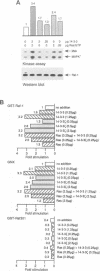
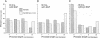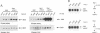Abstract
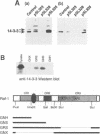
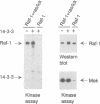
We have identified the beta (beta) isoform of the 14-3-3 family of proteins as an activator of the Raf-1 protein kinase. 14-3-3 was isolated in a yeast two-hybrid screen for Raf-1 kinase domain binding proteins. Purified bovine brain 14-3-3 interacted specifically with both c-Raf-1 and the isolated Raf-1 kinase domain. Association was sensitive to the activation status of Raf-1; 14-3-3 bound to unactivated Raf-1, but not Raf-1 activated by protein kinase C alpha or Ras and Lck. The significance of these interactions under physiological conditions was demonstrated by co-immunoprecipitation of Raf-1 and 14-3-3 from extracts of quiescent, but not mitogen-stimulated, NIH 3T3 cells. 14-3-3 was not a preferred Raf-1 substrate in vitro and did not significantly affect Raf-1 kinase activity in a purified system. However, in cell-free extracts 14-3-3 acted as a Ras-independent activator of both c-Raf-1 and the Raf-1 kinase domain. The same results were obtained in vivo using transfection assays; 14-3-3 enhanced both c-Raf-1- and Raf-1 kinase domain-stimulated expression of AP-1- and NF-kappa B-dependent reporter genes and accelerated Raf-1 kinase domain-triggered differentiation of PC12 cells. We conclude that 14-3-3 is a latent co-activator bound to unactivated Raf-1 in quiescent cells and mediates mitogen-triggered but Ras-independent regulatory effects aimed directly at the kinase domain.
Full text
PDF
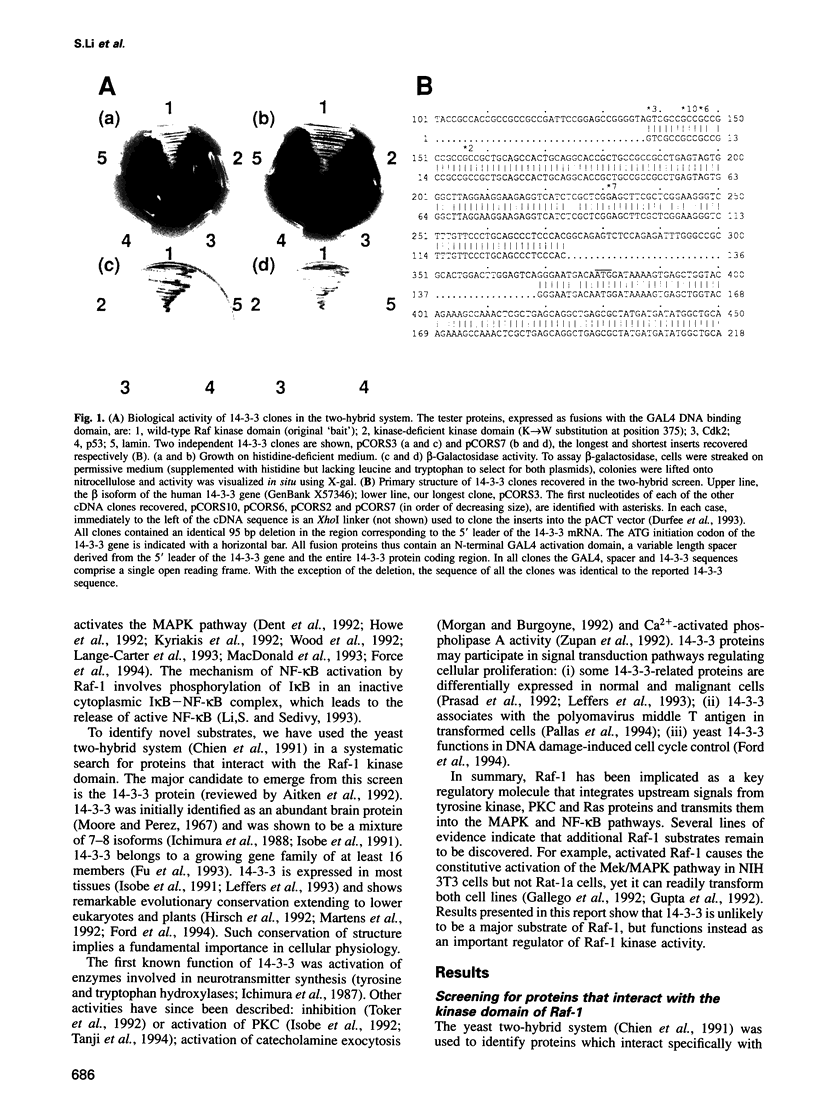
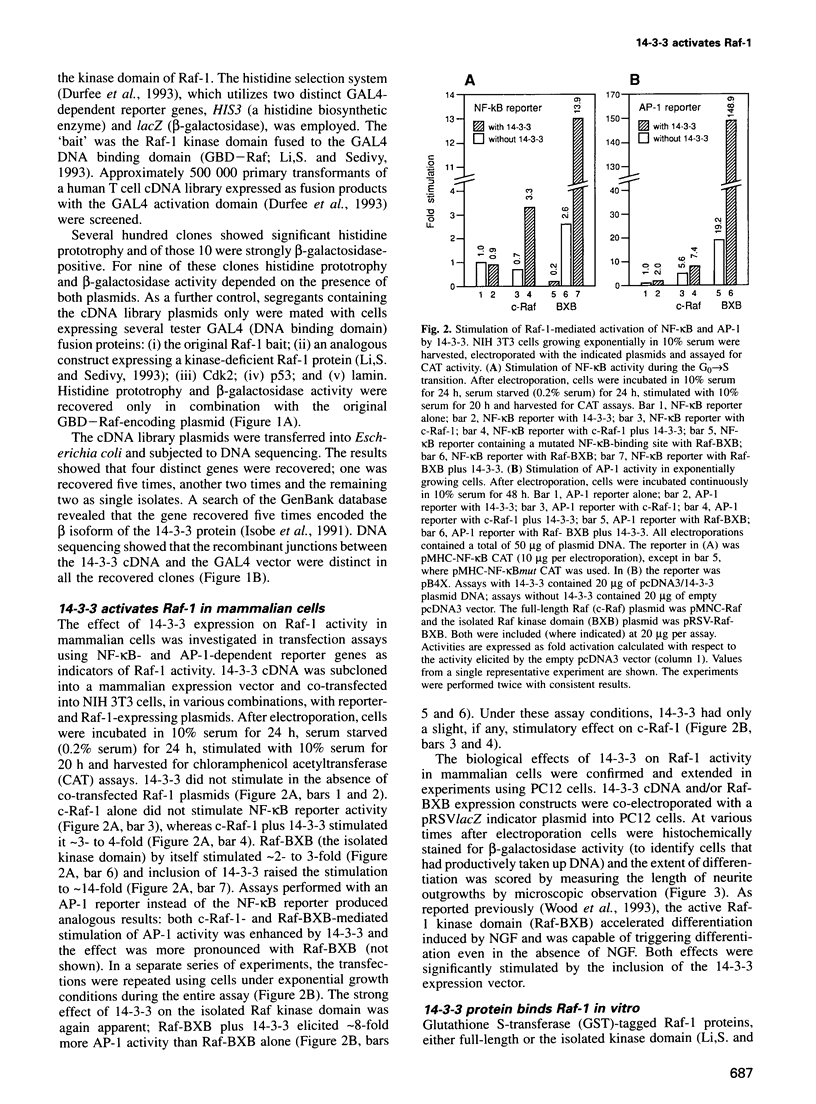
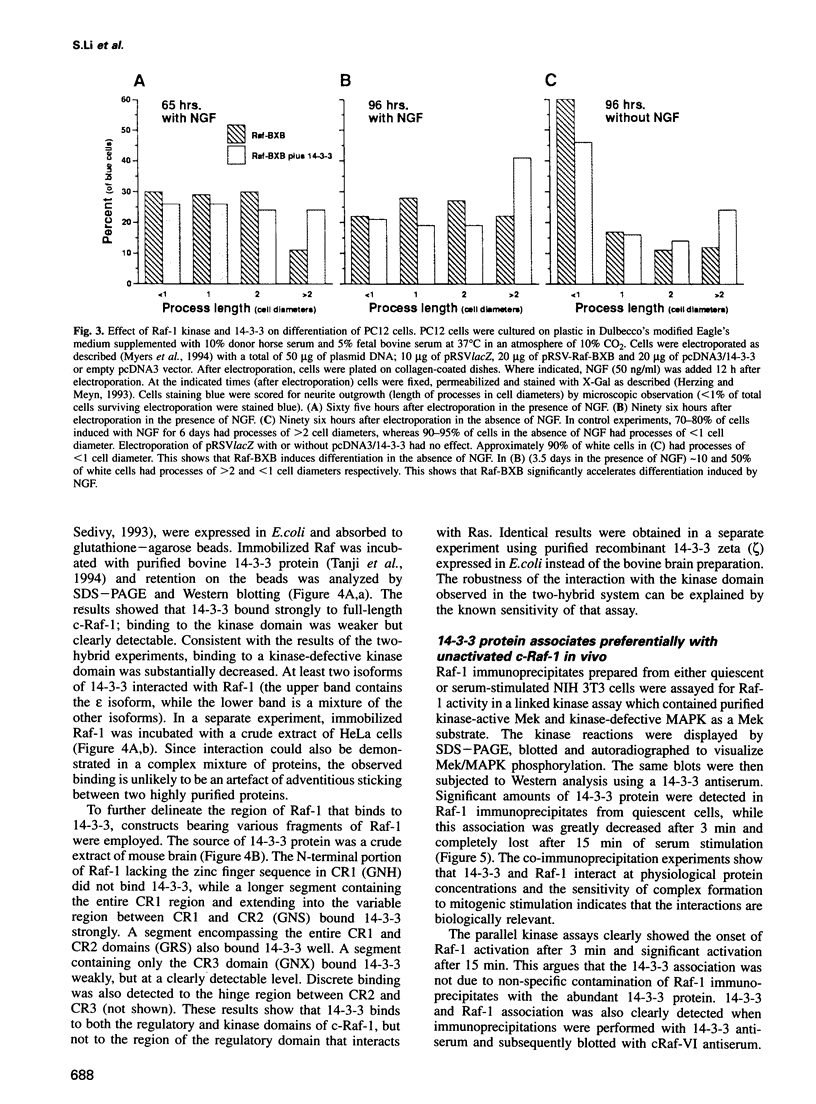







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Collinge D. B., van Heusden B. P., Isobe T., Roseboom P. H., Rosenfeld G., Soll J. 14-3-3 proteins: a highly conserved, widespread family of eukaryotic proteins. Trends Biochem Sci. 1992 Dec;17(12):498–501. doi: 10.1016/0968-0004(92)90339-b. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Azizkhan J. C., Jensen D. E., Beg A. A., Coodly L. R. Induction of NF-kappa B DNA-binding activity during the G0-to-G1 transition in mouse fibroblasts. Mol Cell Biol. 1991 Oct;11(10):4943–4951. doi: 10.1128/mcb.11.10.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder J. T., Heidecker G., Rapp U. R. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992 Apr;6(4):545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- Carroll M. P., May W. S. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J Biol Chem. 1994 Jan 14;269(2):1249–1256. [PubMed] [Google Scholar]
