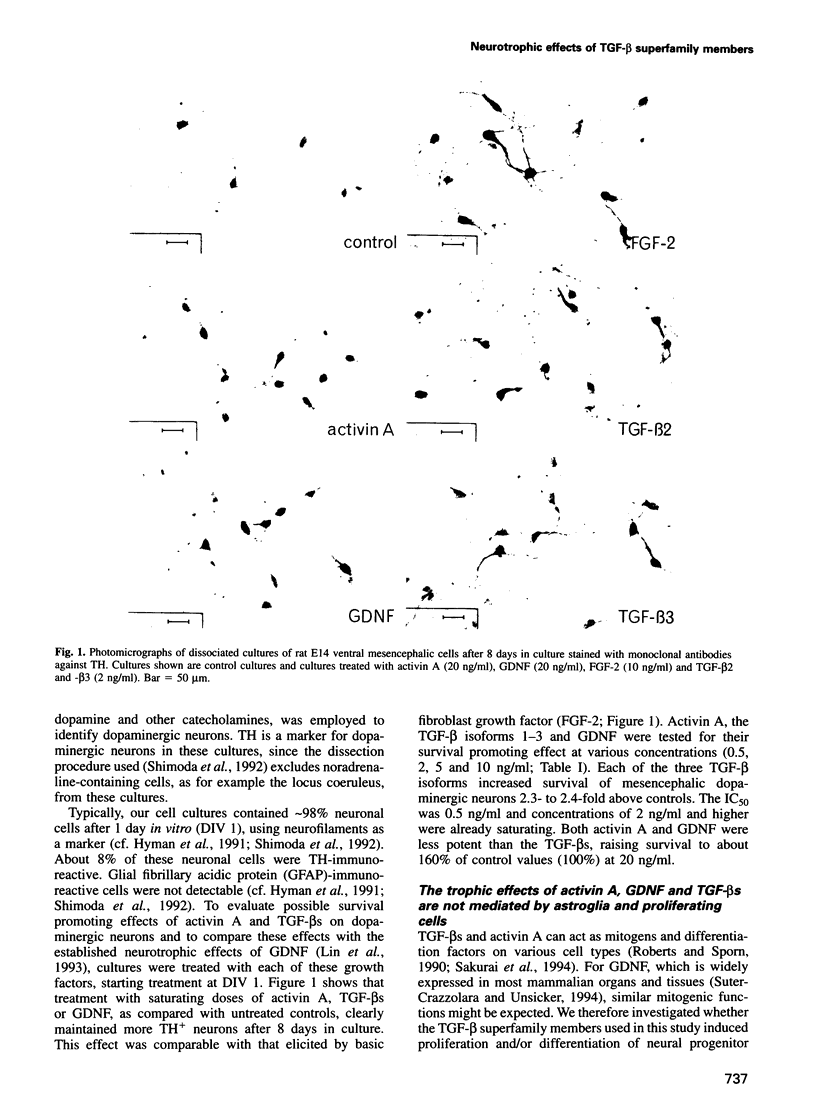
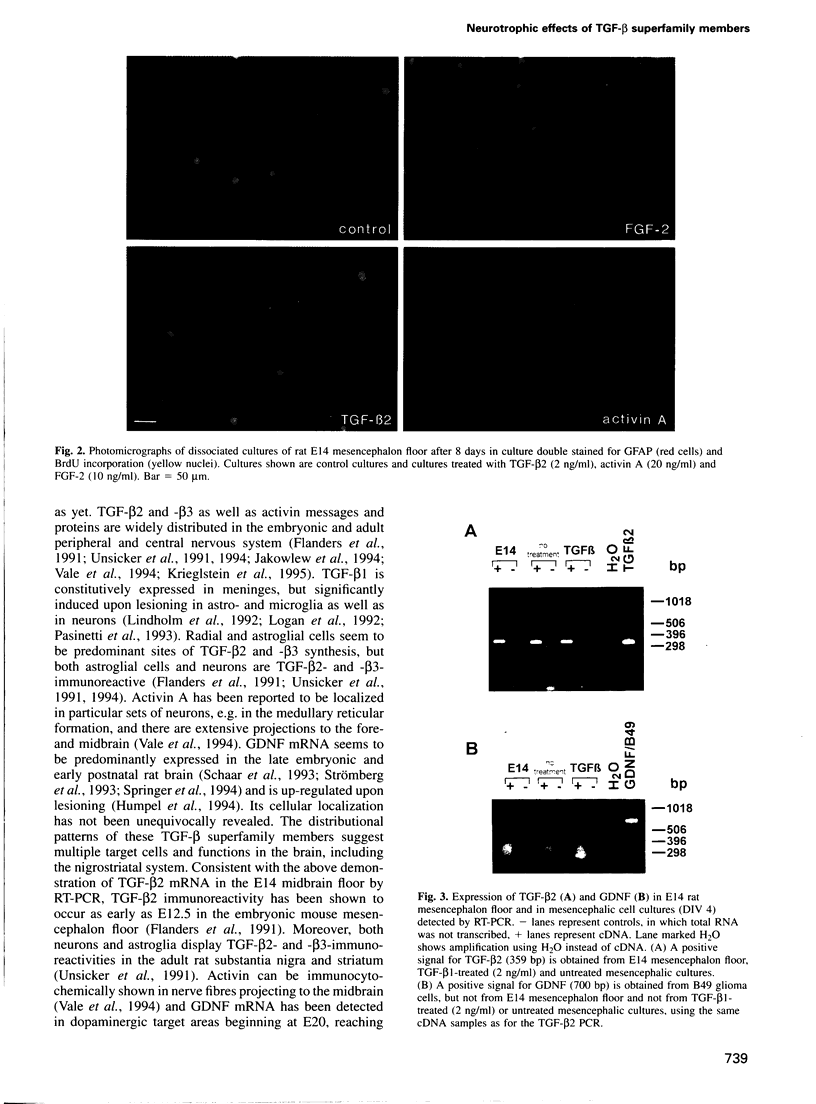
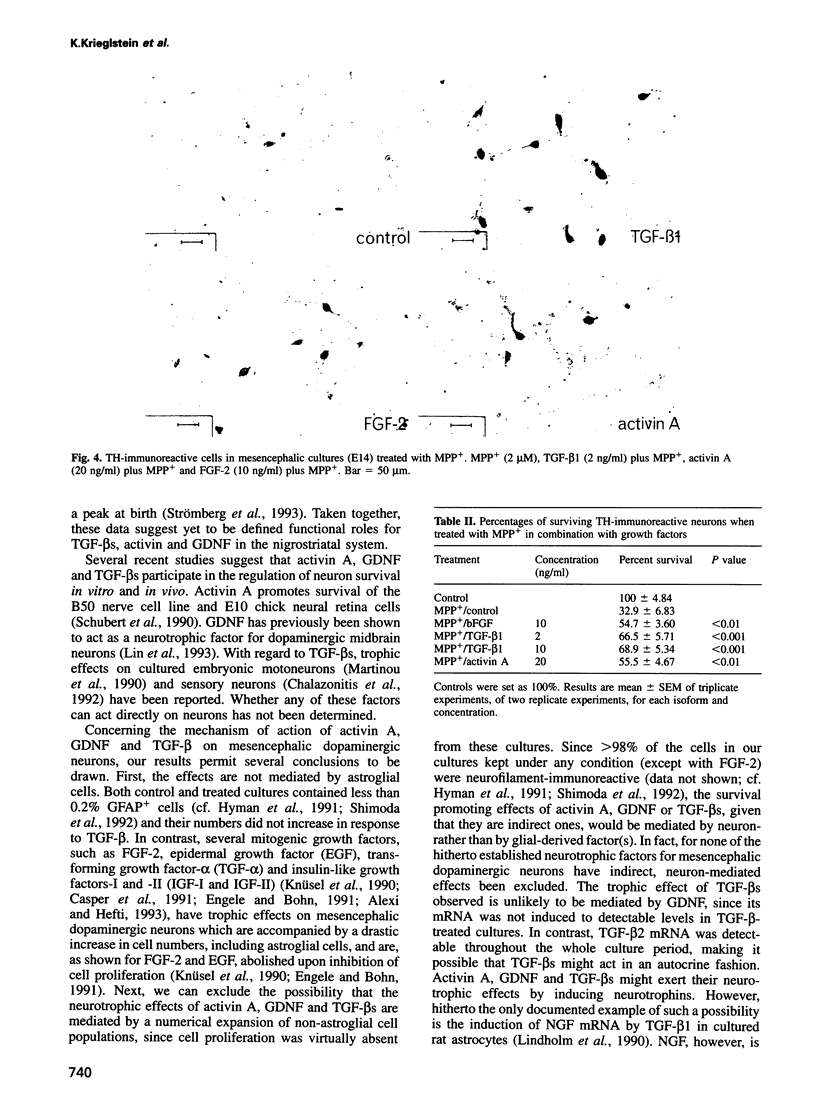
Abstract
The superfamily of transforming growth factors-beta (TGF-beta) comprises an expanding list of multifunctional proteins serving as regulators of cell proliferation and differentiation. Prominent members of this family include the TGF-beta s 1-5, activins, bone morphogenetic proteins and a recently discovered glial cell line-derived neurotrophic factor (GDNF). In the present study we demonstrate and compare the survival promoting and neuroprotective effects of TGF-beta 1, -2 and -3, activin A and GDNF for midbrain dopaminergic neurons in vitro. All proteins increase the survival of tyrosine hydroxylase-immunoreactive dopaminergic neurons isolated from the embryonic day (E) 14 rat mesencephalon floor to varying extents (TGF-beta s 2.5-fold, activin A and GDNF 1.6-fold). TGF-beta s, activin A and GDNF did not augment numbers of very rarely observed astroglial cells visualized by using antibodies to glial fibrillary acidic protein and had no effect on cell proliferation monitored by incorporation of BrdU. TGF-beta 1 and activin A protected dopaminergic neurons against N-methyl-4-phenylpiridinium ion toxicity. Reverse transcription-polymerase chain reaction (RT-PCR) analysis indicated that TGF-beta 2 mRNA, but not GDNF mRNA, is expressed in the E14 rat midbrain floor and in mesencephalic cultures. We conclude that TGF-beta s 1-3, activin A and GDNF share a neurotrophic capacity for developing dopaminergic neurons, which is not mediated by astroglial cells and not accompanied by an increase in cell proliferation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexi T., Hefti F. Trophic actions of transforming growth factor alpha on mesencephalic dopaminergic neurons developing in culture. Neuroscience. 1993 Aug;55(4):903–918. doi: 10.1016/0306-4522(93)90307-2. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Skaper S. D., Varon S. S., Sato G. H. Selective survival of neurons from chick embryo sensory ganglionic dissociates utilizing serum-free supplemented medium. Exp Cell Res. 1980 Jan;125(1):183–190. doi: 10.1016/0014-4827(80)90202-5. [DOI] [PubMed] [Google Scholar]
- Casper D., Mytilineou C., Blum M. EGF enhances the survival of dopamine neurons in rat embryonic mesencephalon primary cell culture. J Neurosci Res. 1991 Oct;30(2):372–381. doi: 10.1002/jnr.490300213. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A., Kalberg J., Twardzik D. R., Morrison R. S., Kessler J. A. Transforming growth factor beta has neurotrophic actions on sensory neurons in vitro and is synergistic with nerve growth factor. Dev Biol. 1992 Jul;152(1):121–132. doi: 10.1016/0012-1606(92)90162-a. