Abstract
E. coli K5 produces heparosan and sheds it into the growth medium in a temperature dependent manner. The shedding is believed to be controlled, at least in part, by enzyme action on the cell-associated capsular polysaccharide, heparosan. One candidate enzyme in such shedding is eliminase. The eliminase gene (elmA) was deleted from the genome of E. coli K5 and its effect on secreted and cell-associated heparosan was investigated. Deletion of the eliminase gene resulted in a significant reduction in heparosan shedding into the medium and heparosan content in the capsule of the cells, indicating its pivotal role in heparosan synthesis and shedding by E. coli K5.
Keywords: E. coli K5, capsular polysaccharide, heparosan, bioengineered heparin, lyase, eliminase
N-Acetyl heparosan (heparosan), the capsular polysaccharide of Escherichia coli O10:K5:H4 (ATCC 23506) (E. coli K5), is similar to heparin, with a higher acetyl content. Since it lacks sulfation and iduronic acid residues, heparosan possesses no antithrombin III binding-site (Linhardt, 2003). Heparosan consists of repeating disaccharide units comprised of [→4) β-D-glucuronic acid (GlcA) (1→4) α-D-N-acetyl-glucosamine (GlcNAc) (1→]n (Wang et al., 2010). Because of its polysaccharide similarity to heparin, heparosan is a promising starting material for the production of heparin by chemo-enzymatic synthesis (Masuko and Linhardt, 2012; Karst and Linhardt, 2003; Xu et al., 2012). Indeed, the synthesis and shedding of large amounts of heparosan by E. coli K5 is essential for the production of bioengineered heparin for potential use as a replacement of the animal-sourced drug (Wang et al., 2010).
The biosynthesis of heparosan in E. coli K5 involves formation of two key metabolic intermediates, UDP-glucose and UDP-N-acetyl-glucosamine, formed by kfiD. This is followed by a series of biotransformations catalyzed by enzymes encoded by two open reading frames, kfiA and kfiC, to synthesize heparosan (Hodson et al., 2000). Heparosan is synthesized in the cytosol, transferred outside the cell by an ABC transporter-dependent mechanism (Whitfield and Roberts, 1999; Whitfield, 2006), and the reducing end is believed to be attached to a phosphatidic acid residue. Two enzymes that have been implicated in the shedding of heparosan by E. coli K5 are K5 lyase (KflA), from the K5A bacteriophage (Clarke et al., 2000) and eliminase (ElmA), from the elmA gene in the E. coli K5 genome (Legoux et al., 1996). These lyases display endo-β-eliminase activity, resulting in a 4,5-unsaturated uronic acid (ΔUA) moiety at the non-reducing end of the heparosan chain. In the present work elmA was deleted to obtain the deletion clone, ΔelmA. The effect of eliminase gene deletion on the production and secretion of heparosan was thus investigated and compared with that of the wild-type strain.
Legoux et al. (1996) previously investigated eliminase by focusing on cloning and overexpression of the enzyme in E. coli K-12 RR1; however, the specific role of eliminase on the heparosan producing strain, E. coli K5, remains unknown. Therefore, we endeavored to elucidate the role of the elmA gene product on heparosan production and shedding from E. coli K5.
