Abstract
A growing number of medically compromised patients are encountered by dentists in their practices. Opportunistic fungal infections such as mucormycosis usually occur in immunocompromised patients but can infect healthy individuals as well. Mucormycosis is an acute opportunistic, uncommon, frequently fatal fungal infection, caused by a saprophytic fungus that belongs to the class of phycomycetes. Among the clinical differential diagnosis we can consider squamous cell carcinoma. Such cases present as chronic ulcers with raised margins causing exposure of underlying bone. There is a close histopathological resemblance between mucormycosis and aspergillosis. Microscopically, aspergillosis has septate branching hyphae, which can be distinguished from mucormycotic hyphae by a smaller width and prominent acute angulations of branching hyphae. A definitive diagnosis of mucormycosis can be made by tissue biopsy that identifies the characteristic hyphae, by positive culture or both. The culture of diseased tissue may be negative and histopathologic examination is essential for early diagnosis. Mucormycosis was long regarded as a fatal infection with poor prognosis. However with early medical and surgical management survival rates are now thought to exceed 80%. In the present case, the fungus was identified by hematoxylin and eosin stain and confirmed by Grocott's silver methenamine special staining technique. Removal of the necrotic bone, which acted as a nidus of infection, was done. Post-operatively patient was advised an obturator to prevent oronasal regurgitation. Since mucormycosis occurs infrequently, it may pose a diagnostic and therapeutic dilemma for those who are not familiar with its clinical presentation.
Key words: fungal infection, mucormycosis, necrotic bone, phycomycetes, squamous cell carcinoma.
Introduction
Mucormycosis is ubiquitous in nature and humans usually have a strong natural resistance to the infection. Mucormycosis becomes pathogenic when the patient's general resistance has been altered by metabolic disorders, immunosuppressive therapy, malignancy or other chronic debilitating disorders.1–3 An underlying disease, frequently diabetes mellitus, is almost always present.4
The organisms are common inhabitants of soil, bread mold and rotten fruit and vegetables and constantly release spores into the atmosphere.5,6 The disease has a worldwide distribution without age or race preference.7 Rhinocerebral form of mucormycosis frequently presents with oral manifestations and may lead to considerable dilemma in clinicians unfamiliar with this entity, which in turn may worsen the prognosis for the patient.8
The maxilla rarely undergoes necrosis due to its rich vascularity. Maxillary necrosis can occur due to bacterial infections such as osteomyelitis, viral infections such as herpes zoster or fungal infections such as mucormycosis, aspergillosis etc.9 Location of mucormycosis on the palate is a rare and late occurance.10 Early diagnosis and treatment of mucormycosis is extremely important. The diagnosis is confirmed by histopathologic demonstration of the organism in affected tissue.11 The present case report is of mucormycosis of the palate presenting as a chronic ulcer with raised margins causing exposure of underlying bone. The aim of this report is to alert the clinicians to be aware of mucormycosis on the importance of early diagnosis and management.
Case Report
A 50-year-old male patient was referred to the Oral and Maxillofacial Surgeon for a progressive non-healing wound with a foul odor and ulceration of the palate and left maxilla of about 2 months duration. The patient had undergone for extraction of left maxillary lateral incisor and canine 3 months earlier due to poor periodontal status. Following extractions the socket never healed completely. Later, he developed headache, sinusitis and noticed a non-healing ulcer on the palate. The patient was moderately built and nourished with normal gait. He also gave a medical history of uncontrolled diabetes mellitus and hypertension. Over the past 2 months the patient was advised various antibiotics and analgesics by different dental practitioners as well as general physicians, and had very limited benefit. The patient also gave a history of tobacco chewing from past 15 years.
Extraorally no swelling was noted and the overlying skin appeared normal with no local rise in temperature or any other secondary changes.
On intraoral examination, an infiltrating ulcer approximately 3×4.5 cm2 with irregular borders was appreciated over the hard palate. Ulcer was covered over by the necrotic slough and on the anterior part of the ulcer; the underlying bone was also exposed. Ulcer was nontender with signs of erythema over the margins (Figure 1). Maxillary left lateral incisor and canine were missing.
Figure 1.
