Abstract
Primary sclerosing cholangitis is a progressive disease, and coincidentally in pregnancy it is rare. It is characterized by progressive inflammation and destruction of bile ducts finally resulting in liver failure. A rare case of primary sclerosing cholangitis in pregnancy is presented. The course of the pregnancy was marked by threatened preterm delivery and exacerbation of cholestasis. She was successfully treated with ursodeoxycholic acid (UDCA). Although, primary sclerosing cholangitis has both maternal and fetal effects on pregnancy, the overall outcome is favorable. Only few cases have been reported using high dose ursodeoxycholic acid for primary sclerosing cholangitis in pregnancy, it often improves pruritus but has no protection against stillbirth. Data on the safety to the fetus or neonate and long-term outcome are scarce.
Key words: pregnancy, sclerosing cholestasis, ursodeoxycholic acid.
Introduction
Although uncommon in pregnancy, liver disease may cause a significant risk for the mother and fetus. Liver diseases presenting during pregnancy are usually specific to pregnancy including preeclampsia, HELLP syndrome (haemolysis, elevated liver enzymes, low platelets), obstetric cholestasis, hyperemesis gravidarum, and acute fatty liver disease.1 The diagnosis of liver disease during pregnancy poses a challenge because of overlapping features of these disorders. In a study of 4377 obstetric patients, Ch'ng et al. reported liver dysfunction in 3% but with no liver-associated maternal mortality.2 Although most of these patients had a pregnancy-related liver disease, liver disease can occur coincidentally in pregnancy.
A rare cause of liver disease in pregnancy is primary sclerosing cholangitis. It is characterized by ongoing inflammation, destruction and fibrosis of intrahepatic and extrahepatic bile ducts. Because of incapability of bile ducts to regenerate effectively or to regenerate at all, the disease often results in cirrhosis, portal hypertension and liver failure. It is more prevalent in men than in women (3:1). The disease normally starts from age 30 to 60, though may begin in childhood.3,4 Symptoms of itching, fatigue, jaundice and weight loss usually indicate progression of the disease, however initially histologic progression can be clinically asymptomatic. Diagnosis is made by magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography and sometimes an additional liver biopsy is performed. Although not proven effective on the natural history of the disease, ursodeoxycholic acid (UDCA) seems to reduce symptoms and improve laboratory findings. In end-stage primary sclerosing cholangitis liver transplantation is the only life extending option at this time.1,3,5
We report a woman with primary sclerosing cholangitis diagnosed prior to pregnancy who developed an exacerbation of sclerosing cholangitis during pregnancy with successful outcome.
Case Report
A 34-year-old woman, gravida 1, para 0, was diagnosed with primary sclerosing cholangitis 12 years before her pregnancy by endoscopic retrograde cholangiopancreatography. Characteristic abnormalities of both the intra and extrahepatic bile ducts, with a long extrahepatic stenosis were evident. This was treated with a 12 cm 10-french endoprothesis for 4 weeks. On repeated colonoscopy there were no signs of associated inflammatory bowel disease. MRCP 4-years before pregnancy showed stenosis of the mid-part of the common hepatic duct without prestenotic dilatation. Based on these findings stenting of the extrahepatic stenosis was repeated for a period of 4 weeks. She had no history of alcohol abuse or viral hepatitis, neither was there any evidence of associated autoimmune hepatitis. She was treated with ursodeoxycholic acid (12 mg/kg/day). In the period from initial diagnoses untill pregnancy, laboratory findings remain stable.
During her pregnancy in the first trimester transaminases were slightly elevated, which normalized in the second trimester with maintenance of ursodeoxycholic acid use (Figure 1). She was admitted in the hospital at 21 weeks of gestational age because of an imminent premature delivery complicated by an urinary tract infection. Transvaginal ultrasound showed cervical shortening and funnelling over 2.5 cm. After successful treatment with antibiotics and tocolysis she was dismissed. At 26 weeks of gestational age symptoms of itching started and 2 weeks later she developed an exacerbation of her sclerosing cholangitis: abnormal liver function tests (Figure 1) and by ultrasound thickening of the common hepatic duct were noted, but no dilatation. Magnetic resonance cholangiopancreatography showed a stenosis of the hepatical duct with prestenotic dilation (Figure 2). Because of our patient’s preference no intervention was performed.
Figure 1.
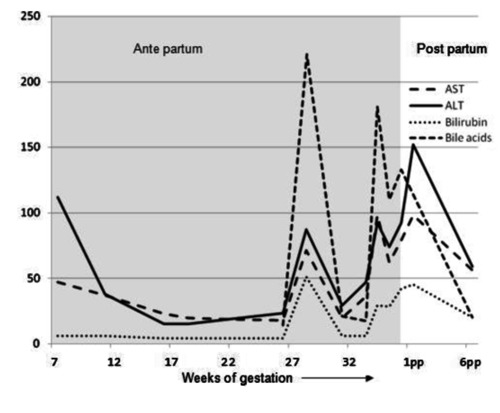
Biochemical levels throughout pregnancy. Showing the levels of aspartate transaminase (AST, U/L), alanine transaminase (ALT, U/L), bilirubin (umol/L) and bile acid (umol/L) throughout the pregnancy and postpartum (pp).