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Engele J., Bohn M. C. The neurotrophic effects of fibroblast growth factors on dopaminergic neurons in vitro are mediated by mesencephalic glia. J Neurosci. 1991 Oct;11(10):3070–3078. doi: 10.1523/JNEUROSCI.11-10-03070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G., Minozzi M. C., Toffano G., Leon A., Skaper S. D. Basic fibroblast growth factor promotes the survival and development of mesencephalic neurons in culture. Dev Biol. 1989 May;133(1):140–147. doi: 10.1016/0012-1606(89)90305-9. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Lüdecke G., Engels S., Cissel D. S., Roberts A. B., Kondaiah P., Lafyatis R., Sporn M. B., Unsicker K. Localization and actions of transforming growth factor-beta s in the embryonic nervous system. Development. 1991 Sep;113(1):183–191. doi: 10.1242/dev.113.1.183. [DOI] [PubMed] [Google Scholar]
- Forno L. S., DeLanney L. E., Irwin I., Langston J. W. Similarities and differences between MPTP-induced parkinsonsim and Parkinson's disease. Neuropathologic considerations. Adv Neurol. 1993;60:600–608. [PubMed] [Google Scholar]
- Franzén P., ten Dijke P., Ichijo H., Yamashita H., Schulz P., Heldin C. H., Miyazono K. Cloning of a TGF beta type I receptor that forms a heteromeric complex with the TGF beta type II receptor. Cell. 1993 Nov 19;75(4):681–692. doi: 10.1016/0092-8674(93)90489-d. [DOI] [PubMed] [Google Scholar]
- Frisén J., Verge V. M., Fried K., Risling M., Persson H., Trotter J., Hökfelt T., Lindholm D. Characterization of glial trkB receptors: differential response to injury in the central and peripheral nervous systems. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4971–4975. doi: 10.1073/pnas.90.11.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach M., Ben-Shachar D., Riederer P., Youdim M. B. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994 Sep;63(3):793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- Heine U. I., Burmester J. K., Flanders K. C., Danielpour D., Munoz E. F., Roberts A. B., Sporn M. B. Localization of transforming growth factor-beta 1 in mitochondria of murine heart and liver. Cell Regul. 1991 Jun;2(6):467–477. doi: 10.1091/mbc.2.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C., Hoffer B., Strömberg I., Bektesh S., Collins F., Olson L. Neurons of the hippocampal formation express glial cell line-derived neurotrophic factor messenger RNA in response to kainate-induced excitation. Neuroscience. 