Abstract
We describe the relation between umbilical cord clamping time and two different enrichment system of CD34+ stem cells from umbilical cord blood with the proliferative ability and bone marrow reconstitution of the stem cells obtained. After an obstetrician performed the cord blood collection, the purification of stem cells was performed either with a combination of monoclonal antibodies (negative selections) using the Stem Sep method, or with a positive cells selection based on their surface CD34 antigens using the Mini Macs system. An excellent recovery of haematopoietic progenitors [Burst Forming Unit Erythroids (BFUE); Colony Forming Unit Granulocytes and Macrophages (CFU-GM); and Colony Forming Unit Granulocytes, Erythroids, Monocytes and Macrophages (CFU-GME)], inversely related to the increase in clamping time, was performed with the Mini Macs system (54% of colonies, with 90% purity). With Stem Sep method, haematopoietic progenitor's recovery was 35% (with 80% purity). By applying early clamping of umbilical cord blood we obtained a greater number of CD34+ cells and their clonogenic activity was increased with enrichment. This is a useful technique considering that the number of CD34+ stem cells usually contained from a unit of placental blood is enough for the transplant to a child, but not for an adult. Thus, using these methods, we can get a larger number of CD34+ stem cells which reduces the risk of Graft versus Host Disease also in adult patients, producing survival rates similar to those obtained with transplantation of bone marrow from unrelated donors.
Key words: CD34+ stem cells, umbilical cord blood, recovery, enrichment.
Introduction
Haematopoietic cells are heterogeneous and belong to different lineages and are at different stages of maturity. The structural and functional integrity of the haematopoietic system is maintained by CD34+ stem cells (Figure 1), which can self-renew, producing other stem cells or differentiate and produce a various haematopoietic lineages.1 Haematopoietic progenitor stem cell transplantation is an important therapy for certain haematological and malignant disorders.2,3,4,5 Umbilical cord blood, also called placental blood, contains a high number of haematopoietic progenitor cells, which are known to possess significant potential for clinical application, and show a high proliferative capacity.6
Figure 1. Fetal stem cells.
In fact, this source of haematopoietic stem cells is characterised by a relative ease of procurement, absence of risk to the donor, and a small likelihood of transmitting clinically important infections, especially Cytomegalovirus and Epstein Barr Virus.7
Recent studies have further indicated that umbilical cord blood can be given in vivo to fully and partially HLA-matched siblings or non-familial recipients for bone marrow reconstitution in genetic disorders as well as malignancies. In comparison to adult peripheral blood, umbilical cord blood has displayed decreased immune responses to alloantigens and has a low incidence of Graft versus Host Disease, but sufficient Graft versus Leukaemia activity.8 These factors are important for the risk of relapse and mortality.9
However, previous experiences show that a unit of placental blood collected from a single donor after processing and testing contains a relatively low number of CD34+ cells. So there may be enough haematopoietic stem cells to reconstitute the bone marrow in children, but the ability to engraft an adult might require ex vivo manipulation.10 Thus, questions arise regarding the efficiency of cord blood collection. The aim of this study was to improve obstetrician-based cord blood collection and the efficiency of CD34+ cell recovery:11 We have developed a technique which actually flushes and collects placental derived stem cells, using two systems of CD34+ cells purification: negative selection with Stem Sep method and positive selection with Mini Macs. In this study the precocity of blood collection will permit a larger number of haematopoietic progenitor cells to be collected avoiding early stasis phenomena and intravascular coagulation, and reduce the transfer of red blood cells. Clamping time was calculated from the tine of birth until the clamping of umbilical cord blood with clips. Umbilical cord blood volume (120 mL) increased with a plateau between 20.30 s.
Materials and Methods
Obstetricians at the Institute of Obstetrics and Gynaecology, University of Catania, S. Bambino Hospital obtained, after informed consent and according to institutional guidelines, 150 samples of umbilical cord blood.
