Abstract
Syringocystadenocarcinoma papilliferum is a rare form of adenocarcinoma of the skin. It is the malignant counterpart of syringocystadenoma papilliferum (SCAP). It usually develops on the scalp in a long-standing lesion identified clinically as SCAP. Twelve cases of syringocystadenocarcinoma papilliferum have been reported in literature, with limited information regarding its clinical and histological characteristics. Sarcomatous change in a case of SCAP has not been reported in literature till date to the best of our knowledge. We present the first case of syringocystadenocarcino-sarcoma papilliferum in a middle-aged female with a satellite lesion over the scalp.
Key words: carcino-sarcoma, syringocystadenoma papilliferum.
Introduction
Syringocystadenoma papilliferum (SCAP) is a proliferating skin tumor with apocrine differentiation. Occasionally eccrine differentiation can be seen. Majority are seen on the face and scalp of young adults, often increasing in size at or around puberty.1 The lesion may be present at birth or appear in early childhood, but may also appear at a later age. In general, these tumors have a characteristic duct-like structure covered by two layers of glandular epithelial cells, located in the upper dermis, with a varying degree of papillomatosis. The deeper dermis has tumor cells arranged in sheets and cord like pattern along with areas of necrosis. These tumors comprise of spindle shaped cells, epithelioid cells, round cells or polygonal cells.2 We report a case of SCAP having characteristic histology which had turned into a carcinoma with a sarcomatous component over a short period of time.
Case Report
A 45-year-old female presented with cauliflower like growth over scalp for one year, which had rapidly increased in size over two weeks. On examination, a 6×3 cm hemispherical swelling was seen on the scalp, which was firm in consistency with a granular surface. Erosions and crustations were seen over its surface. She had associated satellite lesion 2 cm in front of the primary lesion measuring 2x1 cm with similar morphology (Figure 1). There was no associated lymphadenopathy. The rest of the examination was within normal limits. Biopsy from the scalp lesion was consistent with the diagnosis of SCAP progressing into adenocarcinoma. She underwent wide local excision of the tumor along with skin grafting. Histopathologic examination revealed it to be a case of syringocystadenocarcinoma, exhibiting epithelial and spindle cell differentiation like a sarcoma (Figure 2). The margins were free of tumor cells (R0) resection. Immunohistochemistry was positive for pancytokeratin, epithelial membrane antigen (EMA) and also for vimentin (Figure 3). It was negative for CD31, CD34, S-100, CK5/6, and HMB-45. A diagnosis of carcino-sarcoma arising in SCAP was made. No adjuvant therapy was given. Patient is disease free and recurrence free after 1 year of follow up.
Figure 1.
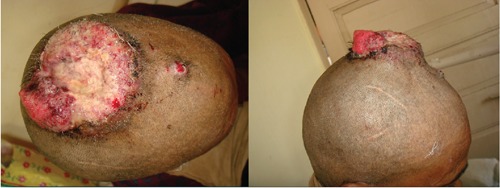
Clinical photograph (superior and lateral view).
Figure 2.
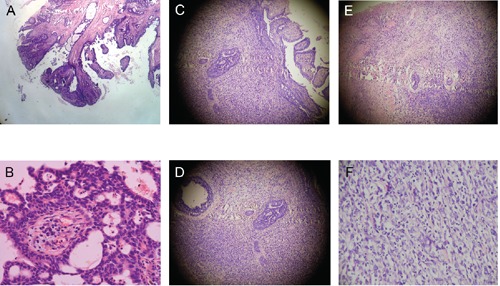
Histology: A) Classic syringocystadenoma papilliferum (SCAP) (H&E stain, 100X magnification); B) Classic SCAP (H&E stain, 400X magnification); C) Carcinomatous change in SCAP (H&E Stain 100X magnification); D) Carcinomatous change in SCAP (H&E Stain 400X magnification); E) Sarcomatous change in SCAP (H&E Stain 100X magnification); F) Sarcomatous change in SCAP (H&E Stain 400X magnification).
Figure 3.
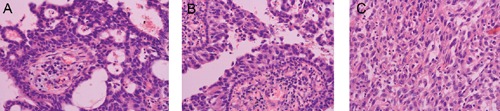
Immunohistochemistry: positive for: A) Pancytokeratin (400X); B) Epithelial membrane antigen; C) Vimentin.
