Abstract
Pemetrexed is an antifolate chemotherapy agent that is active in malignant mesothelioma and non-small cell lung cancer (NSCLC). Pneumonitis is a rare side effect of Pemetrexed. We report the case of 72-year-old female with metastatic poorly differentiated lung adenocarcinoma. She was placed on maintenance pemetrexed and developed gradual progressive dyspnea after first cycle of maintenance of pemextrexed. The computed tomography (CT) of the chest showed ground glass opacity in both lung fields. Transbronchial lung biopsy showed uniform interstitial widening by a cellular chronic infiltrate with areas of type II pneumocyte and exudation of pale eosinophilic edema fluid; features consistent with acute lung injury. Patient improved both clinically and radiological after stopping pemetrexed and starting prednisone. Although pemetrexed induced lung injury is relatively rare, with the increasing use of peme-trexed in first-line treatment and in maintenance therapy of non-small cell lung cancer, awareness of this potential adverse effects is important.
Key words: pemetrexed, lung cancer, pneumonitis.
Introduction
Pemetrexed is an antifolate chemotherapy agent that is active in malignant mesothelioma and non-small cell lung cancer (NSCLC).1,2 Pemetrexed is generally well tolerated and the adverse effect profile includes fatigue, neutropenia and infections.3 Pneumonitis is a known adverse effect of certain chemotherapy agents such as bleomycin. However, Pemetrexed-induced pneumonitis is rare and has not been well characterized.1 We report a patient who developed acute pneumonitis while receiving pemetrexed and review the scientific literature.
Case Report
The patient is a 72-year-old female with metastatic poorly differentiated lung adenocarcinoma. She had attained disease response after 6 cycles of carboplatin and pemetrexed, and was subsequently placed on maintenance with pemetrexed. She had tolerated the doublet chemotherapy well with only mild cytopenias. She had never received radiation therapy during the course. Approximately one month following her first cycle of maintenance pemetrexed, she developed gradually worsening dyspnea. She reported some chills but no fever, cough and hemoptysis. She was hospitalized and required oxygen by nasal cannula for respiratory support. White blood cell count was 8400 per mm3 and hemoglobin was 9.7 g/dL.
Chest computed tomography (CT) scan showed new scattered ground glass infiltrates in both lung fields (Figure 1A). She then underwent bronchoscopy and transbronchial biopsy and bronchoalveolar lavage. Transbronchial biopsy showed uniform interstitial widening by a cellular chronic infiltrate with areas of type II pneumocyte and exudation of pale eosinophilic edema fluid; features consistent with acute lung injury (Figure 2). BAL cultures were negative for PCP, viral and bacterial infections. Pemetrexed was stopped and she was started on oral prednisone dose on 40 mg daily which improved the symptoms clinically within few days. Radiographic evidence of improvement was apparent on a repeat chest CT scan in 4 weeks (Figure 1B).
Figure 1.
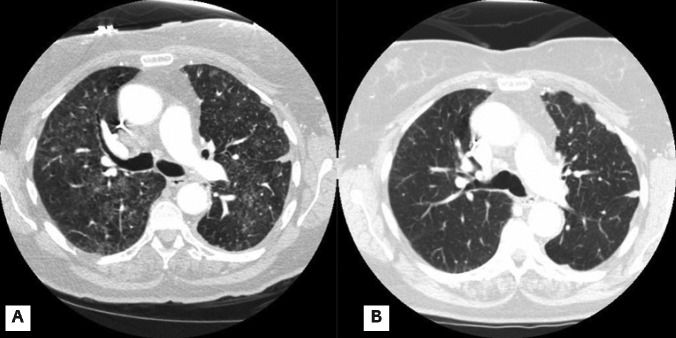
Chest computed tomography scan. A) Bilateral ground glass infiltrates. B) Resolution of infiltrates after stopping pemetrexed and starting prednisone.
Figure 2.
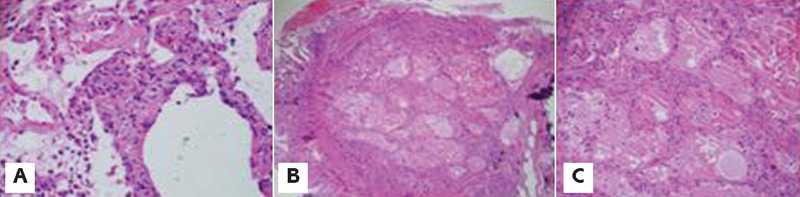
Photomicrograph of transbronchial lung biopsy. A) cellular interstitial pneumonitis with interstitial septal expansion by a mixed inflammatory infiltrate; B) and C) Prominent alveolar edema characterized by pale edema fluid within alveolar spaces (low and high power respectively).