1994 Apr;59(4):791–795. doi: 10.1016/0306-4522(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Hyman C., Hofer M., Barde Y. A., Juhasz M., Yancopoulos G. D., Squinto S. P., Lindsay R. M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991 Mar 21;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Hyman C., Juhasz M., Jackson C., Wright P., Ip N. Y., Lindsay R. M. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994 Jan;14(1):335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. A., Poulsen K., Armanini M., Berkemeier L., Phillips H., Rosenthal A. Neurotrophin-4/5 is a survival factor for embryonic midbrain dopaminergic neurons in enriched cultures. J Neurosci Res. 1994 Jan;37(1):144–154. doi: 10.1002/jnr.490370118. [DOI] [PubMed] [Google Scholar]
- Hüsken-Hindi P., Tsuchida K., Park M., Corrigan A. Z., Vaughan J. M., Vale W. W., Fischer W. H. Monomeric activin A retains high receptor binding affinity but exhibits low biological activity. J Biol Chem. 1994 Jul 29;269(30):19380–19384. [PubMed] [Google Scholar]
- Jakowlew S. B., Ciment G., Tuan R. S., Sporn M. B., Roberts A. B. Expression of transforming growth factor-beta 2 and beta 3 mRNAs and proteins in the developing chicken embryo. Differentiation. 1994 Jan;55(2):105–118. doi: 10.1046/j.1432-0436.1994.5520105.x. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994 Jan;8(2):133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Knusel B., Michel P. P., Schwaber J. S., Hefti F. Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. J Neurosci. 1990 Feb;10(2):558–570. doi: 10.1523/JNEUROSCI.10-02-00558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knüsel B., Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. J Neurochem. 1992 Dec;59(6):1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- Kopin I. J., Markey S. P. MPTP toxicity: implications for research in Parkinson's disease. Annu Rev Neurosci. 1988;11:81–96. doi: 10.1146/annurev.ne.11.030188.000501. [DOI] [PubMed] [Google Scholar]
- Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. 1993 Jul;13(7):2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. F., Doherty D. H., Lile J. D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993 May 21;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lindholm D., Castrén E., Kiefer R., Zafra F., Thoenen H. Transforming growth factor-beta 1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol. 1992 Apr;117(2):395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D., Hengerer B., Zafra F., Thoenen H. Transforming growth factor-beta 1 stimulates expression of nerve growth factor in the rat CNS. Neuroreport. 1990 Sep;1(1):9–12. doi: 10.1097/00001756-199009000-00003. [DOI] [PubMed] [Google Scholar]
