery. 1988;22:864–7. [PubMed] [Google Scholar]
- 2.Ratan SK, Kulshreshtha R, Pandey RM. Predictors of postraumatic convulsions in head-injured children. Pediatr Neurosurg. 1999;30:127–31. doi: 10.1159/000028779. [DOI] [PubMed] [Google Scholar]
- 3.Aldrich EF, Eisenberg HM, Saydjari C. Predictors of mortality in severly head-injured patients with civillian gunshot wounds: a report from the NIH Traumatic Coma Data Bank. Surg Neurol. 1992;38:418–23. doi: 10.1016/0090-3019(92)90109-z. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg HM, Gary HE, Jr, Aldrich EF. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688–98. doi: 10.3171/jns.1990.73.5.0688. [DOI] [PubMed] [Google Scholar]
- 5.Kelly DF, Martin NA, Kordestani R. Cerebral blood flow as a predictor of outcome following traumatic brain injury. J Neurosurg. 1997;86:633–41. doi: 10.3171/jns.1997.86.4.0633. [DOI] [PubMed] [Google Scholar]
- 6.Marmarou A. Increased intracranial pressure in head injury and influence of blood volume. J Neurotrauma. 1992;19:S327–32. [PubMed] [Google Scholar]
- 7.Vespa PM, Nuver MR, Nenow V, et al. Increased incidence and impact of non-convulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750–60. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson H, Ronne-Engström E, Ungerstedt U. Seizure related elevations of extracellular amino acids in human focal epilepsy. Neurosci Lett. 1992;140:30–2. doi: 10.1016/0304-3940(92)90674-v. [DOI] [PubMed] [Google Scholar]
- 9.Collins RC, Tearse RG, Lothman EW. Functional anatomy of limbic seizures: focal discharges from medial endorhinal cortex in rat. Brain Res. 1983;280:25–40. doi: 10.1016/0006-8993(83)91170-8. [DOI] [PubMed] [Google Scholar]
- 10.Engel J, Jr, Kuhl DE, Phelps ME. Local cerebal metabolism during partial seizures. Neurology. 1983;33:400–13. doi: 10.1212/wnl.33.4.400. [DOI] [PubMed] [Google Scholar]
- 11.Hendrick EB, Harris L. Post traumatic epilepsy in children. J Trauma. 1968;8:547–56. doi: 10.1097/00005373-196807000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Lewis RJ, Yee L, Inkelis SH, Gilmore D. Clinical predictors of post-traumatic seizures in children with head trauma. Ann Emerg Med. 1993;22:1114–8. doi: 10.1016/s0196-0644(05)80974-6. [DOI] [PubMed] [Google Scholar]
- 13.Young B, Rapp RP, Norton JA, et al. Failure of prophylactically administered phenytoin to prevent early posttraumatic seizures. J Neurosurg. 1983;58:231–5. doi: 10.3171/jns.1983.58.2.0231. [DOI] [PubMed] [Google Scholar]
- 14.Temkin NR, Dikmen SS, Wilensky AJ. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 15.Schütze M, Dauch WA, Güttinger M, et al. Risikofaktoren für posttraumatische Anfälle und Epilepsie. Zentralbl Neurochir. 1999;60:163–7. [PubMed] [Google Scholar]
- 16.Emanuelson I, Uvebrant P. Occurrence of epilepsy during the first 10 years after traumatic brain injury acquired in childhood up to the age of 18 years in the south western Swedish population-based series. Brain Inj. 2009;23:612–6. doi: 10.1080/02699050902973913. [DOI] [PubMed] [Google Scholar]
- 17.Dawson RE, Webster JE, Gurdjian ES. Serial electroencephalography in acute head injuries. J Neurosurg. 1951;8:613–30. doi: 10.3171/jns.1951.8.6.0613. [DOI] [PubMed] [Google Scholar]
- 18.Jennett WB, Lewin W. Traumatic epilepsy after closed head injuries. J Neurol Neurosurg Psychiatry. 1960;23:295–301. doi: 10.1136/jnnp.23.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annegers JF, Grabow JD, Groover RV, et al. Seizures after head trauma: a population study. Neurology. 1980;30:683–9. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys RP, Jaimovich R, Hendrick B, Hoffman H. Severe head injuries in children. Concepts Pediatr Neurosurg. 1983;4:230–42. [Google Scholar]
- 21.Klonoff H, Low MD, Clark C. Head injuries in children: a prospective five-year follow-up. J Neurol Neurosurg Psychiatry. 1977;40:1211–9. doi: 10.1136/jnnp.40.12.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raimondi AJ, Hirschauer J. Head injury in the infant and toddler. Coma scoring and outcome scale. Childs Brain. 1984;11:12–35. doi: 10.1159/000120157. [DOI] [PubMed] [Google Scholar]
- 23.Thapa A, Chandra SP, Sinha S, et al. Post-traumatic seizures-A prospective study from a tertiary level trauma center in a developing country. Seizure. 2010;19:211–6. doi: 10.1016/j.seizure.2010.02.004. [DOI] [PubMed] [Google Scholar]