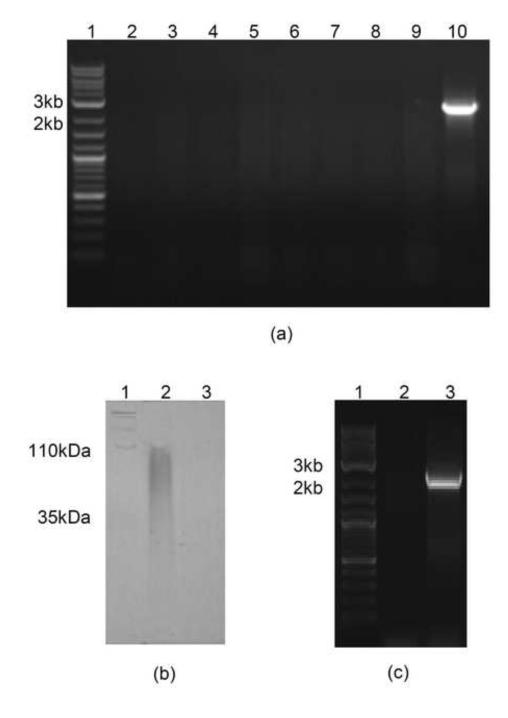
Gene deletion was achieved using the Quick & Easy E. coli Gene Deletion Kit (B-Bridge International, Inc., Cupertino, CA), and elmA gene deletion was confirmed by PCR (Fig. 1). E. coli K5 and E. coli K5 (ΔelmA) were grown in LB medium and the undiluted supernatant was applied to a gradient PAGE gel (4-15%) (Fig. 1), which revealed significant reduction in levels of heparosan shedding by E. coli (ΔelmA). Indeed, no supernatant heparosan was observed in gels stained with Alcian blue, suggesting that eliminase plays a key role in shedding of heparosan from the cells. All further analysis was done using size exclusion chromatography (SEC), since it was not dependent on charge density. Growth was also switched to M9 medium (Sambrook and Russell, 2001) to increase heparosan purity, as LB has been shown previously to generate more impurities (Wang et al., 2010).
Fig. 1.
(a) Gel electrophoresis of PCR products obtained from E. coli K5 (ΔelmA) (lane 2-9) and wild-type E. coli K5 (lane 10) using PCR primers for the eliminase gene (elmA), with 2-log DNA ladder (lane 1); (c) PAGE gel of supernatant heparosan from E. coli K5 (lane 2) and E. coli K5 (ΔelmA) (lane 3), with Hyalose loladder (lane 1); (d) Gel electrophoresis of PCR products obtained from E. coli Nissle 1917 (lane 2) and E. coli K5 (positive control) (lane 3), with 2-log DNA ladder (lane 1).
Prior to SEC analysis, supernatant heparosan was concentrated 10-fold using a 3 kDa MWCO centrifugal concentrator, filtered through a 0.22-μm filter, and applied to a TSKgel G4000-PWxl SEC column (Tosoh Biosciences, Grove City, OH, USA) for analysis. Cell-associated heparosan, concentrated 33-fold when extracted from cell pellets using Tris buffer containing Triton X-100 and sodium deoxycholate, was filtered prior to analysis. Samples were not purified further, since this could result in loss of heparosan. Using a calibration curve and a heparosan standard (10 mg/ml), SEC post-run analysis was performed to determine heparosan concentration, MN, MW, polydispersity (Table 1). SEC results confirmed a reduction in supernatant heparosan levels, with a 50% drop in concentration of heparosan detected in the medium from the E. coli K5 (ΔelmA) strain. Deletion of elmA resulted in a reduction in supernatant heparosan MN (56,450 to 46,480 Da), MW (130,500 to 59,070 Da) and polydispersity (2.31 to 1.27) (Table 1). In the case of cell-associated heparosan, there was ca. 60% drop in heparosan concentration with the ΔelmA mutant. Deletion of elmA also caused a reduction in MN (32,940 to 21,160Da), and MW (64,390 to 45,350 Da), and a slight increase in polydispersity (1.95 to 2.14) in the cell-associated heparosan when compared to wild-type E. coli K5 (Table 1).
Table 1.
Characteristics of heparosan from E. coli K5, E. coli K5 (ΔelmA) and E. coli Nissle 1917.