- Logan A., Frautschy S. A., Gonzalez A. M., Sporn M. B., Baird A. Enhanced expression of transforming growth factor beta 1 in the rat brain after a localized cerebral injury. Brain Res. 1992 Aug 7;587(2):216–225. doi: 10.1016/0006-8993(92)91000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou J. C., Le Van Thai A., Valette A., Weber M. J. Transforming growth factor beta 1 is a potent survival factor for rat embryo motoneurons in culture. Brain Res Dev Brain Res. 1990 Mar 1;52(1-2):175–181. doi: 10.1016/0165-3806(90)90233-o. [DOI] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- McDonald N. Q., Hendrickson W. A. A structural superfamily of growth factors containing a cystine knot motif. Cell. 1993 May 7;73(3):421–424. doi: 10.1016/0092-8674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- Otto D., Unsicker K. Basic FGF reverses chemical and morphological deficits in the nigrostriatal system of MPTP-treated mice. J Neurosci. 1990 Jun;10(6):1912–1921. doi: 10.1523/JNEUROSCI.10-06-01912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto D., Unsicker K. FGF-2-mediated protection of cultured mesencephalic dopaminergic neurons against MPTP and MPP+: specificity and impact of culture conditions, non-dopaminergic neurons, and astroglial cells. J Neurosci Res. 1993 Mar 1;34(4):382–393. doi: 10.1002/jnr.490340403. [DOI] [PubMed] [Google Scholar]
- Pasinetti G. M., Nichols N. R., Tocco G., Morgan T., Laping N., Finch C. E. Transforming growth factor beta 1 and fibronectin messenger RNA in rat brain: responses to injury and cell-type localization. Neuroscience. 1993 Jun;54(4):893–907. doi: 10.1016/0306-4522(93)90583-2. [DOI] [PubMed] [Google Scholar]
- Prehn J. H., Backhauss C., Krieglstein J. Transforming growth factor-beta 1 prevents glutamate neurotoxicity in rat neocortical cultures and protects mouse neocortex from ischemic injury in vivo. J Cereb Blood Flow Metab. 1993 May;13(3):521–525. doi: 10.1038/jcbfm.1993.67. [DOI] [PubMed] [Google Scholar]
- Prehn J. H., Peruche B., Unsicker K., Krieglstein J. Isoform-specific effects of transforming growth factors-beta on degeneration of primary neuronal cultures induced by cytotoxic hypoxia or glutamate. J Neurochem. 1993 May;60(5):1665–1672. doi: 10.1111/j.1471-4159.1993.tb13389.x. [DOI] [PubMed] [Google Scholar]
- Przedborski S., Kostic V., Jackson-Lewis V., Naini A. B., Simonetti S., Fahn S., Carlson E., Epstein C. J., Cadet J. L. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci. 1992 May;12(5):1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Abe Y., Kasuya Y., Takuwa N., Shiba R., Yamashita T., Endo T., Goto K. Activin A stimulates mitogenesis in Swiss 3T3 fibroblasts without activation of mitogen-activated protein kinases. J Biol Chem. 1994 May 13;269(19):14118–14122. [PubMed] [Google Scholar]