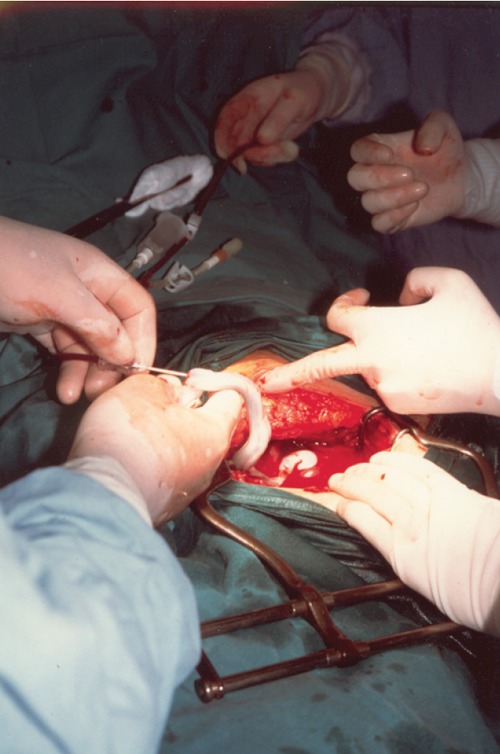
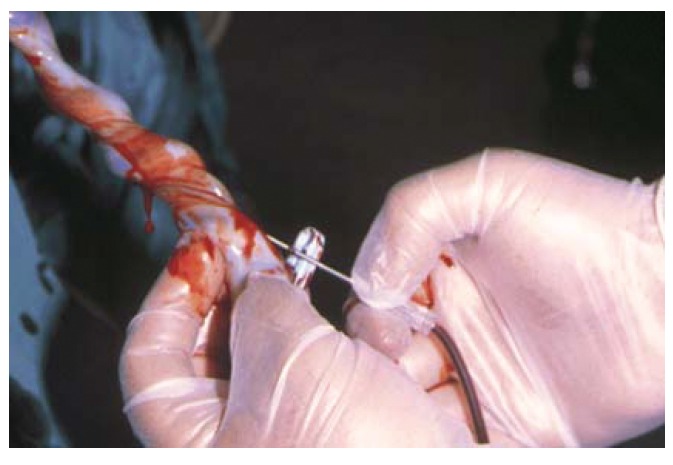
Placental blood units were collected from freshly delivered placenta in preservative containing 21 mL of CPD anticoagulant (Monohydrate citric acid 3.27 g, Citrate sodium 26.30 g, Dihydrogenophosphate 2.51 g, Monohydrate dextrose 25.50 g in 1000 mL of inject able water). Abstracted data were obtained from the mother's interviews and the mother's and infant's medical records. For purpose of consent and analysis, the mother was considered to be the donor. The collection12 was effected after the delivery of an infant (keeping the baby under the level of his mother's perineal region, to increase the blood flow from placenta), after the double ligation of the cord (by applying two haemostatic clamps a few cm from the umbilicus), the cord cutting (in the space between the two clamps), and before the placental detachment. The distal portion of the umbilical cord was cleaned with iodated alcohol (Betadine solution and alcohol), the umbilical vein was cannulised and the sample was collected by gravity into sterile set for placental collection. A higher blood quantity was collected when the sampling was performed before placental detachment, both in vaginal deliveries and in caesarean sections (Figures 2, 3). Residual materno-placental circulation represented of Vis a tergo that permitted blood to flow from the maternal section of placenta. In addition there were uterine contractions during the placental stage. The blood quantity collected depended on: the period of gestation, placental weight, placental infarction, umbilical cord features, foetal respiratory distress, placental weight/neonate weight ratio, the timing of bleeding after delivery, the clamping time and the kind of placental detachment (central or limbic). A greater blood quantity was obtained when the pregnancy was at term and was significatively and directly related to the placental weight and to the absence of infarction. Early blood collection, when other conditions were equal, permitted a collection of a greater blood quantity, despite early stasis phenomena and intravascular coagulation.