Discussion
It is estimated that 10–20 percent of all patients treated with chemotherapeutic agents have some forms of lung toxicity.4,5 The pathogenesis of chemotherapy-induced lung injury is poorly understood and is thought to be due to direct toxicity, cytokine release, oxidative injury and radiation recall.6 The diagnosis of chemotherapy-induced lung injury is usually made on a combination of compatible clinical presentation, and exclusion of infectious or malignant causes in the presence of an offending agent.7,8 Common chemotherapeutic agents that cause pulmonary injury include bleomycin, busulfan, cyclophosphamide, methotrexate, doxorubicin and gemcitabine.7 Methotrexate induced pulmonary toxicity develops in 2–8% and ranges from pneumonitis to possibly neoplastic. Methotrexate induced pneumonitis may present as pemetrexed pneumonitis or may present as BOOP and even fibrosis.9
Large clinical studies of pemetrexed10 did not report any pulmonary toxicity. We performed a literature review and identified 4 case reports of pemetrexed-induced pneumonitis1,2,8,11 (Table 1). There were 2 cases of fatal pemetrexed-induced pneumonitis and both incidents occurred after only 1 dose of pemetrexed.2,8 Conversely, in our case and the two other reported cases,1,11 lung injury occurred after 4–7 exposures of pemetrexed and were non-fatal and reversible. In the above two cases where biopsy were done, one1 showed diffuse alveolar septal thickening with hyaline membranes where as the other8 showed non specific interstitial infiltrates unlike in our case.
Table 1. Review literature of pemetrexed pneumonitis/lung injury.
| Cases/Authors | Biopsy(Y/N) | Radiation(Y/N) | No of cycles | Died(Y/N) | Severity* | Treatment | X ray Resolution | Time of pneumonitis (after last dose) |
|---|---|---|---|---|---|---|---|---|
| Nagata, et al.2 | No | No | 1 | Yes | Grade 5 | Steroids | None | 4 wks |
| Sakamoto, et al.8 | Yes | No | 1 | Yes | Grade 5 | Steroids | Unknown | 1 day |
| Loriot, et al.11 | No | No | 5 | No | Grade 3 | Steroids | Yes | 3 days |
| Kim Ho, et al.1 | Yes | No | 4 | No | Grade 3 | Steroids | Yes | Few days |
Severity grade based on common terminology criteria for adverse events (CTCAE) v 4.
It is standard practice that patients receive dexamethasone 8 mg daily for 3 days starting on the day prior to pemetrexed. This is done mainly to reduce the severity of cutaneous reactions from pemetrexed. Despite the use of high dose dexamethasone (equivalent to prednisone 50 mg), and the long half-life of dexamethasone (36–54 h), lung injury develops relatively quickly after pemetrexed exposure.
Although pemetrexed induced lung injury is relatively rare, with the increasing use of pemetrexed in first-line treatment and in maintenance therapy of non-small cell lung cancer, awareness of this potential adverse effects is important. Early intervention could potentially reduce the morbidity that patients encounter in an already devastating disease such as lung cancer.
References
- 1.Kim HO, Lee SY, Shim JJ, et al. A case of pemetrexed-induced acute lung injury in non small cell lung cancer. J Thorac Oncol. 2010;5:401–2. doi: 10.1097/JTO.0b013e3181c5b198. [DOI] [PubMed] [Google Scholar]
- 2.Nagata K, Kaji R, Tomii K. Fatal pemetrexed-induced ling injury in patients with advanced mesothelioma. J Thorac Oncol. 2010;5:1714–5. doi: 10.1097/JTO.0b013e3181f1378e. [DOI] [PubMed] [Google Scholar]
- 3.Sun JM, Lee KW, Kim JH, et al. Efficacy and toxicity of pemetrexed as a third line treatment for non small cell lung cancer. Jpn J Clin Oncol. 2009;39:27–32. doi: 10.1093/jjco/hyn118. [DOI] [PubMed] [Google Scholar]
- 4.Limper AH, Rosenow EC., 3rd Drug-induced interstitial lung disease. Curr Opin Pulm Med. 1996;2:396–404. doi: 10.1097/00063198-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Snyder LS, Hertz MI. Cytotoxic drug-induced Lung injury. Semin Respir Infect. 1988;3:217–28. [PubMed] [Google Scholar]
- 6.Vahid B, Mark PE. Pulmonary complications of Novel antineoplastic agents for solid tumors. Chest. 2008;133:528–38. doi: 10.1378/chest.07-0851. [DOI] [PubMed] [Google Scholar]
- 7.Limper AH. Chemotherapy-induced lung disease. Clin Chest Med. 2004;25:53–64. doi: 10.1016/S0272-5231(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto S, Kaburaki K, Sakaguchi S, et al. [Case of pemetrexed induced acute lung injury] Nihon Kokyuki Gakkai Zasshi. 2009;47:415–20. [Article in Japanese] [PubMed] [Google Scholar]
- 9.Conaghan PG, Quinn DI, Brooks PM, Day RO. Hazards of low dose methotrexate. Aust N Z J Med. 1995;25:670–3. doi: 10.1111/j.1445-5994.1995.tb02851.x. [DOI] [PubMed] [Google Scholar]
- 10.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 11.Loriot Y, Ferte C, Gomez-Roca C, et al. Pemetrexed-induced penumonitis: a case report. Clin Lung Cancer. 2009;10:364–6. doi: 10.3816/CLC.2009.n.050. [DOI] [PubMed] [Google Scholar]