- Schaar D. G., Sieber B. A., Dreyfus C. F., Black I. B. Regional and cell-specific expression of GDNF in rat brain. Exp Neurol. 1993 Dec;124(2):368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- Schubert D., Kimura H., LaCorbiere M., Vaughan J., Karr D., Fischer W. H. Activin is a nerve cell survival molecule. Nature. 1990 Apr 26;344(6269):868–870. doi: 10.1038/344868a0. [DOI] [PubMed] [Google Scholar]
- Shimoda K., Sauve Y., Marini A., Schwartz J. P., Commissiong J. W. A high percentage yield of tyrosine hydroxylase-positive cells from rat E14 mesencephalic cell culture. Brain Res. 1992 Jul 24;586(2):319–331. doi: 10.1016/0006-8993(92)91642-r. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Watts R. L., Juncos J. L., Torroni A., Wallace D. C. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1991 Sep;30(3):332–339. doi: 10.1002/ana.410300304. [DOI] [PubMed] [Google Scholar]
- Springer J. E., Mu X., Bergmann L. W., Trojanowski J. Q. Expression of GDNF mRNA in rat and human nervous tissue. Exp Neurol. 1994 Jun;127(2):167–170. doi: 10.1006/exnr.1994.1091. [DOI] [PubMed] [Google Scholar]
- Strömberg I., Björklund L., Johansson M., Tomac A., Collins F., Olson L., Hoffer B., Humpel C. Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp Neurol. 1993 Dec;124(2):401–412. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Suter-Crazzolara C., Unsicker K. GDNF is expressed in two forms in many tissues outside the CNS. Neuroreport. 1994 Dec 20;5(18):2486–2488. doi: 10.1097/00001756-199412000-00020. [DOI] [PubMed] [Google Scholar]
- Turski L., Bressler K., Rettig K. J., Löschmann P. A., Wachtel H. Protection of substantia nigra from MPP+ neurotoxicity by N-methyl-D-aspartate antagonists. Nature. 1991 Jan 31;349(6308):414–418. doi: 10.1038/349414a0. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Flanders K. C., Cissel D. S., Lafyatis R., Sporn M. B. Transforming growth factor beta isoforms in the adult rat central and peripheral nervous system. Neuroscience. 1991;44(3):613–625. doi: 10.1016/0306-4522(91)90082-y. [DOI] [PubMed] [Google Scholar]
- Unsicker K. Growth factors in Parkinson's disease. Prog Growth Factor Res. 1994;5(1):73–87. doi: 10.1016/0955-2235(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Zhou X. F., Parada L. F., Soppet D., Rush R. A. Distribution of trkB tyrosine kinase immunoreactivity in the rat central nervous system. Brain Res. 1993 Sep 17;622(1-2):63–70. doi: 10.1016/0006-8993(93)90802-t. [DOI] [PubMed] [Google Scholar]