Discussion
Syringocystadenocarcinoma papilliferum (SCACP) is a rare form of adenocarcinoma of the skin; this is the malignant counterpart of SCAP. It is said to develop in a long-standing case of SCAP. There have been associations of SCACP with malignancies of gastrointestinal tract with distant metastasis.3 However in our case it was not so. Only 12 cases of SCACP have been reported in the literature till date after it was first described in 1980.4 However a case of sarcomatous change in a case of SCACP has not been reported in literature till date. SCAP is usually seen in the middle aged and elderly people with maximum incidence in 47–74 years with equal incidence in both the sexes.5 The most common sites are scalp and neck.6 Lesions involving the extremities and perineum have also been described.3,5
Clinically, the patient presents with skin colored or yellowish papules or nodules of variable size (2.5–13 cm), which remain unchanged for many years but enlarge suddenly. There may be bleeding or ulceration and associated new satellite nodules.7–9 The lesion is usually covered with crusts, which are secretions of apocrine epithelial cells.7 The differential diagnosis includes squamous cell carcinoma, basal cell carcinoma, malignant melanoma or tumors arising from the endothelial lining like an angioma or angiosarcoma.3
Indications for surgical excision include, malignant change characterized by rapid increase in size or number, ulceration, drainage, pruritus, pain, and cosmesis. The standard treatment for syringocystadenoma involves complete surgical excision to the subcutaneous layer to remove all the adenexal structure. The tissue defect is usually closed primarily, but a skin graft may sometimes be required. On histopathology, the tumor shows deep epidermal cystic invaginations containing numerous papillae. Neoplastic cells lining these cystic cavities are connected to the skin surface through funnel-shaped structures lined by infundibular epithelium. The upper part of invaginations is made up of keratinizing squamous epithelium, whereas the lower part and papillae are comprised of two- or multi-layered epithelium. Cells of the inner layer have columnar with oval nuclei and abundant eosinophilic cytoplasm and decapitation on the luminal surface, while the cells of the outer layer are small, cuboidal or flattened and have scanty cytoplasm and oval nuclei.8 Occasionally, numerous normal apocrine glands can be found.5 However, SCACP can be differentiated from SCAP in that, it has an asymmetric structure with ill defined boundaries. The tumor cells often infiltrate the deep dermis or subcutaneous fat. The neoplastic cells exhibit crowded nuclei, with variable degree of nuclear atypia and mitotic figures.7,8 Our case also had findings consistent with SCACP and in addition had transition to the spindle cell variant of a sarcoma. On immunohistochemistry the tumor is usually positive for cytokeratin, carcino-embryonic antigen and EMA.3,10,11 In our case the immunohistochemistry was positive for pancytokeratin and EMA confirming it to be SCACP. In addition the tumor was also positive for vimentin which was consistent with the sarcomatous change of the tumor. Other markers like CD31, CD34, S-100, CK5/6, HMB-45 were negative hence ruling out other differential diagnosis like squamous cell carcinoma, malignant melanoma and endothelial cell lined tumors. A diagnosis of carcino-sarcoma arising in SCAP was made, as the tumor cells were infiltrating into the deep dermis, atypical cells were observed along with the characteristics of SCAP, with immunopositivity for EMA which shows carcinoma component, and dermal tumor cells showed gradual transition into spindle cell forms arranged in intersecting bundles that was positive for vimentin, suggestive of sarcomatous component.
Conclusions
SCACP is a rare variety of skin tumor. The presence of satellite nodule and rapid growth indicates malignant transformation. Sarcomatous transformation can occur in SCACP. Histopathology along with immunohistochemistry helps in establishing diagnosis of carcino-sarcoma in SCAP. Wide excision is the treatment of choice. More studies are required for understanding the development of these lesions.
References
- 1.Mammino JJ, Vidmar DA. Syringocystadenoma papilliferum. Int J Dermatol. 1991;30:763–6. doi: 10.1111/j.1365-4362.1991.tb04780.x. [DOI] [PubMed] [Google Scholar]
- 2.Kazakov DV, Requena L, Kutzner H, et al. Morphologic diversity of syringocystadenocarcinoma papilliferum based on a clinic pathologic study of 6 cases and review of the literature. Am J Dermatopathol. 2010;32:340–7. doi: 10.1097/DAD.0b013e3181b96c0c. [DOI] [PubMed] [Google Scholar]
- 3.Ishida-Yamamoto A, Sato K, Wada T, et al. Syringocystadenocarcinoma papilliferum: case report and immuno histochemical comparison with its benign counterpart. J Am Acad Dermatol. 2001;45:755–9. doi: 10.1067/mjd.2001.117723. [DOI] [PubMed] [Google Scholar]
- 4.Dissanayake RV, Salm R. Sweat-gland carcinomas: prognosis related to histological type. Histopathology. 1980;4:445–66. doi: 10.1111/j.1365-2559.1980.tb02939.x. [DOI] [PubMed] [Google Scholar]
- 5.Park SH, Shin YM, Shin DH, et al. Syringocystadenocarcinoma papilliferum: a case report. J Korean Med Sci. 2007;22:762–5. doi: 10.3346/jkms.2007.22.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leeborg N, Thompson M, Rossmiller S. Diagnostic pitfalls in syringo cystadenocarcinoma papilliferum: case report and review of the literature. Arch Pathol Lab Med. 2010;134:1205–9. doi: 10.5858/2009-0399-CR.1. [DOI] [PubMed] [Google Scholar]
- 7.Requena L, Kiryu H, Ackerman AB. Philadelphia, PA: Lippincott-Raven; 1998. Ackerman's histologic diagnosis of neoplastic skin disease: a method by pattern analysis: neoplasms with apocrine differentiation; pp. 665–75. [Google Scholar]
- 8.Chi CC, Tsai RY, Wang SH. Syringocystadenocarcinoma papilliferum: successfully treated with Mohs micrographic surgery. Dermatol Surg. 2004;30:468–71. doi: 10.1111/j.1524-4725.2004.30023.x. [DOI] [PubMed] [Google Scholar]
- 9.Seco Navedo MA, Fresno Forcelledo M, Orduña Domingo A, et al. Syringocystadenoma papilliferum with malignant evolution: presentation of a case [in French] Ann Dermatol Venereol. 1982;109:685–9. [PubMed] [Google Scholar]
- 10.Bonadi R, Urso C. Syringocystadenocarcinoma papilliferum. Histopathology. 1996;28:475–7. doi: 10.1046/j.1365-2559.1996.t01-4-297345.x. [DOI] [PubMed] [Google Scholar]
- 11.Arai Y, Kusakabe H, Kiyokane K. A case of syringocystadenocarcinoma papilliferum in situ occurring partially in syringocystadenoma papilliferum. J Dermatol. 2003;30:146–50. doi: 10.1111/j.1346-8138.2003.tb00363.x. [DOI] [PubMed] [Google Scholar]


