Abstract
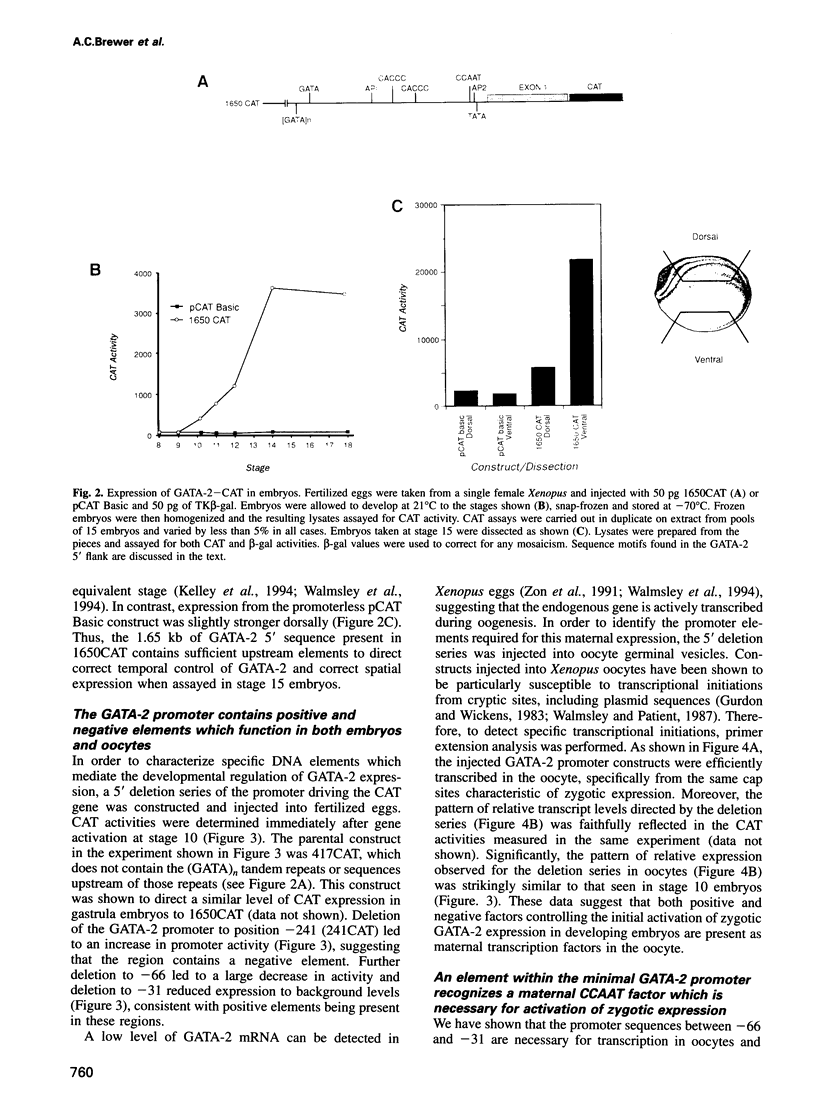
The transcription factor GATA-2 is present in blood cell precursors and plays a pivotal role in the control of erythroid differentiation. In Xenopus embryos, low levels of GATA-2 mRNA are maternally derived, while the onset of zygotic GATA-2 expression coincides with commitment to haematopoietic lineages. However, its initial transcriptional activation is not restricted to the presumptive blood islands, but occurs throughout ventral and lateral regions, in all three germ layers. In order to determine how this expression pattern is controlled, we have isolated and characterized the Xenopus GATA-2 gene. We show that 1.65 kb of 5' flanking sequences are sufficient to direct both correct transcriptional initiation in oocytes and appropriate temporal and spatial gene expression in early embryos. The transgene is activated during gastrulation and by neurula stages in predominantly expressed in the ventral hemisphere. We demonstrate that a CCAAT element is necessary for gene activity in both systems and that extracts prepared from oocytes and embryos contain a factor which specifically recognizes this element. We also show that cytoplasmic localization inhibits the function of this CCAAT factor until the beginning of gastrulation, when the zygotic GATA-2 gene is activated. These observations extend our understanding of the mechanisms by which maternal factors control the temporal activation of transcription in early vertebrate embryos.