- Chien C. T., Bartel P. L., Sternglanz R., Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Dent P., Sturgill T. W. Activation of (His)6-Raf-1 in vitro by partially purified plasma membranes from v-Ras-transformed and serum-stimulated fibroblasts. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9544–9548. doi: 10.1073/pnas.91.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P., Wu J., Romero G., Vincent L. A., Castle D., Sturgill T. W. Activation of the mitogen-activated protein kinase pathway in Triton X-100 disrupted NIH-3T3 cells by p21 ras and in vitro by plasma membranes from NIH 3T3 cells. Mol Biol Cell. 1993 May;4(5):483–493. doi: 10.1091/mbc.4.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee T., Becherer K., Chen P. L., Yeh S. H., Yang Y., Kilburn A. E., Lee W. H., Elledge S. J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993 Apr;7(4):555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Fabian J. R., Daar I. O., Morrison D. K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993 Nov;13(11):7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finco T. S., Baldwin A. S., Jr Kappa B site-dependent induction of gene expression by diverse inducers of nuclear factor kappa B requires Raf-1. J Biol Chem. 1993 Aug 25;268(24):17676–17679. [PubMed] [Google Scholar]
- Force T., Bonventre J. V., Heidecker G., Rapp U., Avruch J., Kyriakis J. M. Enzymatic characteristics of the c-Raf-1 protein kinase. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1270–1274. doi: 10.1073/pnas.91.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J. C., al-Khodairy F., Fotou E., Sheldrick K. S., Griffiths D. J., Carr A. M. 14-3-3 protein homologs required for the DNA damage checkpoint in fission yeast. Science. 1994 Jul 22;265(5171):533–535. doi: 10.1126/science.8036497. [DOI] [PubMed] [Google Scholar]
- Freed E., Symons M., Macdonald S. G., McCormick F., Ruggieri R. Binding of 14-3-3 proteins to the protein kinase Raf and effects on its activation. Science. 1994 Sep 16;265(5179):1713–1716. doi: 10.1126/science.8085158. [DOI] [PubMed] [Google Scholar]