Figure 2. Umbilical cord blood collection in caesarean section.
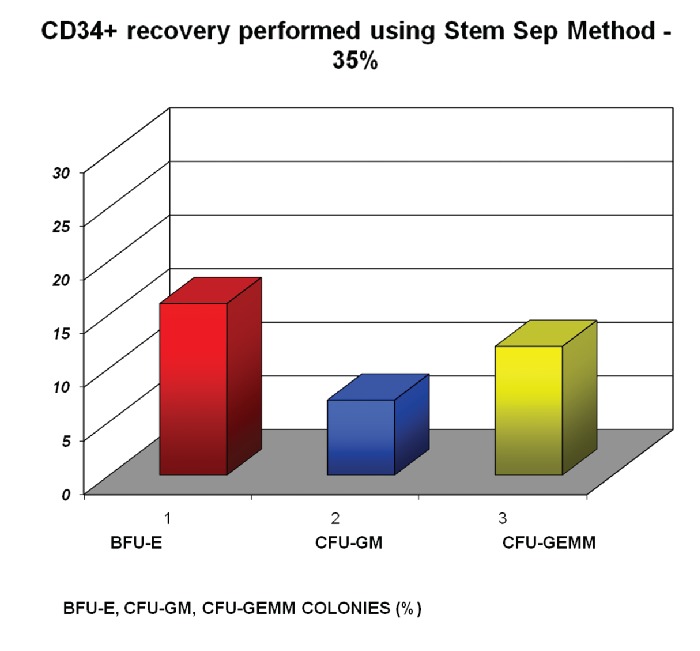
Figure 3. Umbilical cord blood collection in vaginal delivery.
Of the 150 sampling collected, 131 were analysed. The remaining were not useful for the study, being less than 60 mL, (and then too much diluted with the anticoagulant), or showing precocious coagulation phenomena.
Once collected, the blood was separated over a Ficoll Hypaque density gradient (d-1.077) at 400 g for 40 min at 20°C. Interface mononuclear cells were collected, twice washed, and resuspended in Iscove's modified Dulbecco's medium. Cell vitality was assessed by the trypan blue exclusion test, and cell mortality never exceeded 10%. Cord blood mononuclear cells were resuspended in Iscove's modified Dulbecco's medium containing 20% heat-inactivated foetal calf serum at a cellular density of 2×106 cells/mL and then treated with overnight plastic adherence cycles. Cells were washed three times, resuspended in PBS and prepared for either Mini Macs or Stem Sep separation. CD34+ cells were then resuspended in a small volume of medium, counted, and plated in semisolid medium for clonogenic assays and liquid culture as indicated below. Purification of CD34+ cells from total cord blood was obtained either with a combination of monoclonal antibodies, glycoforina A, CD3, CD2, CD24, CD19, CD14, CD16, CD66 negative selections, using the Stem Sep method or positive selection of CD34-expressing cells using a Mini Macs system. An excellent recovery of haematopoietic progenitors (with a CD34+ purity of 99%) and haematopoietic colony forming cells (Burst Forming Unit Erythroids; and Colony Forming Unit Granulocyte and Macrophages) were obtained with the Mini Macs system. Purified cells, with Mini Macs or Stem Sep system, were plated in methylcellulose culture at a density of 1×103 cells (two plates per point), in 0.9 % methylcellulose, and 3.0 IU/mL erytopoietin, Granulocyte and Macrophage Cell Stem Factor (10 ng/mL), and Interleukine 3 (100 U/mL) in Iscove's modified Dulbecco's medium supplemented with A-thioglycerolo (10–4 mol/mL) at 37°C in a 5% CO2/O2 humidified atmosphere.
Colonies were categorised after 14 days.