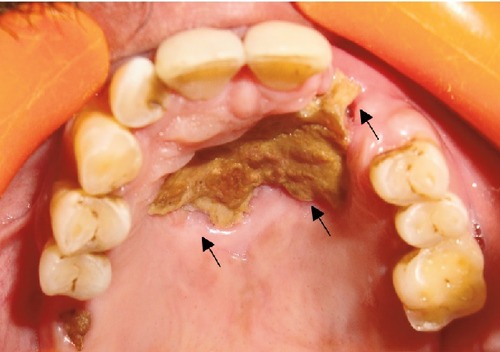
Clinical photograph showing ulcer with necrosis in the anterior palate extending to alveolar bone of left lateral incisor and canine (arrows showing the necrotic bone).
A provisional diagnosis of squamous cell carcinoma was made. The differential diagnosis of midline lethal granuloma and fungal infection were thought.
Later the patient was sent for blood investigations, which revealed raised ESR (34 mm/1st hour) and random blood glucose level 460 mg/100mL. Tridot, HBs test and chest X ray were non-contributory. The computed tomography (CT) scan revealed focal destruction of the bone of anterior palate and in the left anterior maxillary region (Figure 2).
Figure 2.
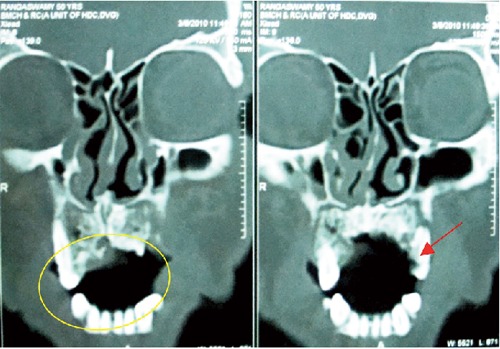
Computed tomography scan showing focal destruction of the bone of anterior palate (circled area) to the left anterior maxillary region (arrow).
The patient underwent a partial maxillectomy (Figure 3) and the specimen was creamish white, non-vital, measured about 5×3 cm with rough surface (Figure 4). Microscopic examination of the specimen revealed bony spicules and dead lamellated bone around the fatty marrow showing ischemic necrosis. Numerous non-septate, broad, branching hyphae were seen within the necrotic marrow tissue (Figures 5 and 6). Thus, a final diagnosis of mucormycosis was arrived. A strict diabetic control and a course of amphotericin B were advised. He was rehabilitated with an obturator for the oro-nasal fistula (Figures 7 and 8) and was being followed up for the past 6 months and has no further complaints.
Figure 3.
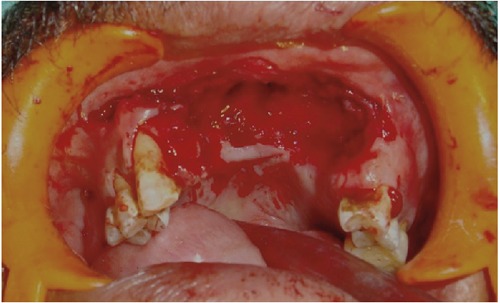
Intra-operative image showing the area after the removal of necrotic bone.
Figure 4.
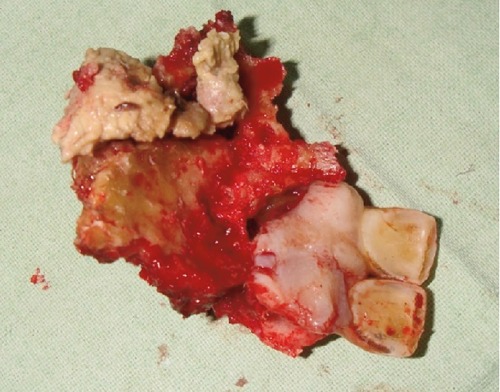
Photograph showing superior view of surgical specimen with involved teeth.
Figure 5.