| Strain | Location | MN | MW | Polydispersity | Concentration (mg/ml) |
Specific productivity (mg/g cells) |
|---|---|---|---|---|---|---|
| E. coli K5 | Cell-associated | 32,940 | 64,390 | 1.95 | 0.16 | |
| Supernatant | 56,450 | 130,500 | 2.31 | 0.10 | 100 | |
| E. coli K5 (ΔelmA) | Cell-associated | 21,160 | 45,350 | 2.14 | 0.06 | |
| Supernatant | 46,480 | 59,070 | 1.27 | 0.05 | 50 | |
| E. coli Nissle 1917 | Cell-associated | 46,620 | 77,930 | 1.67 | 0.08 | |
| Supernatant | 46,020 | 75,420 | 1.64 | 0.25 | 250 |
These observations suggest that deletion of elmA may result in feedback inhibition in the heparosan synthetic pathway, thereby causing a reduction in heparosan production and a reduction in chain elongation for cell-associated and shed heparosan. Indeed, the total heparosan production by the deletion strain was ca. 45% of that of the wild-type E. coli K5. These findings are in agreement with the suggestion by Roberts and coworkers that eliminase may play a regulatory role in capsule synthesis (Clarke et al., 2000), and promoting the accumulation of heparosan.
Not all K5 strains contain eliminase, PCR analysis indicated that E. coli Nissle 1917 did not possess the gene for eliminase (Fig. 1) and, thus, this strain was used for comparison. Interestingly, SEC analysis indicated that supernatant heparosan produced by E. coli Nissle 1917 exhibited greater similarity to E. coli K5 (ΔelmA) than wild-type E. coli K5. Thus, longer heparosan chains in the supernatant of wild-type E. coli K5 fermentation may indeed be due to eliminase, while the intermediate-length chains obtained with E. coli K5 (ΔelmA) and E. coli Nissle 1917 may be due to one or more other, as yet unidentified, enzymes.
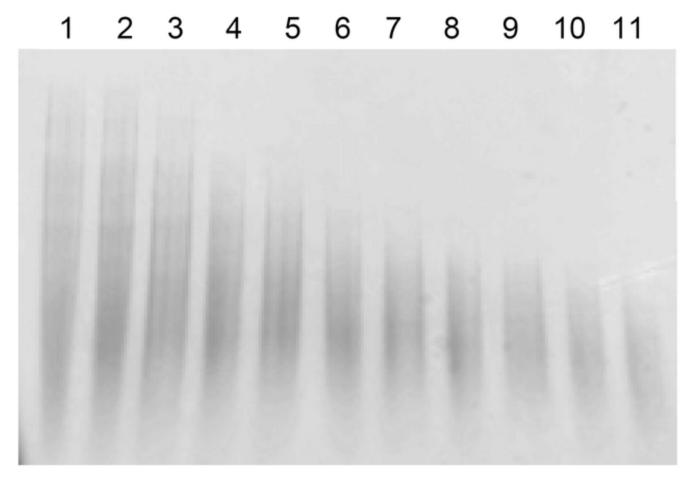
E. coli Nissle 1917 was found to generate higher levels of heparosan in the supernatant, producing 0.25 mg/ml heparosan (250 mg/g cells) as compared to 0.10 mg/ml (100 mg/g cells) generated by wild-type E. coli K5. It may be that eliminase further degrades heparosan once shed, leaving mainly unreacted high molecular weight heparosan and small oligosaccharides and disaccharides; a hypothesis supported by the ability of eliminase to degrade heparosan in vitro (Fig. 2). An alternative hypothesis is that E. coli Nissle 1917 possesses a more effective mechanism for heparosan shedding, generating heparosan of lower polydispersity, a mechanism that may be downregulated by eliminase in E. coli K5. The results do indicate that deletion of elmA resulted in the similarity between E. coli K5 (ΔelmA) and E. coli Nissle 1917 supernatant heparosan. It should be noted that it is possible that the activity of eliminase caused an underestimation of heparosan levels in the supernatant of E. coli K5, since supernatant heparosan from E. coli Nissle 1917 had similar MN and MW to E. coli K5 (ΔelmA).
Fig. 2.
PAGE gel of supernatant heparosan degradation by E. coli K5 cell lysate, samples taken after 0, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 5.0 h incubation at 37 °C and 220 rpm.
