Abstract
Meckel’s diverticulum occurs in 2% of the general population and majority of patients remain asymptomatic. Gastrointestinal bleeding is the most common presentation in the paediatric population. While asymptomatic and incidentally found Meckel’s diverticulum may be left alone, surgery is essential for treating a symptomatic patient. Despite advances in imaging and technology, pre-operative diagnosis is often difficult. We present a first report of an unusual mechanism of small bowel obstruction due to Meckel’s diverticulitis in a paediatric patient. The diagnosis was only apparent at laparotomy.
Key words: Meckel’s diverticulum, Meckel’s diverticulitis, small bowel obstruction.
Introduction
Meckel’s diverticulum is the most common congenital abnormality of the small intestine.1 It arises due to an incomplete obliteration of the omphalomesenteric duct. The majority of patients remain asymptomatic; with only 4–16% of patients experiencing symptoms.2 Gastrointestinal bleeding is the most common presentation in the paediatric population while intestinal obstruction is the most common complication in adults. We present a first report of an unusual mechanism of small bowel obstruction due to Meckel’s diverticulitis in a paediatric patient.
Case Report
A 15-year-old girl presented with one day history of colicky central abdominal pain associated with loss of appetite and nausea. There was no other history to note. She was afebrile with normal hemodynamic parameters. She was mildly dehydrated and abdominal examination revealed tenderness over the periumbilical and suprapubic regions. Bowel sounds were active and the abdomen was not distended. Digital rectal examination was unremarkable. Laboratory tests showed leukocytosis with left shift (16,600 white blood cells/mm3, 92.8% neutrophils), raised serum amylase (468 U/L) and raised urinary amylase (769 U/L). Serum lipase, electrolytes, creatinine and liver function tests were unremarkable. Chest and abdominal films were unremarkable. The patient underwent computed tomography (CT) scan of her abdomen/pelvis on the first day of admission which showed mild dilatation of the small bowels, particularly in the distal jejunum and proximal ileum with thickening of the bowel wall and submucosal oedema. No transition point was seen on the CT scan (Figure 1).
Figure 1.
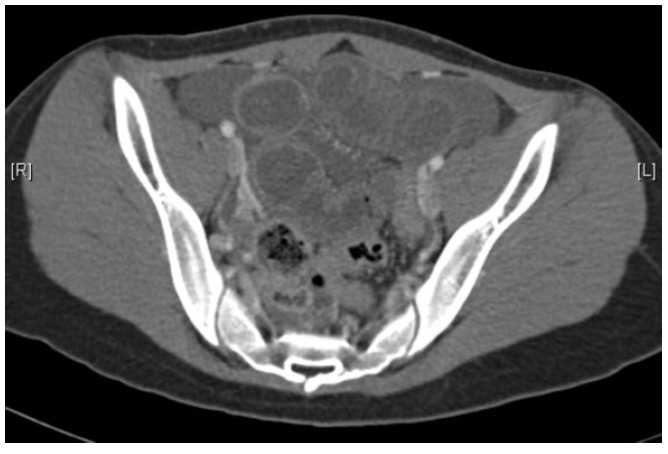
Computed tomography scan showing dilated small bowel loops and mucosal oedema. The bowel wall is well enhancing.
She received symptomatic treatment. However, her abdomen became increasingly distended and she developed vomiting over next three days. A repeat CT scan of the abdomen/pelvis was performed on the fourth day of admission and this showed interval worsening of small bowel dilatation along with a transition point in the distal ileum (Figure 2). She underwent emergency exploratory laparotomy. Intra-operatively, gangrenous 6 cm long Meckel’s diverticulum was found with omentum adherent at its tip (Figure 3). The small bowel loop was obstructed secondary to the omental band. The terminal ileum distal to the band was collapsed. The small bowel was otherwise viable. A short segment of the terminal ileum containing the Meckel’s diverticulum and adhesion band was resected and stapled anastamosis with linear staples was performed. The histology showed an infracted Meckel’s diverticulum lined by small intestine-type mucosa. No gastric-type mucosa or pancreatic tissue was identified. Her recovery was prolonged due to ileus. She was discharged on post-operative day 8 and was well at her clinic follow-up visit.
Figure 2.
