Abstract
Infection with cryptococcal meningitis is uncommon in immunocompetent patients. The major virulence factor is the polysaccharide capsule, while nonencapsulated mutants are generally considered nonpathogenic. The authors present a case of hydrocephalus caused by meningitis from an indolent, nonencapsulated Cryptococcus sp. requiring placement and multiple revisions of a ventriculoperitoneal shunt (VPS). The patient presented with progressively worsening occipital headaches. Computed tomography and magnetic resonance imaging showed significant hydrocephalus with no apparent cause. Her symptoms initially resolved after placement of a VPS, but returned four months later. Cultures of the shunt tubing and cerebrospinal fluid (CSF) showed no bacterial infection. When the symptoms failed to resolve, CSF fungal culture revealed Cryptococcus-like yeast, although the organisms were nonencapsulated, and the cryptococcal antigen was negative. After antibiotic therapy, the symptoms resolved. The unusual clinical presentation delayed the diagnosis, highlighting the importance of understanding the detection, diagnosis, and treatment of meningeal infections caused by C. neoformans.
Key words: Cryptococcus neoformans, hydrocephalus, ventriculoperitoneal shunt, meningitis.
Introduction
Cryptococcus neoformans is a budding, non-mycelial yeast that is a known cause of opportunistic infection in patients with human immunodeficiency virus (HIV) or other immunocompromised states such as malignancy or autoimmune disease. Worldwide, Cryptococcus is the most frequent cause of fungal meningitis. The encapsulated form of this organism has been shown to cause meningitis in immunocompromised hosts, but infection with the nonencapsulated form in an immunocompetent patient is rare and requires a high index of suspicion for correct diagnosis.1 Here we review the case of a young woman with a competent immune system who presented with hydrocephalus resulting from infection with a nonencapsulated species of Cryptococcus. Cerebrospinal fluid (CSF) fungal culture aided in the diagnosis after cryptococcal antigen testing came back with negative findings. The patient required shunt placement and multiple revisions along with long-term antifungal therapy. Although infection with C. neoformans can cause serious and permanent neurological deficits, treatment with antifungal therapy and shunting if deemed necessary can lead to improvement even if the infection is not discovered at initial presentation. However, diagnosis of nonencapsulated cryptococcal meningitis requires high clinical suspicion as routine fungal antigen testing does not always reveal the offending organism.
Case Report
A 27-year-old woman presented to the emergency department with 3 weeks of progressively worsening occipital headaches preceded by flashes of light and worsened by noise, straining, and tension. The patient complained of nausea and vomiting for several days but denied parasthesias, weakness, or numbness. The patient's temperature was 35.5°C. There were no meningeal signs, and the results of a neurological examination were normal. No papilledema was noted on fundoscopic examination. The patient's symptoms prompted a computed tomography (CT) scan of the head, which showed significant hydrocephalus with dilation of the third, fourth, and lateral ventricles, and obliteration of the basilar cisterns (Figure. 1). After this scan, an external ventricular drain (EVD) was placed for CSF diversion, and the patient was found to have elevated intracranial pressures in the range of 20–30 cm H20. Magnetic resonance imaging (MRI) performed with and without contrast administration after EVD placement showed no clear underlying cause for hydrocephalus. A CSF sample showed 15 white blood cells/µL with 80% lymphocytes and only 5 red blood cells/µL. Protein and glucose levels were within normal limits, and CSF culture was negative for pathogens. This was suggestive of viral meningitis, which was thought to have precipitated the hydrocephalus.
Figure 1.
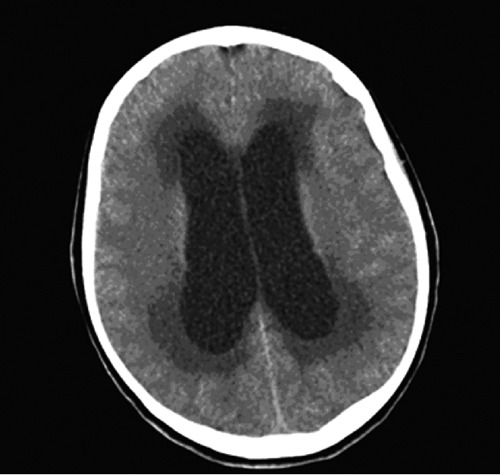
Computed tomography scan of the head without contrast enhancement demonstrating dilation of the lateral ventricles.
Because the patient continued to experience persistent headaches, she could not be weaned from the EVD, and she required placement of a right frontal ventriculoperitoneal shunt with a medium pressure valve. A head CT scan on postoperative day 1 showed decompression of the ventricles (Figure 2). A repeat MRI with and without contrast enhancement showed no associated mass lesion. At the time of discharge three days later, the patient was asymptomatic. One month after shunt placement, she reported good resolution of her headaches.