Results
We studied the effect of clamping time on the number of CD34+ cells isolated. The modification of umbilical cord blood depending on clamping time was investigated. Clamping time was calculated from a fetus birth till the clamping of umbilical cord blood with clips. The umbilical cord blood volume collected increased when clamping was performed earlier, with a plateau between 20 and 30 s. The volume of detected umbilical cord blood was equal when clamping time was increased over 30 s.
White blood cells, red blood cells and the haematocrit of the newborn increased as clamping time increased. A bigger blood quantity was obtained when bleeding was performed before placental detachment, both in vaginal deliveries and in Caesarean sections under ultrasonic guidance. Residual materno-placental circulation represented of a Vis a tergo that permitted blood to flow from the foetal sections of the placenta to the umbilical cord; in addition there were uterine contractions during the placental stage. Anyway early blood collection permitted a larger quantity collection, avoiding the early stasis phenomena and intravascular coagulation.
Haematopoietic progenitors were purified from cord blood using two methods: Mini Macs separation (the final index of purification was 99%); and Stem Sep method (obtaining a purity of 80%).
The effect of clamping time on the clonogenic capacity was related to now early the blood was collected. The purified umbilical cord blood progenitors included a large majority of Burst Forming Unit Erythroid and Colony Forming Unit Granulocytes and Macrophage colonies and a minority of multipotent progenitors (Colony Forming Unit Granulocytes, Erythroids, Monocytes and Macrophages). These progenitors gave rise to large colonies in clonogenic culture. The number of colonies obtained with Mini Macs system was larger (54%) than those obtained with the selection to the negative using the Stem Sep system (35%) In both cases each colony contained 105 cells (Figure 4).
Figure 4. CD34+ recovery performed using Stem Sep method - 35%.
Discussion
Haematopoietic stem cells are increasingly used for treatment of both malignant and non-malignant disorders.13–15 Various attempts have been made in recent years to expand and manipulate these cells in order to increase their therapeutic potential. In particular, cord blood is an attractive alternative to bone marrow or to peripheral blood cells mobilised by growth factors as a source of transplantable haematopoietic tissue.16,17 This study was directed to improve the feasibility of obstetrician-based cord blood collection system and enrichment of CD34+ progenitor cord blood to obtain the optimal haematopoietic progenitor recovery.18 It has been reported that the earlier collection permits collection of a larger quantity, (avoiding early stasis phenomena and intravascular coagulation). This method has several clinical advantages: i) if a newborn is underweight (preterm) is necessary to immediately cut the umbilical cord to avoid risks related to abrupt circulatory overload and to permit reanimation as soon as possible, ii) immediate umbilical cord clamping is needed in red cell materno-fetal isoimmunisation to reduce the transfer of foetal blood containing red cells with superficial antigens to his mother, iii) in addition, if the mother is Rh negative, it is helpful not to occlude the umbilical cord near the placenta and to reduce the possible passage of foetal red cells to the maternal circulation. With this study, we found that a more efficient recovery of CD34+ cells from UCB was obtained by early clamping. The blood volume collected was related to the decrease in clamping time, with a plateau between 20 and 30 s. Using the magnetic cell sorting Mini Macs or Stem Sep system to isolate CD34+ cells we demonstrated an excellent recovery of haematopoietic progenitors and haematopoietic colony forming cells when an umbilical cord blood cut was made early. We can state that by applying early clamping of the umbilical cord, we obtained a greater number of CD34+ cells; than their clonogenic activity increased with enrichment and, finally, that by using a positive selection (Mini Macs method) we got better results (54% of colonies) than by using a negative selection (Stem Sep system 35% of colonies), obtaining a yield of 99% instead of 80%. This is particularly useful considering that the number of CD34+ stem cells contained in one unit of placental blood is enough for transplanting a child, but not for an adult. Thus, by using these methods, we increased the possibility of engraftment in adult patients, and the possibility of producing survival rates similar to those obtained with the transplantation of bone marrow from unrelated donors. Umbilical cord blood may be considered a useful source of haematopoietic stem cells for patients who do not have an HLA-related donor.