Full text
PDF


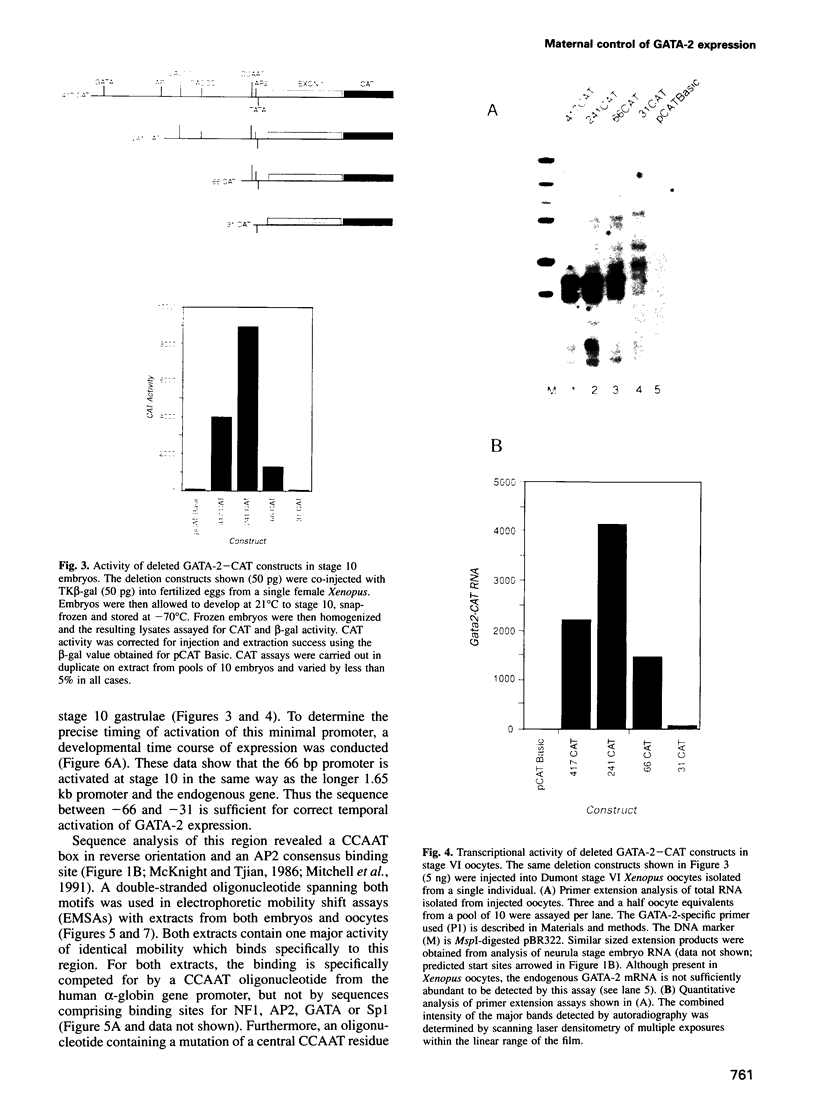
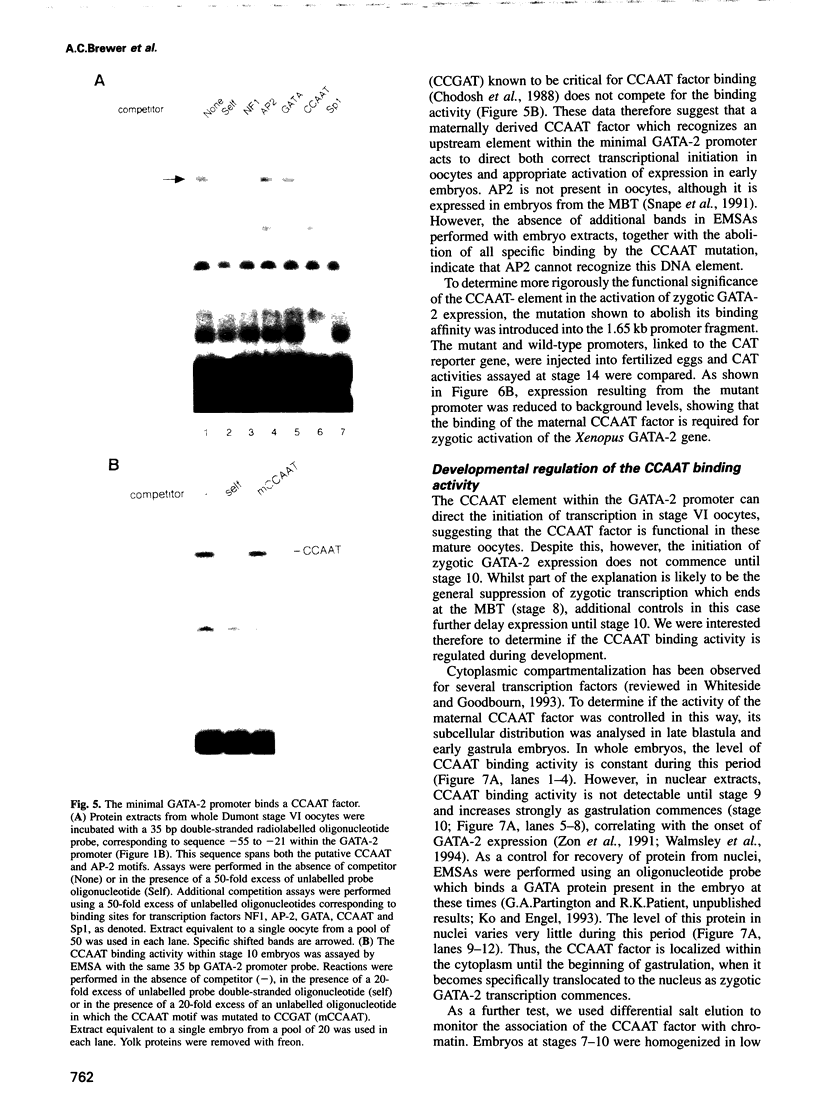
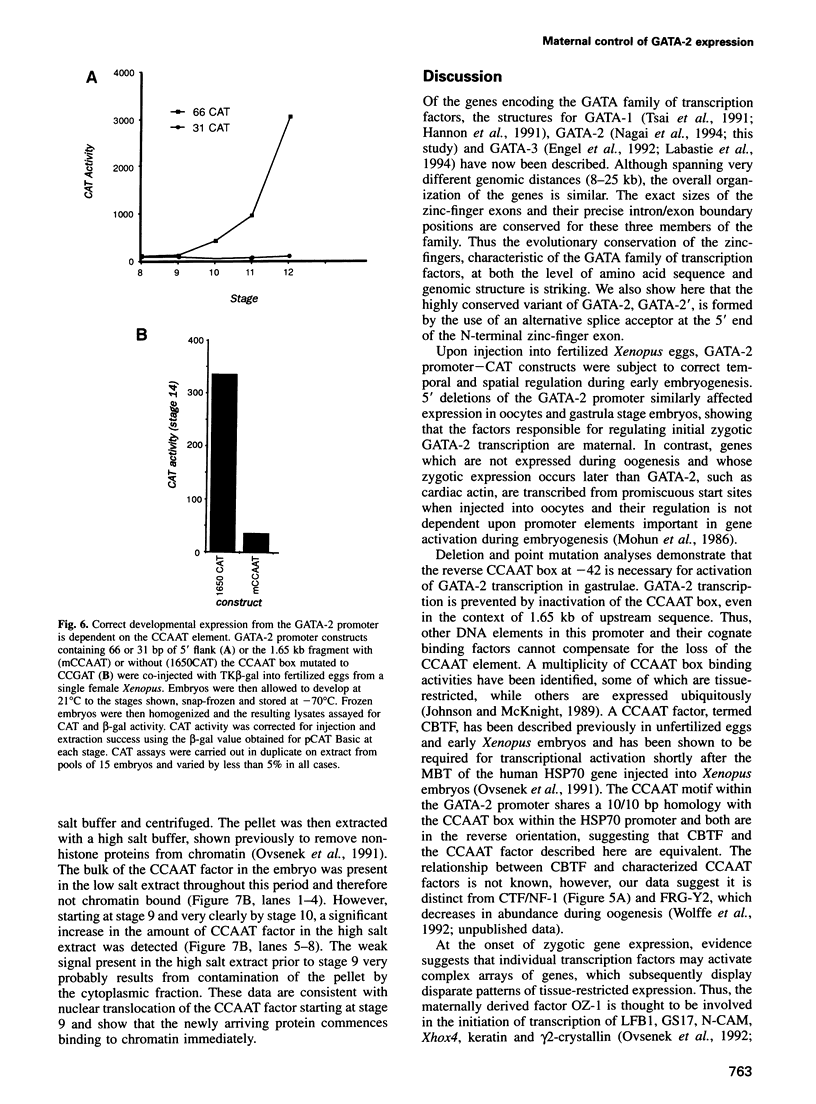
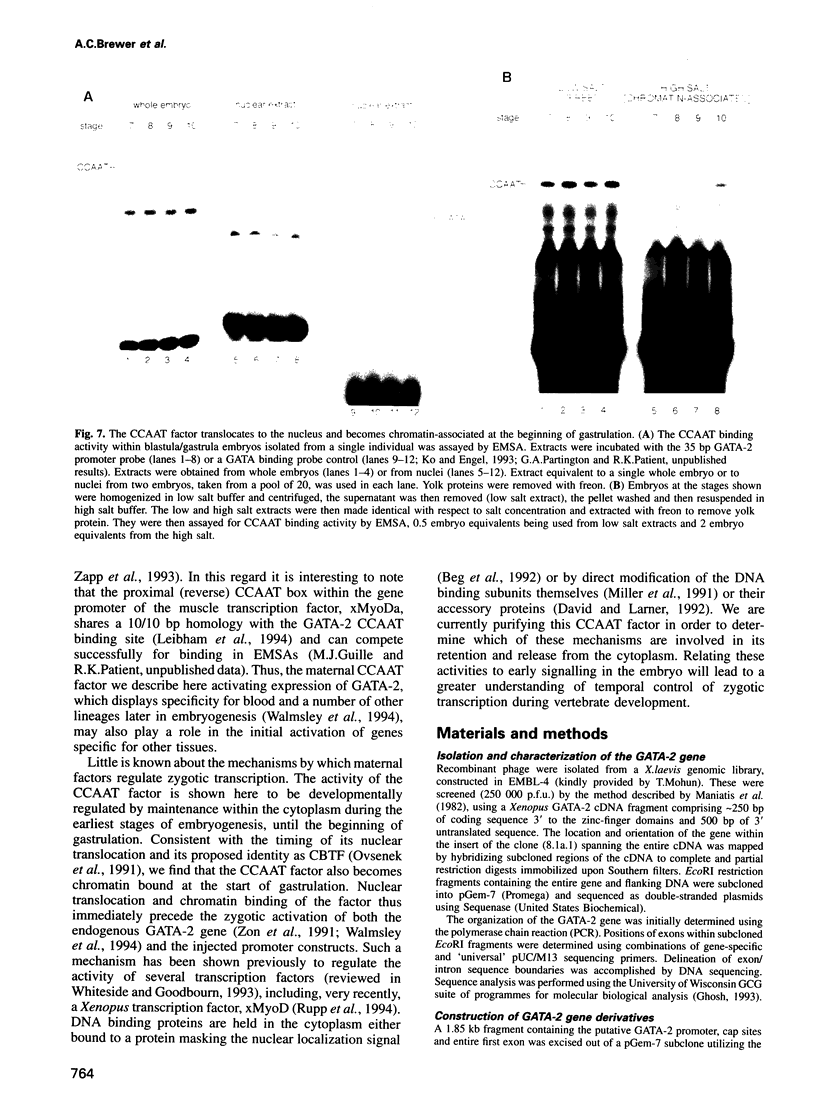


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beg A. A., Ruben S. M., Scheinman R. I., Haskill S., Rosen C. A., Baldwin A. S., Jr I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev. 1992 Oct;6(10):1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- Briegel K., Lim K. C., Plank C., Beug H., Engel J. D., Zenke M. Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev. 1993 Jun;7(6):1097–1109. doi: 10.1101/gad.7.6.1097. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- David M., Larner A. C. Activation of transcription factors by interferon-alpha in a cell-free system. Science. 1992 Aug 7;257(5071):813–815. doi: 10.1126/science.1496402. [DOI] [PubMed] [Google Scholar]
- Devine J. M., Tsang A. S., Williams J. G. Differential expression of the members of the discoidin I multigene family during growth and development of Dictyostelium discoideum. Cell. 1982 Apr;28(4):793–800. doi: 10.1016/0092-8674(82)90058-7. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Beug H., LaVail J. H., Zenke M. W., Mayo K., Leonard M. W., Foley K. P., Yang Z., Kornhauser J. M., Ko L. J. cis and trans regulation of tissue-specific transcription. J Cell Sci Suppl. 1992;16:21–31. doi: 10.1242/jcs.1992.supplement_16.4. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Activation of globin genes during chicken development. Cell. 1981 May;24(2):393–401. doi: 10.1016/0092-8674(81)90329-9. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Hannon R., Evans T., Felsenfeld G., Gould H. Structure and promoter activity of the gene for the erythroid transcription factor GATA-1. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3004–3008. doi: 10.1073/pnas.88.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Karlinsey J., Stamatoyannopoulos G., Enver T. Simultaneous purification of DNA and RNA from small numbers of eukaryotic cells. Anal Biochem. 1989 Aug 1;180(2):303–306. doi: 10.1016/0003-2697(89)90435-1. [DOI] [PubMed] [Google Scholar]