Figure 2.
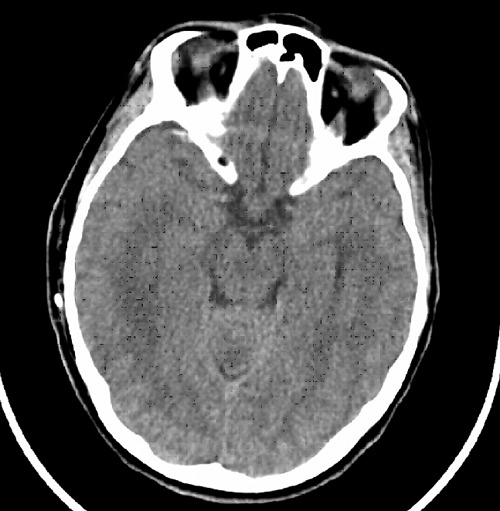
Postoperative computed tomography scan without contrast enhancement showing decompression of the ventricles.
The patient returned to the emergency room four months after surgery complaining of three days of headaches without blurred vision, photophobia, numbness, or weakness. Her temperature was 35.9°C. She also complained of weight loss and abdominal pain. A head CT showed ventricular enlargement (Figure 3), and an abdominal CT showed a loculated fluid collection associated with the tip of the peritoneal catheter. The patient underwent revision of the distal shunt catheter. The shunt tubing and CSF were cultured. The white blood cell count was noted to be 203/µL, but no bacterial infection was found. The total protein and glucose levels were within normal limits. No fungal cultures were obtained.
Figure 3.
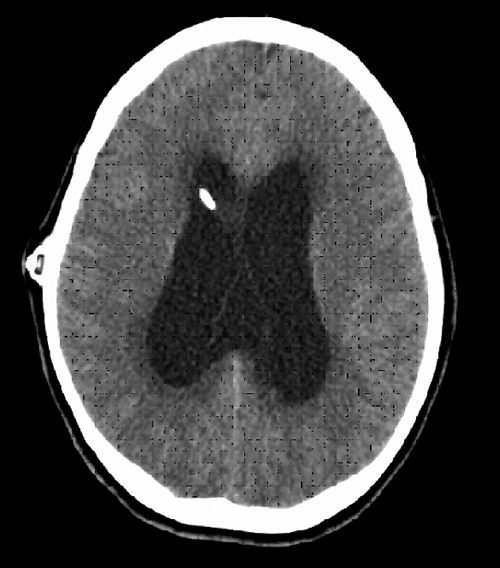
Computed tomography scan of the head without contrast enhancement demonstrating hydrocephalus.
Two weeks after catheter revision, the patient's headaches had again largely resolved, but she now complained of double vision and problems with coordination. On examination, she demonstrated nystagmus in all directions and dysmetria on finger-to-nose testing. She also demonstrated marked gait instability, especially with tandem walk and Romberg testing. A head CT revealed hydrocephalus with moderate dilation of all ventricles. An abdominal CT showed another pseudocyst surrounding the distal end of the shunt catheter.
At this time, the decision was made to externalize the shunt and to obtain cultures from both the cyst and the CSF to rule out shunt infection as a cause for the recurrent pseudocyst. CSF was sent for fungal culture, and direct microscopic analysis revealed Cryptococcus-like yeast, although the organisms seen were nonencapsulated, and the cryptococcal antigen was negative. Final cultures ultimately revealed infection with C. neoformans as the most likely cause of the hydrocephalus and the shunt malfunctions. The CSF white blood cell count at this time was 2/µL with normal lymphocyte subpopulations, and the total protein level was again within normal limits. A complete immune work-up, including HIV serologies, was negative. The infectious disease service was consulted, and a two-week course of amphotericin B and flucytosine was begun, followed by a six-week course of fluconazole.
One month after shunt externalization, the patient underwent placement of a left ventriculoperitoneal shunt. She tolerated this procedure extremely well, and on follow-up a month later reported few headaches and a gradual diminishment of her other neurological symptoms, particularly the blurred vision. MRI scan performed at this follow-up visit showed her ventricles to be decreased in size.