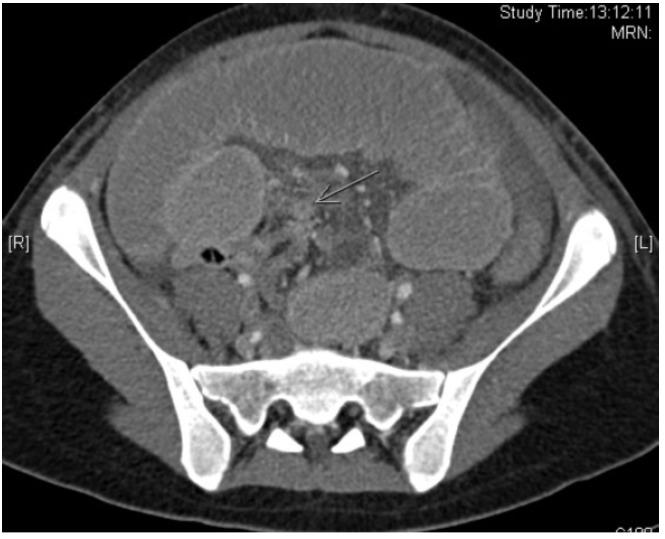
Computed tomography scan showing worsening small bowel dilatation and a transition point (green arrow) at the ileum.
Figure 3.
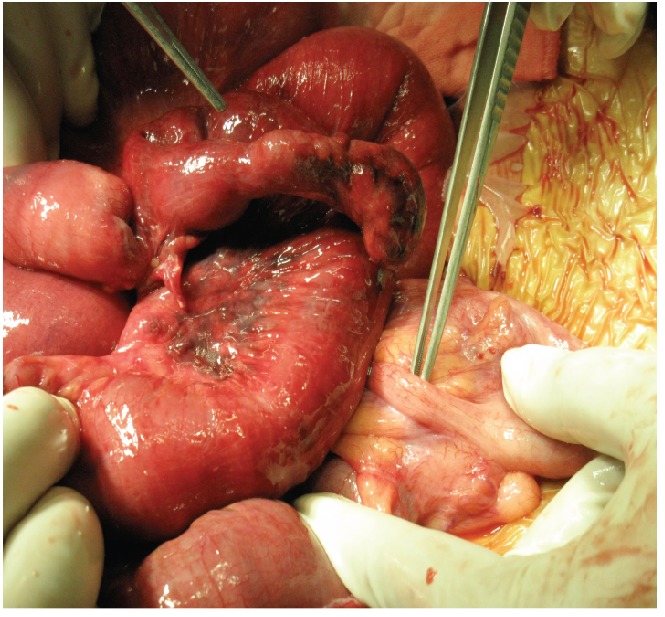
Intra-operative photograph showing normal appendix and gangrenous Meckel’s diverticulum. Miniature photograph of resected specimen.
Discussion
Meckel’s diverticulum was first described by Fabricus Heldanus in 1650,3 then reported by Levator in 16714 and by Ruysch in 1730.5 However, its embryonic origin was established by Johann Friedrich Meckel much later in 1809 and since then bears his name.6
During the first few weeks of gestation, the midgut loop remains in open connection with the yolk sac by way of the vitelline duct. Normally, the vitelline duct obliterates. Meckel’s diverticulum represents a common vestigial remnant of the omphalomesenteric duct (also known as the vitelline duct). Persistence of the duct may also rarely lead to (i) fistula between umbilicus and ileum when the entire duct remains patent; (ii) umbilical sinus when the umbilical end of the duct is not obliterated and (iii) fibrous cord between the umbilicus and the ileum representing an obliterated duct. It is a true diverticulum containing all layers of the intestinal wall and is usually situated 60–100 cm from the ileocecal valve on the anti-mesenteric border of the terminal ileum. The apex of the diverticulum may be free or attached by a fibrous band to the umbilicus or to the mesentery, in which case it can cause intestinal obstruction. As the cells lining the vitelline duct are pluripotent, it is not uncommon to find heterotopic tissue within a Meckel’s diverticulum. In one study, heterotopic gastric mucosa was found in 62% of cases, pancreatic tissue was found in 6% while pancreatic tissue and gastric mucosa were found in 5%.7 The raised serum amylase levels in our patient were reflective of intestinal ischaemia rather than ectopic pancreatic tissue. Most people with Meckel’s diverticulum remain asymptomatic with only 4–16% developing symptoms arising from complications such as intestinal obstruction, gastrointestinal bleeding, and diverticulitis.2,8 In a large series of 1476 cases of Meckel’s diverticulum found intra-operatively in a single institution over a span of 52 years, it was found that 16% were symptomatic with a mean age of 31 years and a male:female ratio of 3:1.8 In the paediatric patient population, the commonest complication is gastrointestinal bleeding arising from peptic ulceration due to acid secreted by heterotopic gastric mucosa.9 On the other hand, intestinal obstruction is the most frequent complication in the adult patient population. Our case is an infrequent case where intestinal obstruction occurred in a paediatric patient. Intestinal obstruction occurs by various mechanisms: omphalomesenteric band, internal hernia through vitelline duct remnants, volvulus around vitelline duct remnant, intussusceptions, incarceration within a hernia sac (Littre’s hernia) or chronic Meckel’s diverticulitis.2 Enteroliths formed in the diverticulum causing intestinal obstruction have also been reported.10,11 The small bowel obstruction in our patient was not explained by any of the above mechanisms. This is the first report of an omental band adherent to Meckel’s diverticulum causing intestinal obstruction.