References
- 1.Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanism in stem cell biology. Cell. 1997;88:287–8. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 2.Mimiero R, Busca A, Roncarolo MG, et al. HLA-haploidentical umbilical cord blood stem cell transplantation in a child with advanced leukemia: clinical outcome and analysis of hematopoietic recovery. Bone Marrow Transplant. 1995;16:229–40. [PubMed] [Google Scholar]
- 3.Katzenstein HM, Morgan ER, Olsewski M, et al. Haploidentical related umbilical cord blood stem cell transplant in a child with acute nonlymphocytic leukemia. Bone Marrow Transplant. 1997;19:765–9. doi: 10.1038/sj.bmt.1700751. [DOI] [PubMed] [Google Scholar]
- 4.Arcese W, Aversa F, Bandini G, et al. Clinical use of alligeneic hematopoietic stem cell from sources other than bone marrow. Haematologica. 1998;83:159–82. [PubMed] [Google Scholar]
- 5.Engelfriet CP, Reesink HW, Wagner JE, et al. Use of cord blood progenitor cells as an alternative for bone marrow transplantation. Vox Sang. 1998;75:156–72. [PubMed] [Google Scholar]
- 6.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. New Engl J Med. 1998;339:1565–77. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 7.Almici C, Wagner JE, Mangoni L, et al. Biologic and phenotypic analysis of early haematopoietic progenitor cells in umbilical cord blood. Leukemia. 1997;11:2143–9. doi: 10.1038/sj.leu.2400871. [DOI] [PubMed] [Google Scholar]
- Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engrafment and acute graft versus host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- David S, Boiron JM, Rice A, et al. Expansion of blood CD34+ cells: committed precursor expansion does not affect immature haematopoietic progenitors. J Hematother. 1997;6:151–8. doi: 10.1089/scd.1.1997.6.151. [DOI] [PubMed] [Google Scholar]
- 10.Farley TJ, Ahmed T, Fitzgerald M, Preti RA. Optimisation of CD34+cell selection using immunomagnet beads: implications for use in cryopreserved peripheral blood stem cell collection. J Hematother. 1997;6:53–60. doi: 10.1089/scd.1.1997.6.53. [DOI] [PubMed] [Google Scholar]
- 11.Denning-Kendall PA, Horsley H, Donaldson C, et al. Different behaviour of fresh and cultured CD34+ cells during immunomagnetic separation. Br J Hematol. 1999;105:780–5. doi: 10.1046/j.1365-2141.1999.01397.x. [DOI] [PubMed] [Google Scholar]
- 12.Morikawa H, Yoshida T, Fujimura Y, Kawamoto Y. Collection of umbilical cord blood to separate the peripheral blood stem cells during the third stage of labor. Rinsho Byori. 1999;Suppl 110:16–20. [PubMed] [Google Scholar]
- 13.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–66. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 14.Wagner JE. Umbilical cord blood transplantation: overview of the clinical experience. Blood Cells. 1994;20:227–33. [PubMed] [Google Scholar]
- 15.Cairo MS, Wagner JE. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood. 1997;90:4665–78. [PubMed] [Google Scholar]
- 16.Gluckman E. Umbilical cord blood biology and transplantation. Curr Opin Hematol. 1995;2:413–6. doi: 10.1097/00062752-199502060-00003. [DOI] [PubMed] [Google Scholar]
- 17.Sirchia G, Rebulla P. Placental/umbilical cord blood transplantation. Haematologica. 1999;84:738–47. [PubMed] [Google Scholar]
- 18.Wall DA, Noffsinger JM, Mueckl KA, et al. Feasibility of an obstetrician-based cord blood collection network for unrelated donor umbilical cord blood banking. J Matern Fetal Med. 1997;6:320–3. doi: 10.1002/(SICI)1520-6661(199711/12)6:6<320::AID-MFM4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]