- Kelley C., Yee K., Harland R., Zon L. I. Ventral expression of GATA-1 and GATA-2 in the Xenopus embryo defines induction of hematopoietic mesoderm. Dev Biol. 1994 Sep;165(1):193–205. doi: 10.1006/dbio.1994.1246. [DOI] [PubMed] [Google Scholar]
- Kenten J. H., Molgaard H. V., Houghton M., Derbyshire R. B., Viney J., Bell L. O., Gould H. J. Cloning and sequence determination of the gene for the human immunoglobulin epsilon chain expressed in a myeloma cell line. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6661–6665. doi: 10.1073/pnas.79.21.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff C. GATA tandem repeats detect minisatellite regions in blowfly DNA (Diptera: Calliphoridae). Chromosoma. 1988;96(2):107–111. doi: 10.1007/BF00331042. [DOI] [PubMed] [Google Scholar]
- Ko L. J., Engel J. D. DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol. 1993 Jul;13(7):4011–4022. doi: 10.1128/mcb.13.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Developmental regulation of a gastrula-specific gene injected into fertilized Xenopus eggs. EMBO J. 1985 Dec 16;4(13A):3463–3471. doi: 10.1002/j.1460-2075.1985.tb04105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labastie M. C., Bories D., Chabret C., Grégoire J. M., Chrétien S., Roméo P. H. Structure and expression of the human GATA3 gene. Genomics. 1994 May 1;21(1):1–6. doi: 10.1006/geno.1994.1217. [DOI] [PubMed] [Google Scholar]
- Laughlin M. A., Zeichner S., Kolson D., Alwine J. C., Seshamma T., Pomerantz R. J., Gonzalez-Scarano F. Sodium butyrate treatment of cells latently infected with HIV-1 results in the expression of unspliced viral RNA. Virology. 1993 Oct;196(2):496–505. doi: 10.1006/viro.1993.1505. [DOI] [PubMed] [Google Scholar]