Charles Mayo once remarked Meckel’s diverticulum is frequently suspected, often looked for and seldom found.12 Preoperative diagnosis of symptomatic Meckel’s diverticulum is difficult, especially in patients presenting with symptoms other than per-rectal bleeding. In a study of 776 patients, 88% of patients with Meckel’s diverticulum presenting with per-rectal bleeding had a correct preoperative diagnosis while only 11% of patients who presented with other symptoms were rightly diagnosed preoperatively.13 Plain X-ray, CT scans and barium studies are rarely useful in pre-operative diagnosis of Meckel’s. In our patient, CT scan was done twice and it failed to recognize the presence of inflamed Meckel’s diverticulum on two occasions. Hence pre-operative diagnosis is many times not possible and a high index of suspicion is essential in dealing with an undiagnosed abdominal pain. When the patient presents with gastrointestinal bleeding, technetium-99m pertechnate scan is a useful non-invasive investigation. In children, it has a sensitivity of 80–90%, specificity of 95% and accuracy of 90%14 but in the adults, it is less reliable with a sensitivity of 62.5%, specificity of 9% and accuracy of 46%.15 As the technetium-99 m pertechnate scan is specific to ectopic gastric mucosa and not specifically to Meckel’s diverticulum, it may be positive in gut duplication cysts with ectopic gastric mucosa.16
Management of incidentally detected Meckel’s diverticulum is controversial. Resection of incidentally found Meckel’s diverticulum has been justified due to a potential for morbidity and mortality throughout life.17–20 In 1976, Soltero et al. first opposed routine resection of incidentally found Meckel’s diverticulum, demonstrating that there was only a small chance of a truly asymptomatic Meckel’s diverticulum causing disease in later life.21 Peoples et al. also discouraged the resection of incidentally found Meckel’s diverticulum as they found that the lifetime risk of developing symptoms from a Meckel’s diverticulum do not significantly outweigh the surgical morbidity and mortality of resection.22 Many others advocate a case-specific approach. Incidentally found Meckel’s diverticulum with a broad base or of a short length should be left in situ23,24 while the palpability of mucosal heterotopia would steer a surgeon towards resection.9,25,26 Park et al. also recommend a selective approach, advising resection of incidentally detected Meckel’s diverticulum in the following cases: (i) patients younger than 50 years of age; (ii) male patients; (iii) diverticulum longer than 2 cm; (iv) detection of abnormal features inside the diverticulum.8 A recent meta-analysis does not support routine resection of incidentally detected Meckel’s diverticulum.27
The definitive treatment of symptomatic Meckel’s diverticulum is surgery, via laparotomy, laparoscopic or laparoscopic-assisted approaches. The extent of resection is guided by the type of complication encountered and the intra-operative findings. A narrow-base omphalomesenteric remnant without any palpable mass in the lumen may be treated with a simple wedge resection of the diverticulum and closure of the ileal defect.28 In cases where the diverticulum has a wide base or palpable ectopic tissue or where there is inflammatory or ischemia changes in adjacent ileum, it is preferable to resect the involved bowel with end-to-end bowel anatamosis.24,29 Segmental ileal resection is also required for treatment of patients with gastrointestinal bleeding as the site of bleeding is usually in the adjacent ileum. Involvement of the diverticulum by benign tumors can be dealt with a simple diverticulectomy, depending on the site and size of the lesion. Where malignant tumors are involved, wide intestinal and mesenteric resection would be required.30,31
In conclusion, Meckel’s diverticulum is not uncommon and hence should be considered as a differential diagnosis in a patient with an unestablished cause of intestinal obstruction.