Figure 2.
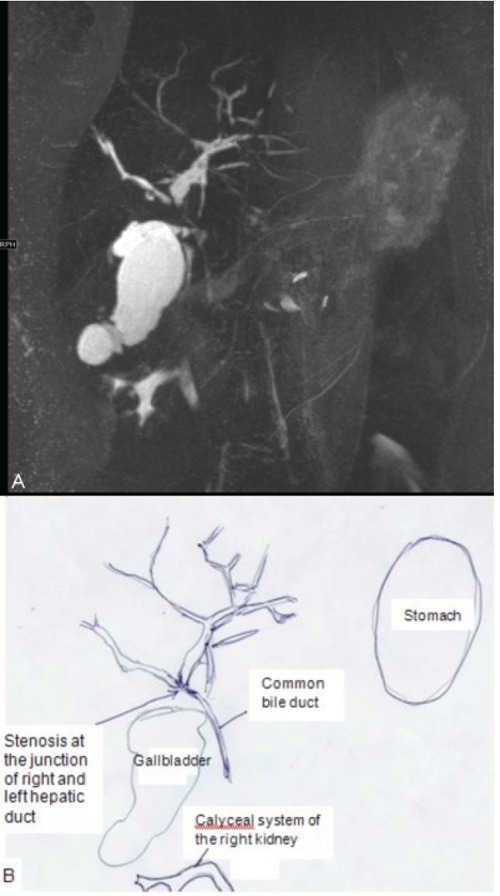
A) Magnetic resonance cholangiopancreatomography. The biliary ducts are alternatingly dilated and stenosed. The major stenosis is found at the level of the junction of the right and left hepatic duct. The common bile duct is normal. This MRCP image in a 35-year old pregnant woman is very suspect for primary sclerosing cholangitis. B) Explanatory picture of MRCP.
She was admitted again at 32 3/7 weeks of gestation because of jaundice: progression of pruritus and dark urine. Blood pressure was 120/75 mmHg and heart rate 88 bpm. No fever was present. Liver function test including serum bile acids were elevated (Figure 1) and serology was negative for hepatitis A, B and C viruses and HIV. Other laboratory tests included hemoglobin 12.9 g/dL (12–16 g/dL), platelet count 610×109/L (150–400×109/L), uric acid 0.17 mmol/L (0.12–0.34 mmol/L), glucose 6.3 mmol/L, no proteinuria. In comparison with previous ultrasound no changes were noted. In conclusion: the preexisting liver disease combined with laboratory results and clinical presentation could account for a diagnosis of exacerbation of primary sclerosing cholangitis with obstetric cholestasis. She was treated with increased dosage of ursodeoxycholic acid (35 mg/kg/day). Frequent controls of the activated partial thromboplastin time were normal; therefore vitamin K was not added. At 36 weeks she spontaneously delivered a healthy boy of 2660 gram (p60)5 with meconium-stained amniotic fluid.
Postpartum liver functions worsened initially and recovered. Mother and son were discharged six days post partum. One month after delivery her liver tests were still elevated with elevated bilirubin but pruritis had disappeared. Twenty-two months post-partum the child appears to develop normal. Further follow up showed that bilirubin levels and liver tests returned to her pre-pregnancy values and remained stable with a follow up of two years.
Discussion
Primary sclerosing cholangitis is a progressive disease, which is rare in pregnancy. Our patient suffered from multiple peripartal exacerbations and had fetal complications including preterm labor and meconium-stained amniotic fluid. Besides idiopathic cholestasis of pregnancy other causes of liver disease due to pregnancy had been ruled out. The maternal and fetal effects of primary sclerosing cholangitis are similar to the effects of idiopathic cholestasis of pregnancy: pruritis, elevated bile acids, placental insufficiency, preterm labor and sudden fetal death.