- Laverriere A. C., MacNeill C., Mueller C., Poelmann R. E., Burch J. B., Evans T. GATA-4/5/6, a subfamily of three transcription factors transcribed in developing heart and gut. J Biol Chem. 1994 Sep 16;269(37):23177–23184. [PubMed] [Google Scholar]
- Leibham D., Wong M. W., Cheng T. C., Schroeder S., Weil P. A., Olson E. N., Perry M. Binding of TFIID and MEF2 to the TATA element activates transcription of the Xenopus MyoDa promoter. Mol Cell Biol. 1994 Jan;14(1):686–699. doi: 10.1128/mcb.14.1.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard M. W., Lim K. C., Engel J. D. Expression of the chicken GATA factor family during early erythroid development and differentiation. Development. 1993 Oct;119(2):519–531. doi: 10.1242/dev.119.2.519. [DOI] [PubMed] [Google Scholar]
- Mayor R., Essex L. J., Bennett M. F., Sargent M. G. Distinct elements of the xsna promoter are required for mesodermal and ectodermal expression. Development. 1993 Nov;119(3):661–671. doi: 10.1242/dev.119.3.661. [DOI] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Means A. L., Farnham P. J. Transcription initiation from the dihydrofolate reductase promoter is positioned by HIP1 binding at the initiation site. Mol Cell Biol. 1990 Feb;10(2):653–661. doi: 10.1128/mcb.10.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos G. L., Matthaei K. I., Reed K. C. Occurrence of the (GATA)n sequences in vertebrate and invertebrate genomes. Chromosoma. 1989 Sep;98(3):194–200. doi: 10.1007/BF00329683. [DOI] [PubMed] [Google Scholar]