Discussion
This case demonstrates a rare example of an immunocompetent patient who was found to have an infection with a nonencapsulated form of C. neoformans resulting in hydrocephalus necessitating shunt placement. Although no structural lesion was seen on contrasted imaging scans, it is possible that the indolent nature of this infection and circulating organisms in the CSF caused dysfunction of the arachnoid villi and impaired CSF absorption, resulting in ventricular dilation. Additionally, the presence of dilated lateral, third, and fourth ventricles on the CT scans could imply some scarring of the subarachnoid spaces surrounding the brain stem due to chronic C. neoformans colonization, resulting in the obstructive tetraventricular hydrocephalus seen in our patient. Chronic infection around the brain stem with hydrocephalus stemming from infection with C. neoformans was recently reported by Sancesario et al.,2 although the patient in their report was infected with the encapsulated form of this organism. Despite the indolent nature of the infection, shunt malfunctions occurred because of ongoing colonization, and this ultimately resulted in the correct diagnosis of the infection. However, failure to recognize this nonencapsulated variant resulted in a delay in initiating treatment. Earlier diagnosis of this entity is vital to prevent excess morbidity associated with fungal meningitis caused by C. neoformans.
The virulence of C. neoformans is mediated predominantly by a polysaccharide capsule surrounding its cell wall,1 which provides a physical barrier that interferes with normal phagocytosis by macrophages and clearance by the immune system. The C. neoformans capsule inhibits the production of proinflammatory cytokines and reduces leukocyte migration to sites of inflammation.1 The capsule also constitutes the major diagnostic feature of Cryptococcus, as it can be visualized with light microscopy using India ink staining in 50–70% of cases. Detection of the capsular antigen in CSF by latex agglutination tests is also used and is positive in 90% of cases. CSF findings are consistent with lymphocytic meningitis, with generally <150 white cells, elevated protein, and decreased glucose. As the capsule appears to be the main virulence factor of this organism, reports of meningeal infection with the nonencapsulated form are rare.3 Predisposing factors for the non-encapsulated variant of C. neoformans appear to be similar to those of the encapsulated form and include HIV infection, post-transplant immunocompromise, exogenous corticosteroid therapy, sarcoidosis, and collagen vascular diseases. In addition to fungal meningitis, there have also been isolated case reports of nonencapsulated Cryptococcus causing pulmonary infection in an immunocompetent host.4 Symptoms are usually consistent with an indolent pneumonia. The symptoms of cryptococcal meningitis include headache, mental status changes, cranial nerve abnormalities (optic atrophy, extraocular palsy, hearing loss), meningeal signs, and fever. Infection with C. neoformans should be considered in an immunocompetent patient if any of these symptoms are present and other more common causes of central nervous system (CNS) infection (e.g., bacterial meningitis) have been excluded.
Capsule-deficient Cryptococcus poses a treatment challenge, as routine CSF examination often fails to reveal infection with this organism. One possible explanation is that capsule-deficient Cryptococcus is susceptible to phagocytosis and may be engulfed by macrophages before it reaches the CSF space.5 In the case of our patient, yeast forms were eventually seen on CSF culture, although only after repeated shunt malfunctions and multiple CSF analyses. The latex agglutination test is not useful in the case of capsule-deficient Cryptococcus, as this test detects capsular polysaccharide antigens with the antibody affixed to latex particles.5 Nonencapsulated cultured cells will stain positive with indirect immunofluorescence using diluted sera from another patient diagnosed with cryptococcal meningitis.5 Additionally, inoculation of colonies cultured from human CSF into mice has been shown to result in formation of capsular C. neoformans after approximately 2 weeks.5 When cryptococcal meningitis is suspected, a high index of suspicion is needed for the capsule-deficient variant, as routine diagnostic testing may fail to reveal the presence of this organism.
Several other reasons for false-negative cryptococcal antigen testing have been proposed by To et al.6 A low level of antigen production by the Cryptococcus organism may lead to a false-negative result, and in fact the same result can occur with a very high antigen level. In the latter case, the prozone effect results in masking of the presence of a particular antigen because of a larger aggregate of nonbinding antibodies. The prozone phenomenon can be averted by performing very high dilutions to determine the presence of the specific antigen in question.6 Another proposed reason for a false-negative antigen test is the small-colony variant of C. neoformans, which is also less susceptible to antibiotics and more prone to relapse.6
Cryptococcus can be treated with amphotericin, at a dose of 0.4 to 0.6 mg/kg/day for 6 weeks. If this treatment does not produce a response, 5-fluorocytosine (150 mg/kg/day) can be added along with 0.3 to 0.4 mg/kg of amphotericin. Up to 70% of patients respond to more than one course of treatment,7 while the remainder relapse before they are subsequently cured. The mortality rate of cryptococcal meningitis approaches 35%, while one third to one half of patients may develop permanent neurological deficits.