References
- 1.Anderson DJ. Carcinoid tumour in Meckel’s diverticulum: laparoscopic treatment and review of literature. J Am Osteopath Assoc. 2000;100:432–4. [PubMed] [Google Scholar]
- 2.Prall RT, Bannon MO, Bharucha AE. Meckel’s diverticulum causing intestinal obstruction. Am J Gastroenterol. 2001;96:3246–7. doi: 10.1111/j.1572-0241.2001.05344.x. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri TK, Christie JH. False positive Meckel’s diverticulum scan. Surgery. 1972;71:313–313. [PubMed] [Google Scholar]
- 4.Dalinka MK, Wunder JF. Meckel’s diverticulum and its complications, with emphasis on roentgenologic demonstration. Radiology. 1973;106:295–8. doi: 10.1148/106.2.295. [DOI] [PubMed] [Google Scholar]
- 5.Duszynski DO. Radionuclide imaging of gastrointestinal disorders. Semin Nucl Med. 1972;2:383–6. doi: 10.1016/s0001-2998(72)80028-x. [DOI] [PubMed] [Google Scholar]
- 6.Opitz JM, Schultka R, Gobbel L. Meckel on developmental pathology. Am J Med Genet A. 2006;140:115–28. doi: 10.1002/ajmg.a.31043. [DOI] [PubMed] [Google Scholar]
- 7.Elsayes KM, Menias CO, Harvin HJ, Francis IR. Imaging manifestations of Meckel’s diverticulum. AJR Am J Roentgenol. 2007;189:81–8. doi: 10.2214/AJR.06.1257. [DOI] [PubMed] [Google Scholar]
- 8.Park JJ, Wolff BG, Tollefson MK, et al. Meckel diverticulum: the Mayo Clinic experience with 1476 patients (1950–2002) Ann Surg. 2005;241:529–33. doi: 10.1097/01.sla.0000154270.14308.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St-Vil D, Brandt ML, Panic S, et al. Meckel’s diverticulum in children: a 20-year review. J Pedatr Surg. 1991;26:1289–92. doi: 10.1016/0022-3468(91)90601-o. [DOI] [PubMed] [Google Scholar]
- 10.Srinivas GN, Cullen P. Intestinal obstruction due to meckel’s diverticulum: a rare presentation. Acta Chir Belg. 2007;107:64–6. doi: 10.1080/00015458.2007.11680014. [DOI] [PubMed] [Google Scholar]
- 11.Trésallet C, Renard-Penna R, Nguyen-Thanh Q, et al. Intestinal obstruction by an enterolith from a perforated giant Meckel’s diverticulum: diagnosis with CT reconstructed images. Int Surg. 2007;92:125–7. [PubMed] [Google Scholar]
- 12.Russell RCG, Williams NS, Bulstrode CJK. The small and large intestines. In: Russell RCG, Williams NS, Bulstrode CJK, editors. Bailey and Love’s Short Practice of Surgery. 23rd ed. London: Arnold: 2000. pp. 1033–1033. [Google Scholar]
- 13.Higaki S, Saito Y, Akazawa A, et al. Bleeding Meckel’s diverticulum in an adult. Hepatogastroenterology. 2001;48:1628–30. [PubMed] [Google Scholar]
- 14.Kong MS, Chen CY, Tzen KY, et al. Technetium-99m pertechnetate scan for ectopic gastric mucosa in children with gastrointestinal bleeding. J Formos Med Assoc. 1993;92:717–20. [PubMed] [Google Scholar]
- 15.Lin S, Suhocki PV, Ludwig KA, Shetzline MA. Gastrointestinal bleeding in adult patients with Meckel’s diverticulum: the role of technetium 99m pertechnetate scan. South Med J. 2002;95:1338–41. [PubMed] [Google Scholar]
- 16.Kumar R, Tripathi M, Chandrashekar N, et al. Diagnosis of ectopic gastric mucosa using 99Tcm-pertechnetate: spectrum of scintigraphic findings. Br J Radiol. 2005;78:714–20. doi: 10.1259/bjr/16678420. [DOI] [PubMed] [Google Scholar]