- Miller I. J., Bieker J. J. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Krüppel family of nuclear proteins. Mol Cell Biol. 1993 May;13(5):2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Reddy B. A., Kloc M., Li X. X., Dreyer C., Etkin L. D. The nuclear-cytoplasmic distribution of the Xenopus nuclear factor, xnf7, coincides with its state of phosphorylation during early development. Development. 1991 Oct;113(2):569–575. doi: 10.1242/dev.113.2.569. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Timmons P. M., Hébert J. M., Rigby P. W., Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 1991 Jan;5(1):105–119. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- Mohun T. J., Garrett N., Gurdon J. B. Upstream sequences required for tissue-specific activation of the cardiac actin gene in Xenopus laevis embryos. EMBO J. 1986 Dec 1;5(12):3185–3193. doi: 10.1002/j.1460-2075.1986.tb04628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthon M. A., Bernard O., Mitjavila M. T., Romeo P. H., Vainchenker W., Mathieu-Mahul D. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood. 1993 Feb 1;81(3):647–655. [PubMed] [Google Scholar]
- Myers R. M., Tilly K., Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986 May 2;232(4750):613–618. doi: 10.1126/science.3457470. [DOI] [PubMed] [Google Scholar]
- Nagai T., Harigae H., Ishihara H., Motohashi H., Minegishi N., Tsuchiya S., Hayashi N., Gu L., Andres B., Engel J. D. Transcription factor GATA-2 is expressed in erythroid, early myeloid, and CD34+ human leukemia-derived cell lines. Blood. 1994 Aug 15;84(4):1074–1084. [PubMed] [Google Scholar]
- Orkin S. H. GATA-binding transcription factors in hematopoietic cells. Blood. 1992 Aug 1;80(3):575–581. [PubMed] [Google Scholar]
- Ovsenek N., Karn H. A., Heikkila J. J. Analysis of CCAAT box transcription factor binding activity during early Xenopus laevis embryogenesis. Dev Biol. 1991 Jun;145(2):323–327. doi: 10.1016/0012-1606(91)90130-u. [DOI] [PubMed] [Google Scholar]