Both cystic and granulomatous lesions have been identified.8 The cystic lesions are actually pseudocysts, as they lack a capsule and contain thick gelatinous or mucoid material. Surrounding edema may or may not be present. Intraventricular lesions favor the choroid plexus.8
Hydrocephalus is a known complication of chronic fungal meningitis. Symptoms, including decreased mental acuity and gait ataxia, are often attributed to the underlying infection. In addition, while the indications for shunting in cryptococcal infection are not well understood, most groups suggest early shunt placement to avoid permanent neurological damage.7 This advice is not universal, however, as other groups have suggested that shunting should be delayed until after the infection is controlled to avoid shunt malfunction or dissemination of the infection via the shunt.9 In our patient, early shunting was performed, although the lack of a diagnosis for the symptoms led to multiple shunt malfunctions before the C. neoformans infection was discovered. In retrospect, had fungal cultures been obtained at the time of initial presentation, the shunt revisions may have been avoided. Fortunately, although the patient required nearly six months of treatment and several shunt revisions, she did experience sustained neurological improvement. This implies that while infection with C. neoformans can cause permanent neurological sequelae, treatment with antifungal therapy and/or shunting can lead to improvement even if there is a delay in identifying the presence of infection with this organism.
Conclusions
Capsule-deficient C. neoformans is a rare cause of fungal meningitis in an immunocompetent host. False-negative cryptococcal antigen testing can occur for a number of different reasons and may result in treatment delay. Although uncommon, hydrocephalus is a known complication of infection with C. neoformans. It is crucial that management of patients with cryptococcal meningitis include careful observation for neurological symptoms that may indicate hydrocephalus. It is also important to recognize that not all patients infected with this organism have impaired immune function and that CSF fungal culture may be a valuable diagnostic tool in the case of persistent, unexplained hydrocephalus in an immunocompetent host. Whether or not CSF evaluation for fungus should be performed on all patients with unexplained hydrocephalus undergoing shunt placement remains unresolved, but we note it would have been beneficial in this case. Although fungal CNS infection with nonencapsulated C. neoformans is treatable with antifungal therapy and CSF diversion if necessary, clearly the difficulty in diagnosing capsule-deficient Cryptococcus requires a high index of suspicion by the clinician to prevent a lengthy delay between diagnosis and treatment.
Acknowledgments:
the authors would like to thank Kristin Kraus, M.Sc., for editorial assistance with this paper.
References
- 1.Buchanan KL, Murphy JW. What makes Cryptococcus neoformans a pathogen? Emerg Infect Dis. 1998;4:71–83. doi: 10.3201/eid0401.980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sancesario G, Palmieri G, Viola G, et al. Difficulty diagnosing chronic cryptococcal meningitis in idiopathic CD4+ lymphocytopenia. Neurol Sci. 2011;32:519–24. doi: 10.1007/s10072-011-0496-5. [DOI] [PubMed] [Google Scholar]
- 3.Lacaz CS, Heins-Vaccari EM, Melo NT, et al. [Neurocryptococcosis caused by nonencapsulated Cryptococcus neoformans] Arq Neuropsiquiatr. 1993;51:395–8. doi: 10.1590/s0004-282x1993000300019. [Article in Portuguese] [DOI] [PubMed] [Google Scholar]
- 4.Cheon WS, Eom KS, Yoo BK, et al. A case of pulmonary cryptococcosis by capsule-deficient Cryptococcus neoformans. Korean J Intern Med. 2006;21:83–7. doi: 10.3904/kjim.2006.21.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiura Y, Homma M, Yamamoto T. Difficulty in diagnosing chronic meningitis caused by capsule-deficient Cryptococcus neoformans. J Neurol Neurosurg Psychiatry. 2005;76:1460–1. doi: 10.1136/jnnp.2004.052662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To KK, Cheng VC, Tang BS, et al. False-negative cerebrospinal fluid cryptococcal antigen test due to small-colony variants of Cryptococcus neoformans meningitis in a patient with cystopleural shunt. Scand J Infect Dis. 2006;38:1110–4. doi: 10.1080/00365540600664118. [DOI] [PubMed] [Google Scholar]
- 7.Tang LM. Ventriculoperitoneal shunt in cryptococcal meningitis with hydrocephalus. Surg Neurol. 1990;33:314–9. doi: 10.1016/0090-3019(90)90198-x. [DOI] [PubMed] [Google Scholar]
- 8.Penar PL, Kim J, Chyatte D, Sabshin JK. Intraventricular cryptococcal granuloma. Report of two cases. J Neurosurg. 1988;68:145–8. doi: 10.3171/jns.1988.68.1.0145. [DOI] [PubMed] [Google Scholar]
- 9.Chan KH, Mann KS, Yue CP. Neurosurgical aspects of cerebral cryptococcosis. Neurosurgery. 1989;25:44–7. doi: 10.1097/00006123-198907000-00008. discussion 7–8. [DOI] [PubMed] [Google Scholar]