Conversely, the profile of cell-associated heparosan extracted from E. coli Nissle 1917 resembled that of wild-type E. coli K5 (Table 1), although E. coli Nissle 1917 produced significantly less cell-associated heparosan (50%) than wild-type E. coli K5, suggesting that the presence of eliminase results in upregulation of capsular heparosan production to cope with the demands of eliminase. This could explain the relatively higher ratio of cell-associated heparosan to supernatant heparosan in E. coli K5 compared with E. coli K5 (ΔelmA) and E. coli Nissle 1917. The variation in capsule thickness could also explain why E. coli K5 is an opportunistic pathogen and E. coli Nissle 1917 is a probiotic strain, since E. coli K5 would more likely be prone to biofilm formation and E. coli Nissle 1917 would likely be motile, as suggested by Zorraquoino et al. (2013).
In conclusion, deletion of the gene encoding eliminase from the genome of E. coli K5 resulted in a dramatic change in the shedding and physical characteristics of E. coli K5 heparosan, with significant reduction in accumulation of heparosan in the cell capsule and in the culture supernatant.
Deletion of eliminase results in a ca. 50% drop in heparosan production by the cell.
Eliminase exerts considerable control over production and shedding of heparosan by E. coli K5.
Eliminase is not solely responsible for heparosan shedding, but appears to be the major contributor.
ACKNOWLEDGEMENTS
This work was funded by the National Institutes of Health through a Bioengineering Research Partnership Grant (HL096972).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Clarke BR, Esumeh F, Roberts IS. Cloning, Expression, and Purification of the K5 Capsular Polysaccharide Lyase (KflA) from Coliphage K5A: Evidence for Two Distinct K5 Lyase Enzymes. J. Bacteriol. 2000;182:3761–3766. doi: 10.1128/jb.182.13.3761-3766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson N, Griffiths G, Cook N, Pourhossein M, Gottfridson E, Lind T, Lidholt K, Roberts IS. Identification That KfiA, a Protein Essential for the Biosynthesis of the Escherichia coli K5 Capsular Polysaccharide, Is an (α)-UDP-GlcNAc Glycosyltransferase. J. Biol. Chem. 2000;275:27311–27315. doi: 10.1074/jbc.M004426200. [DOI] [PubMed] [Google Scholar]
- Karst NA, Linhardt RJ. Recent Chemical and Enzymatic Approaches to the Synthesis of Glycosaminoglycan Oligosaccharides. Curr. Med. Chem. 2003;10:1993–2031. doi: 10.2174/0929867033456891. [DOI] [PubMed] [Google Scholar]
- Legoux R, Lelong P, Jourde C, Feuillerat C, Capdevielle J, Sure V, Ferran E, Kaghad M, Delpech B, Shire D, Ferrara P, Loison G, Salomé M. N-acetyl-heparosan lyase of Escherichia coli K5: gene cloning and expression. J. Bacteriol. 1996;178:7260–7264. doi: 10.1128/jb.178.24.7260-7264.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhardt RJ. Heparin: Structure and Activity. J.Med.Chem. 2003;46:2551–2564. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- Masuko S, Linhardt RJ. Chemoenzymatic synthesis of the next generation of ultralow MW heparin therapeutics. Future Med. Chem. 2012;4:289–296. doi: 10.4155/fmc.11.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 2nd Edition Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2001. [Google Scholar]
- Wang Z, Ly M, Zhang F, Zhong W, Suen A, Hickey AM, Dordick JS, Linhardt RJ. E. coli K5 fermentation and the preparation of heparosan, a bioengineered heparin precursor. Biotechnol. Bioeng. 2010;107:964–973. doi: 10.1002/bit.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C. Biosynthesis and Assembly of Capsular Polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- Whitfield C, Roberts IS. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 1999;31:1307–1319. doi: 10.1046/j.1365-2958.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Pempe EH, Liu J. Chemoenzymatic Synthesis of Heparin Oligosaccharides with both Anti-Xa and Anti-IIa Activities. J. Biol. Chem. 2012:29054–29061. doi: 10.1074/jbc.M112.358523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorraquino V, García B, Latasa C, Echeverz M, Toledo-Arana A, Valle J, Lasa I, Solano C. Coordinated Cyclic-Di-GMP Repression of Salmonella Motility through YcgR and Cellulose. J. Bacteriol. 2013;195:417–428. doi: 10.1128/JB.01789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]