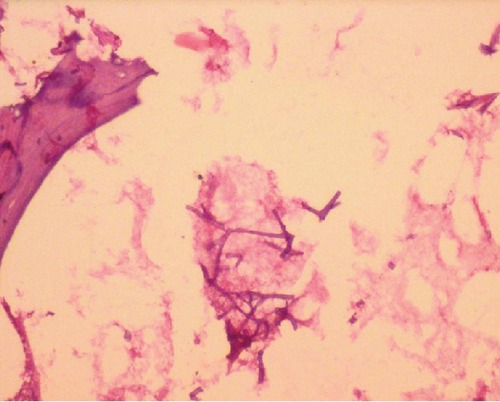
Microphotograph showing non-septate branched fungal hyphae in a necrotic tissue (H & E stain, Original magnification ×250).
Figure 6.
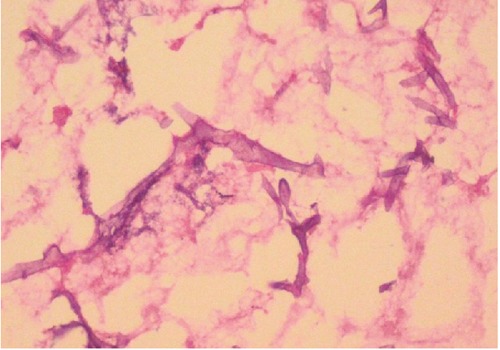
Microphotographs showing non-septate, broad, branched fungal hyphae (H & E stain, Original magnification ×400).
Figure 7.
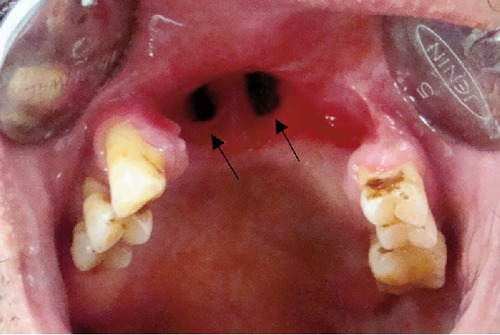
Post-operative photograph showing the oro-nasal fistula (arrows).
Figure 8.

Photograph showing the obturator for the oro-nasal fistula.
Discussion
Mucormycosis is a saprophytic aerobic fungus commonly found in the environment in bread moulds or decaying vegetation. This organism is frequently found to colonize the oral mucosa, nasal mucosa, paranasal sinuses and pharyngeal mucosa of asymptomatic patients. These fungi do not usually cause disease in healthy people with intact immune systems, but patients with a number of conditions can be predisposed to the development of invasive fungal disease.12,13
In 1885, Paltauf14 documented the first histologic description of generalized mucormycosis in a 52-year-old patient. Since then, opportunistic infections caused by fungi of the order Mucorales have been recognized in association with diabetes, hematologic malignant diseases, immunosuppressive therapy, thermal burns and surgery.15
Infection arises through inhalation of spores and contamination of the traumatized tissue, ingestion and direct inoculation.15,16 The hallmarks of disease caused by these organisms are angioinvasion, thrombosis, ischemia and necrosis of involved tissue.17–19
High incidence of mucormycosis is seen in uncontrolled diabetic patients, because they produce the enzyme ketoreductase, which allows them to utilize the patient's ketone bodies. It is also likely that the hyperglycemia stimulates fungal growth and the reduced chemotaxis and phagocytic efficiency permit innocuous organisms to proliferate.4
Oral manifestations of mucormycosis are frequently the first clinical signs to arise, probably because of the highly vascularized structure of oral soft tissues. Furthermore, it has been suggested that vascular ruptures and bleeding due to dental extractions may create a portal of entry for fungi into the maxillofacial regions.5,20 Intraorally, the hard palate is usually affected because of its proximity to the infection of the nasal fossa and paranasal sinuses. In addition, isolated intraoral involvement is extremely rare.21
The diagnosis of mucormycosis is usually based on a clinical picture revealing the invasive course of the disease and is confirmed by biopsy, where the specimen will show broad, non-septate hyphae with branching at right angles and invading tissue.1 However, negative culture results often appear despite positive histologic findings.22
Differential diagnosis of destructive lesions of the palate and maxilla should include squamous cell carcinoma, nasopharyngeal carcinoma, peripheral T-cell lymphoma (formerly termed midline lethal granuloma), chronic granulomatous diseases like tuberculosis, tertiary syphilis, wegener's granulomatosis and other deep fungal infection.4,8,9,18,22
The present case was provisionally diagnosed as squamous cell carcinoma of the palate which presented as chronic necrotizing ulcer with raised margins and exposure of the underlying bone. Similar cases are reported in the literature by few authors recently.23,24