- Ovsenek N., Zorn A. M., Krieg P. A. A maternal factor, OZ-1, activates embryonic transcription of the Xenopus laevis GS17 gene. Development. 1992 Jun;115(2):649–655. doi: 10.1242/dev.115.2.649. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Rutherford T. R., Partington G. A. Transcriptional analysis of human zeta globin genes. EMBO J. 1984 Jul;3(7):1533–1540. doi: 10.1002/j.1460-2075.1984.tb02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp R. A., Snider L., Weintraub H. Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev. 1994 Jun 1;8(11):1311–1323. doi: 10.1101/gad.8.11.1311. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Mathers P. H. Analysis of class II gene regulation. Methods Cell Biol. 1991;36:347–365. doi: 10.1016/s0091-679x(08)60287-3. [DOI] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Snape A. M., Winning R. S., Sargent T. D. Transcription factor AP-2 is tissue-specific in Xenopus and is closely related or identical to keratin transcription factor 1 (KTF-1). Development. 1991 Sep;113(1):283–293. doi: 10.1242/dev.113.1.283. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992 Jan 24;68(2):201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Tsai F. Y., Keller G., Kuo F. C., Weiss M., Chen J., Rosenblatt M., Alt F. W., Orkin S. H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994 Sep 15;371(6494):221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Strauss E., Orkin S. H. Functional analysis and in vivo footprinting implicate the erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Genes Dev. 1991 Jun;5(6):919–931. doi: 10.1101/gad.5.6.919. [DOI] [PubMed] [Google Scholar]
- Walmsley M. E., Guille M. J., Bertwistle D., Smith J. C., Pizzey J. A., Patient R. K. Negative control of Xenopus GATA-2 by activin and noggin with eventual expression in precursors of the ventral blood islands. Development. 1994 Sep;120(9):2519–2529. doi: 10.1242/dev.120.9.2519. [DOI] [PubMed] [Google Scholar]