- 17.Arnold JF, Pellicane JV. Meckel’s diverticulum: a ten-year experience. Am Surg. 1997;63:354–5. [PubMed] [Google Scholar]
- 18.Bani-Hani KE, Shatnawi NJ. Meckel’s diverticulum: comparison of incidental and symptomatic cases. World J Surg. 2004;28:917–20. doi: 10.1007/s00268-004-7512-3. [DOI] [PubMed] [Google Scholar]
- 19.Michas CA, Cohen SE, Wolfman EF., Jr Meckel’s diverticulum: should it be excised incidentally at operation? Am J Surg. 1975;129:682–5. doi: 10.1016/0002-9610(75)90345-1. [DOI] [PubMed] [Google Scholar]
- 20.Cullen JJ, Kelly KA, Moir CR, et al. Surgical management of Meckel’s diverticulum. An epidemiologic, population-based study. Ann Surg. 1994;220:564–9. doi: 10.1097/00000658-199410000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soltero MJ, Bill AH. The natural history of Meckel’s Diverticulum and its relation to incidental removal. A study of 202 cases of diseased Meckel’s diverticulum found in King County, Washington, over a fifteen year period. Am J Surg. 1976;132:168–173. doi: 10.1016/0002-9610(76)90043-x. [DOI] [PubMed] [Google Scholar]
- 22.Peoples JB, Lichtenberger EJ, Dunn MM. Incidental Meckel's diverticulectomy in adults. Surgery. 1995;118:649–52. doi: 10.1016/s0039-6060(05)80031-5. [DOI] [PubMed] [Google Scholar]
- 23.Robijn J, Sebrechts E, Miserez M. Management of incidentally found Meckel’s diverticulum a new approach: resection based on a Risk Score. Acta Chir Belg. 2006;106:467–70. doi: 10.1080/00015458.2006.11679933. [DOI] [PubMed] [Google Scholar]
- 24.Varcoe RL, Wong SW, Taylor CF, Newstead GL. Diverticulectomy is inadequate treatment for short Meckel’s diverticulum with heterotopic mucosa. ANZ J Surg. 2004;74:869–72. doi: 10.1111/j.1445-1433.2004.03191.x. [DOI] [PubMed] [Google Scholar]
- 25.Simms MH, Corkery JJ. Meckel’s diverticulum: its association with congenital malformation and the significance of atypical morphology. Br J Surg. 1980;67:216–9. doi: 10.1002/bjs.1800670316. [DOI] [PubMed] [Google Scholar]
- 26.Groebli Y, Bertin D, Morel P. Meckel’s diverticulum in adults: retrospective analysis of 119 cases and historical review. Eur J Surg. 2001;167:518–24. doi: 10.1080/110241501316914894. [DOI] [PubMed] [Google Scholar]
- 27.Zani A, Eaton S, Rees CM, Pierro A. Incidentally detected Meckel diverticulum: to resect or not to resect? Ann Surg. 2008;247:276–81. doi: 10.1097/SLA.0b013e31815aaaf8. [DOI] [PubMed] [Google Scholar]
- 28.Sharma RK, Jain VK. Emergency surgery for Meckel’s diverticulum. World J Emerg Surg. 2008;3:27–27. doi: 10.1186/1749-7922-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukai M, Takamatsu H, Noguchi H, et al. Does the external appearance of a Meckel’s diverticulum assist in choice of the laparoscopic procedure? Pediatr Surg Int. 2002;18:231–3. doi: 10.1007/s003830100663. [DOI] [PubMed] [Google Scholar]
- 30.Sutter PM, Canepa MG, Kuhrmeier F, et al. [Carcinoid tumor in Meckel’s diverticulum: case presentation and review of literature] Schweiz med Wochenschr Suppl. 1997;89:20S–24S. [PubMed] [Google Scholar]
- 31.Kovács M, Davidovics S, Gyurus P, Rácz I. [Identification of a Meckel’s diverticulum bleeding by urgent capsule endoscopy] Orv Hetil. 2006;147:2003–6. [PubMed] [Google Scholar]