- Fu H., Coburn J., Collier R. J. The eukaryotic host factor that activates exoenzyme S of Pseudomonas aeruginosa is a member of the 14-3-3 protein family. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2320–2324. doi: 10.1073/pnas.90.6.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego C., Gupta S. K., Heasley L. E., Qian N. X., Johnson G. L. Mitogen-activated protein kinase activation resulting from selective oncogene expression in NIH 3T3 and rat 1a cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7355–7359. doi: 10.1073/pnas.89.16.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A. M., Vaillancourt R. R., Johnson G. L. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase by G protein and tyrosine kinase oncoproteins. J Biol Chem. 1993 Aug 25;268(24):17896–17901. [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Gallego C., Johnson G. L., Heasley L. E. MAP kinase is constitutively activated in gip2 and src transformed rat 1a fibroblasts. J Biol Chem. 1992 Apr 25;267(12):7987–7990. [PubMed] [Google Scholar]
- Hallberg B., Rayter S. I., Downward J. Interaction of Ras and Raf in intact mammalian cells upon extracellular stimulation. J Biol Chem. 1994 Feb 11;269(6):3913–3916. [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Huleihel M., Cleveland J. L., Kolch W., Beck T. W., Lloyd P., Pawson T., Rapp U. R. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol Cell Biol. 1990 Jun;10(6):2503–2512. doi: 10.1128/mcb.10.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Kölch W., Morrison D. K., Rapp U. R. The role of Raf-1 phosphorylation in signal transduction. Adv Cancer Res. 1992;58:53–73. doi: 10.1016/s0065-230x(08)60290-0. [DOI] [PubMed] [Google Scholar]
- Herzing L. B., Meyn M. S. Novel lacZ-based recombination vectors for mammalian cells. Gene. 1993 Dec 31;137(2):163–169. doi: 10.1016/0378-1119(93)90002-k. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Marais R., John S., Wynne J., Dalton S., Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993 Apr 23;73(2):395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- Hirsch S., Aitken A., Bertsch U., Soll J. A plant homologue to mammalian brain 14-3-3 protein and protein kinase C inhibitor. FEBS Lett. 1992 Jan 20;296(2):222–224. doi: 10.1016/0014-5793(92)80384-s. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Häfner S., Adler H. S., Mischak H., Janosch P., Heidecker G., Wolfman A., Pippig S., Lohse M., Ueffing M., Kolch W. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol. 1994 Oct;14(10):6696–6703. doi: 10.1128/mcb.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T., Isobe T., Okuyama T., Takahashi N., Araki K., Kuwano R., Takahashi Y. Molecular cloning of cDNA coding for brain-specific 14-3-3 protein, a protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7084–7088. doi: 10.1073/pnas.85.19.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T., Isobe T., Okuyama T., Yamauchi T., Fujisawa H. Brain 14-3-3 protein is an activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+,calmodulin-dependent protein kinase II. FEBS Lett. 1987 Jul 13;219(1):79–82. doi: 10.1016/0014-5793(87)81194-8. [DOI] [PubMed] [Google Scholar]