- Walmsley M. E., Patient R. K. Highly efficient beta globin transcription in the absence of both a viral enhancer and erythroid factors. Development. 1987 Dec;101(4):815–827. doi: 10.1242/dev.101.4.815. [DOI] [PubMed] [Google Scholar]
- Walters M., Martin D. I. Functional erythroid promoters created by interaction of the transcription factor GATA-1 with CACCC and AP-1/NFE-2 elements. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10444–10448. doi: 10.1073/pnas.89.21.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside S. T., Goodbourn S. Signal transduction and nuclear targeting: regulation of transcription factor activity by subcellular localisation. J Cell Sci. 1993 Apr;104(Pt 4):949–955. doi: 10.1242/jcs.104.4.949. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Tafuri S., Ranjan M., Familari M. The Y-box factors: a family of nucleic acid binding proteins conserved from Escherichia coli to man. New Biol. 1992 Apr;4(4):290–298. [PubMed] [Google Scholar]
- Wyllie A. H., Laskey R. A., Finch J., Gurdon J. B. Selective DNA conservation and chromatin assembly after injection of SV40 DNA into Xenopus oocytes. Dev Biol. 1978 May;64(1):178–188. doi: 10.1016/0012-1606(78)90069-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ko L. J., Leonard M. W., Beug H., Orkin S. H., Engel J. D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- Zapp D., Bartkowski S., Holewa B., Zoidl C., Klein-Hitpass L., Ryffel G. U. Elements and factors involved in tissue-specific and embryonic expression of the liver transcription factor LFB1 in Xenopus laevis. Mol Cell Biol. 1993 Oct;13(10):6416–6426. doi: 10.1128/mcb.13.10.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon L. I., Mather C., Burgess S., Bolce M. E., Harland R. M., Orkin S. H. Expression of GATA-binding proteins during embryonic development in Xenopus laevis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10642–10646. doi: 10.1073/pnas.88.23.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]