Primary sclerosing cholangitis may represent an extreme alteration of maternal-fetal bile salt metabolism as in obstetric cholestasis. The mechanism of obstetric cholestasis has been suggested to be related to hormonal influences of increased estrogens on the bile salt export pump and the basolateral membrane of hepatocytes resulting in impaired transfer of bile acids from mother to fetus across the placenta, leading to potentially toxic levels of bile acids in the fetus. The elevation of the bile acid levels possibly also affects myometrial contractility and may cause vasoconstriction of chorionic veins in the placenta, possibly resulting in preterm deliveries and fetal distress. Transporter gene mutations in the hepatocyte may play an important role as disease modifiers in primary sclerosing cholangitis. It is conceivable that interaction of these mechanisms may result in severe exacerbations of primary sclerosing cholestasis.5,6 Management of idiopathic cholestasis of pregnancy is 2-fold: symptomatic treatment (ursodeoxycholic acid) and delivery of the fetus. Ursodeoxycholic acid improves maternal pruritus and cholestatic enzymes with no fetal effect.1,5,7–8
A total of seventeen cases are described of primary sclerosing cholangitis in pregnancy. The course of primary sclerosing cholangitis is highly variable; a flare of activity may occur throughout pregnancy or post partum. In all cases the main presenting symptom was severe pruritus and in two cases the pruritus was the reason to induce labor (30 and 38 weeks gestation). One women had a successful stenting soon after delivery and another had a liver transplantation six years after her last pregnancy. In three cases signs of fetal asphyxia were noted (either meconium staining or low Apgar scores). Three women had a preterm delivery (<37 weeks gestation). One woman had a dysmature child with birth weight 2140 gram at 38 weeks gestational age. In only two women treatment with ursodeoxycholic acid was reported.9–13 It is proposed that ursodeoxycholic acid can displace more hydrophobic endogenous bile salts from the bile acid pool and thereby protect the hepatocyte membrane from their damaging toxicity, enhance bile acid clearance across the placenta from the fetus and protect in vitro rat cardiomyocytes from damage by endogenous bile salts.14 Low dose UDCA has shown relief of pruritus and improved liver tests along with less prematurity. Furthermore it is more effective in reducing pruritus, aminotransferase activity and bile acid levels than cholestyramine or dexamethasone with fewer preterm deliveries or side effects.1 More recently Lindor et al. suggested that long term higher dosage of UDCA may improve serum liver tests, but does not improve survival and may have severe adverse effects.15 Data are insufficient to support the widespread use of high dose ursodeoxycholic acid outside of clinical trials. Women should be aware of the lack of robust data concerning improvement in pruritus, protection against stillbirth and safety to the fetus or neonate.14 Since primary sclerosing cholangitis may represent an extreme alteration of maternal-fetal bile acid metabolism as in obstetric cholestasis, we treated our case with ursodeoxycholic acid with success.
Although, long term follow up of women with primary sclerosing cholangitis is lacking and few cases are described, these cases suggest that primary sclerosing cholangitis has both maternal and fetal effects on pregnancy: exacerbation of primary sclerosing cholangitis, increased risk of fetal asphyxia and preterm birth (15%).10–13 The association of exacerbation of primary sclerosing cholangitis and obstetric cholestasis implies a high recurrence rate in a subsequent pregnancy.
In conclusion, primary sclerosing cholangitis coincidental with pregnancy is rare. Although, primary sclerosing cholangitis has both maternal and fetal effects on pregnancy, the overall outcome is favorable. Only few cases have been reported using ursodeoxycholic acid for primary sclerosing cholangitis. It often improves pruritus but data on protection against stillbirth and safety to the fetus or neonate are scarce.
Acknowledgement:
we would like to acknowledge Dr. J.B.C.M. Puijlaert, for providing the magnetic resonance cholangiopancreatography and explanatory image.
References
- 1.Hay JE. Liver disease in pregnancy. Hepatology. 2008;47:1067–76. doi: 10.1002/hep.22130. [DOI] [PubMed] [Google Scholar]
- 2.Ch’ng CL, Morgan M, Hainsworth I, Kingham JG. Prospective study of liver dysfunction in pregnancy in Southwest Wales. Gut. 2002;51:876–80. doi: 10.1136/gut.51.6.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332:924–33. doi: 10.1056/NEJM199504063321406. [DOI] [PubMed] [Google Scholar]
- 4.Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15:897–906. doi: 10.3748/wjg.15.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–31. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 6.Zollner G, Trauner M. Mechanisms of cholestasis. Clin Liver Dis. 2008;12:1–26. doi: 10.1016/j.cld.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Brites D. Intrahepatic cholestasis of pregnancy: changes in maternal-fetal bile acid balance and improvement by ursodeoxycholic acid. Ann Hepatol. 2002;1:20–8. [PubMed] [Google Scholar]
- 8.Rodrigues CMP, Marin JJ, Brites D. Bile acid patterns in meconium are influenced by cholestasis of pregnancy and not altered by ursodeoxycholic acid treatment. Gut. 1999;45:446–52. doi: 10.1136/gut.45.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloosterman GJ. On intrauterine growth. The significance of prenatal care. Int J Gynaecol Obstet. 1970;8:895–912. [Google Scholar]
- 10.Janczewska I, Olsson R, Hultcrantz R, Broomé U. Pregnancy in patients with primary sclerosing cholangitis. Liver. 1996;16:326–30. doi: 10.1111/j.1600-0676.1996.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 11.Landon MB, Soloway RD, Freedman LJ, Gabbe SG. Primary sclerosing cholangitis and pregnancy. Obstet Gynecol. 1987;69:457–60. [PubMed] [Google Scholar]
- 12.Nolan DG, Martin LS, Natarajan S, Hume RF., Jr Fetal compromise associated with extreme fetal bile academia and maternal primary sclerosing cholangitis. Obstet Gynecol. 1994;84:695–6. [PubMed] [Google Scholar]
- 13.Gossard AA, Lindor KD. Pregnancy in a patient with primary sclerosing cholangitis. J Clin Gastroenterol. 2002;35:353–55. doi: 10.1097/00004836-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Royal College of Obstetricians and Gynecologists. Obstetric Cholestasis. Guideline no. 43. 2006 Jan; [Google Scholar]
- 15.Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–14. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]