- Irie K., Gotoh Y., Yashar B. M., Errede B., Nishida E., Matsumoto K. Stimulatory effects of yeast and mammalian 14-3-3 proteins on the Raf protein kinase. Science. 1994 Sep 16;265(5179):1716–1719. doi: 10.1126/science.8085159. [DOI] [PubMed] [Google Scholar]
- Isobe T., Hiyane Y., Ichimura T., Okuyama T., Takahashi N., Nakajo S., Nakaya K. Activation of protein kinase C by the 14-3-3 proteins homologous with Exo1 protein that stimulates calcium-dependent exocytosis. FEBS Lett. 1992 Aug 17;308(2):121–124. doi: 10.1016/0014-5793(92)81257-m. [DOI] [PubMed] [Google Scholar]
- Isobe T., Ichimura T., Sunaya T., Okuyama T., Takahashi N., Kuwano R., Takahashi Y. Distinct forms of the protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. J Mol Biol. 1991 Jan 5;217(1):125–132. doi: 10.1016/0022-2836(91)90616-e. [DOI] [PubMed] [Google Scholar]
- Jamal S., Ziff E. Transactivation of c-fos and beta-actin genes by raf as a step in early response to transmembrane signals. Nature. 1990 Mar 29;344(6265):463–466. doi: 10.1038/344463a0. [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Lloyd P., Rapp U. R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991 Jan 31;349(6308):426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Kortenjann M., Thomae O., Shaw P. E. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol. 1994 Jul;14(7):4815–4824. doi: 10.1128/mcb.14.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Force T. L., Rapp U. R., Bonventre J. V., Avruch J. Mitogen regulation of c-Raf-1 protein kinase activity toward mitogen-activated protein kinase-kinase. J Biol Chem. 1993 Jul 25;268(21):16009–16019. [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Leevers S. J., Paterson H. F., Marshall C. J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994 Jun 2;369(6479):411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- Leffers H., Madsen P., Rasmussen H. H., Honoré B., Andersen A. H., Walbum E., Vandekerckhove J., Celis J. E. Molecular cloning and expression of the transformation sensitive epithelial marker stratifin. A member of a protein family that has been involved in the protein kinase C signalling pathway. J Mol Biol. 1993 Jun 20;231(4):982–998. doi: 10.1006/jmbi.1993.1346. [DOI] [PubMed] [Google Scholar]