On the contrary, a recent report suggests that mucormycosis confined to the oral tissues without any systemic involvement is very rare. When mucormycosis presents as an isolated intraoral infection, the ulcers may also have a white necrotic-apperaring tissue without black scab which may be due to a necrotic infarction of the palate. The lesion will be usually deep and causes denudation of the palate along with osteomyelitis of the affected bones.18,25,26 In such situations; the differential diagnoses should also include acute necrotizing gingivitis or periodontitis, herpetic infections and traumatic ulcers. In more severe cases, the extensive mucormycosis may resemble malignancies, cancrum oris, pyogenic orbital cellulitis and other systemic mycoses.27
Treatment of mucormycosis consists of surgical debridement; systemic antifungal therapy and treatment of any underlying condition are most effective methods.1,5,21 Control and prevention of opportunistic fungal infection in patients suffering from debilitating diseases such as diabetic ketoacidosis, immunodepression, blood dyscrasia, solid organ transplant, patients on long term steroids and bone marrow transplant is very important. Once mucormycosis infection diagnosed in debilitated patients, it must be treated proptly, without any delay, by different modalities, medically and surgically.
Conclusions
Mucormycosis is a life-threatening fungal infection that frequently occurs in immunocompromised patients. These infections are becoming increasingly common, yet survival remains poor. Since mucormycosis occurs rarely in the oral cavity, it may pose a diagnostic and therapeutic dilemma for those who are not familiar with its clinical presentation. A greater understanding of the pathogenesis of the disease may lead to future therapies. Early diagnosis and management of the lesion will localize and lessen the chances of spreading to the adjacent tissue and may have great advantages.
The case presented here emphasizes the concept that simple procedures such as dental extractions can cause cataclysmic complications in susceptible patients. In the present scenario, dental professionals may encounter patients whose general health is compromised. We must remain cautious in our efforts to perform procedures and follow such patients to be certain that appropriate healing occurs.
References
- 1.Ruoppi P, Dietz A, Nikanne E, et al. Paranasal sinus mucormycosis: a report of two cases. Acta Otolaryngol. 2001;121:948–52. [PubMed] [Google Scholar]
- 2.Ketenci I, Unlü Y, Sentürk M, Tuncer E. Indolent mucormycosis of the sphenoid sinus. Otolaryngol Head Neck Surg. 2005;132:341–2. doi: 10.1016/j.otohns.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya AK, Deshpande AR, Nayak SR, et al. Rhinocerebral mucormycosis: an unusual case presentation. J Laryngol Otol. 1992;106:48–9. doi: 10.1017/s0022215100118584. [DOI] [PubMed] [Google Scholar]
- 4.Marx RE, Stern D. 1st ed. Hanover Park, IL: Quintessence Publishing Co, Inc; 2006. Oral and maxillofacial pathology: a rationale for diagnosis and treatment; pp. 104–106. [Google Scholar]
- 5.Salisbury PL, 3rd, Caloss R, Jr, Cruz JM, et al. Mucormycosis of the mandible after dental extractions in a patient with acute myelogenus leukemia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:340–4. doi: 10.1016/s1079-2104(97)90240-7. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Fortson JK, Cook HE. A fatal outcome from rhinocerebral mucormycosis after dental extractions: a case report. J Oral Maxillofac Surg. 2001;59:693–7. doi: 10.1053/joms.2001.23407. [DOI] [PubMed] [Google Scholar]
- 7.Yousem DM, Galetta SL, Gusnard DA, Goldberg HI. MR findings in rhinocerebral mucormycosis. J Comput Assist Tomogr. 1989;13:878–82. doi: 10.1097/00004728-198909000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Shetty SR, Punnya VA. Palatal mucormycosis:a rare clinical dilemma. Oral Surg. 2008;1:145–48. [Google Scholar]