- Li P., Wood K., Mamon H., Haser W., Roberts T. Raf-1: a kinase currently without a cause but not lacking in effects. Cell. 1991 Feb 8;64(3):479–482. doi: 10.1016/0092-8674(91)90228-q. [DOI] [PubMed] [Google Scholar]
- Li S., Sedivy J. M. Raf-1 protein kinase activates the NF-kappa B transcription factor by dissociating the cytoplasmic NF-kappa B-I kappa B complex. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9247–9251. doi: 10.1073/pnas.90.20.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald S. G., Crews C. M., Wu L., Driller J., Clark R., Erikson R. L., McCormick F. Reconstitution of the Raf-1-MEK-ERK signal transduction pathway in vitro. Mol Cell Biol. 1993 Nov;13(11):6615–6620. doi: 10.1128/mcb.13.11.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Martens G. J., Piosik P. A., Danen E. H. Evolutionary conservation of the 14-3-3 protein. Biochem Biophys Res Commun. 1992 May 15;184(3):1456–1459. doi: 10.1016/s0006-291x(05)80046-4. [DOI] [PubMed] [Google Scholar]
- Moodie S. A., Willumsen B. M., Weber M. J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993 Jun 11;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Exo1 and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature. 1992 Feb 27;355(6363):833–836. doi: 10.1038/355833a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Heidecker G., Rapp U. R., Copeland T. D. Identification of the major phosphorylation sites of the Raf-1 kinase. J Biol Chem. 1993 Aug 15;268(23):17309–17316. [PubMed] [Google Scholar]
- Pallas D. C., Fu H., Haehnel L. C., Weller W., Collier R. J., Roberts T. M. Association of polyomavirus middle tumor antigen with 14-3-3 proteins. Science. 1994 Jul 22;265(5171):535–537. doi: 10.1126/science.8036498. [DOI] [PubMed] [Google Scholar]
- Prasad G. L., Valverius E. M., McDuffie E., Cooper H. L. Complementary DNA cloning of a novel epithelial cell marker protein, HME1, that may be down-regulated in neoplastic mammary cells. Cell Growth Differ. 1992 Aug;3(8):507–513. [PubMed] [Google Scholar]
- Rapp U. R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991 Apr;6(4):495–500. [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D., Grover-Bardwick A., Heidecker G., Rapp U. R., Karin M. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992 Aug;12(8):3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Macdonald S. G., Cadwallader K., Symons M., Hancock J. F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994 Jun 3;264(5164):1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- Sözeri O., Vollmer K., Liyanage M., Frith D., Kour G., Mark G. E., 3rd, Stabel S. Activation of the c-Raf protein kinase by protein kinase C phosphorylation. Oncogene. 1992 Nov;7(11):2259–2262. [PubMed] [Google Scholar]