- 9.Auluck A. Maxillary necrosis by mucormycosis. a case report and literature review. Med Oral Patol Oral Cir Bucal. 2007;12:E360–4. [PubMed] [Google Scholar]
- 10.Kyrmizakis DE, Doxas PG, Hajiioannou JK, Papadakis CE. Palate ulcer due to mucormycosis. J Laryngol Otol. 2002;116:146–7. doi: 10.1258/0022215021909917. [DOI] [PubMed] [Google Scholar]
- 11.Succar MB, Nichols RD, Burch KH. Rhinocerebral mucormycosis. Arch Otolaryngol. 1979;105:212–4. doi: 10.1001/archotol.1979.00790160046012. [DOI] [PubMed] [Google Scholar]
- 12.Bhansali A, Bhadada S, Sharma A, et al. Presentation and outcome of rhinoorbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80:670–4. doi: 10.1136/pgmj.2003.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNulty JS. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope. 1982;92:1140–3. [PubMed] [Google Scholar]
- 14.Jones AC, Bentsen TY, Freedman PD. Mucormycosis of the oral cavity. Oral Surg Oral Med Oral Pathol. 1993;75:455–60. doi: 10.1016/0030-4220(93)90170-9. [DOI] [PubMed] [Google Scholar]
- 15.Rosen PP. Opportunistic fungal infections in patients with neoplastic diseases. Pathol Annu. 1976;11:255–315. [PubMed] [Google Scholar]
- 16.Sugar AM. Mucormycosis. Clin Infect Dis. 1992;14(Suppl 1):S126–9. doi: 10.1093/clinids/14.supplement_1.s126. [DOI] [PubMed] [Google Scholar]
- 17.Tryfon S, Stanopoulos I, Kakavelas E, et al. Rhinocerebral mucormycosis in a patient with latent diabetes mellitus: a case report. J Oral Maxillofac Surg. 2002;60:328–30. doi: 10.1053/joms.2002.30600. [DOI] [PubMed] [Google Scholar]
- 18.Jayachandran S, Krithika C. Mucor-mycosis presenting as palatal perforation. Indian J Dent Res. 2006;17:139–42. doi: 10.4103/0970-9290.29873. [DOI] [PubMed] [Google Scholar]
- 19.Damante JH, Fleury RN. Oral and rhinoorbital mucormycosis: case report. J Oral Maxillofac Surg. 1998;56:267–71. doi: 10.1016/s0278-2391(98)90883-7. [DOI] [PubMed] [Google Scholar]
- 20.Spellberg B, Walsh TJ, Kontoyiannis DP, et al. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48:1743–51. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samaranayake LP, Cheung LK, Samaranayake YH. Candidiasis and other fungal diseases of the mouth. Dermatol Ther. 2002;15:252–70. [Google Scholar]
- 22.Breiman A, Sadowsky D, Friedman J. Mucormycosis. Discussion and report of a case involving the maxillary sinus. Oral Surg Oral Med Oral Pathol. 1981;52:375–8. doi: 10.1016/0030-4220(81)90333-9. [DOI] [PubMed] [Google Scholar]
- 23.Bonifaz A, Macias B, Paredes-Farrera F, et al. Palatal zygomycosis: experience of 21 cases. Oral Dis. 2008;14:569–74. doi: 10.1111/j.1601-0825.2007.01433.x. [DOI] [PubMed] [Google Scholar]
- 24.Tugsel Z, Sezer B, Akalin T. Facial swelling and palatal ulceration in a diabetic patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:630–6. doi: 10.1016/j.tripleo.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Hauman CH, Raubenheimer EJ. Orofacial mucormycosis. Oral Surg Oral Med Oral Pathol. 1989;68:624–7. doi: 10.1016/0030-4220(89)90251-x. [DOI] [PubMed] [Google Scholar]
- 26.Berger CJ, Disque FC, Topazian RG. Rhinocerebral mucormycosis: diagnosis and treatment. Report of two cases. Oral Surg Oral Med Oral Pathol. 1975;40:27–33. doi: 10.1016/0030-4220(75)90339-4. [DOI] [PubMed] [Google Scholar]
- 27.Dogan MC, Leblebisatan G, Haytac MC, et al. Oral mucormycosis in children with leukemia: report of 2 cases. Quintessence Int. 2007 Jun;38:515–20. [PubMed] [Google Scholar]