- Tanji M., Horwitz R., Rosenfeld G., Waymire J. C. Activation of protein kinase C by purified bovine brain 14-3-3: comparison with tyrosine hydroxylase activation. J Neurochem. 1994 Nov;63(5):1908–1916. doi: 10.1046/j.1471-4159.1994.63051908.x. [DOI] [PubMed] [Google Scholar]
- Thompson P. A., Ledbetter J. A., Rapp U. R., Bolen J. B. The Raf-1 serine-threonine kinase is a substrate for the p56lck protein tyrosine kinase in human T-cells. Cell Growth Differ. 1991 Dec;2(12):609–617. [PubMed] [Google Scholar]
- Toker A., Sellers L. A., Amess B., Patel Y., Harris A., Aitken A. Multiple isoforms of a protein kinase C inhibitor (KCIP-1/14-3-3) from sheep brain. Amino acid sequence of phosphorylated forms. Eur J Biochem. 1992 Jun 1;206(2):453–461. doi: 10.1111/j.1432-1033.1992.tb16946.x. [DOI] [PubMed] [Google Scholar]
- Troppmair J., Bruder J. T., Munoz H., Lloyd P. A., Kyriakis J., Banerjee P., Avruch J., Rapp U. R. Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation by oncogenes, serum, and 12-O-tetradecanoylphorbol-13-acetate requires Raf and is necessary for transformation. J Biol Chem. 1994 Mar 4;269(9):7030–7035. [PubMed] [Google Scholar]
- Van Aelst L., Barr M., Marcus S., Polverino A., Wigler M. Complex formation between RAS and RAF and other protein kinases. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B., Heidecker G., Huleihel M., Rapp U. R. Expression of raf oncogenes activates the PEA1 transcription factor motif. Mol Cell Biol. 1989 May;9(5):2247–2250. doi: 10.1128/mcb.9.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. W., Qi H., D'Arcangelo G., Armstrong R. C., Roberts T. M., Halegoua S. The cytoplasmic raf oncogene induces a neuronal phenotype in PC12 cells: a potential role for cellular raf kinases in neuronal growth factor signal transduction. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5016–5020. doi: 10.1073/pnas.90.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993 Jul 22;364(6435):308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]
- Zupan L. A., Steffens D. L., Berry C. A., Landt M., Gross R. W. Cloning and expression of a human 14-3-3 protein mediating phospholipolysis. Identification of an arachidonoyl-enzyme intermediate during catalysis. J Biol Chem. 1992 May 5;267(13):8707–8710. [PubMed] [Google Scholar]