SUMMARY
IκB kinase α (IKKα) activity is required for ErbB2-induced mammary tumorigenesis. Here, we show that IKKα and its activator, NF-κB-inducing kinase (NIK), support the expansion of tumor-initiating cells (TICs) that copurify with a CD24medCD49fhi population from premalignant ErbB2-expressing mammary glands. Upon activation, IKKα enters the nucleus, phosphorylates the cyclin-dependent kinase (CDK) inhibitor p27/Kip1, and stimulates its nuclear export or exclusion. Reduced p27 expression rescues mammary tumorigenesis in mice deficient in IKKα kinase activity and restores TIC self-renewal. IKKα is also likely to be involved in human breast cancer, where its expression shows an inverse correlation with metastasis-free survival, and its presence in the nucleus of invasive ductal carcinomas (IDCs) is associated with decreased nuclear p27 abundance.
INTRODUCTION
Breast cancer (BCa), the leading cause of cancer deaths in women (Jemal et al., 2011), can be classified into Luminal A, Luminal B, ERBB2/HER2 positive, triple-negative, and normal types based on estrogen receptor (ER), progesterone receptor (PR), and ERBB2 expression. Genomic amplification of the ERBB2 locus and/or overexpression of its product occur in 20%–30% of BCa and correlate with poor prognosis (Borg et al., 1989). Trastuzumab, a humanized monoclonal ERBB2 antibody, is effective in treating ERBB2-postitive BCa. However, most patients develop resistance to such drugs, necessitating identification of new therapeutic strategies that target tumor and metastasis initiating cells.
The tumor-initiating cells (TICs) of ERBB2-induced breast and mammary cancers remain elusive. Within normal and lactating mammary glands, the mouse mammary epithelium consists of CD24hiCD49floCD29lo lineage-free (L−) luminal cells, CD24medCD49f+CD29hiL− basal cells, and alveolar cells (Shackleton et al., 2006; Stingl et al., 2006). Luminal epithelial cells comprise CD61+ luminal progenitors and CD61− mature luminal cells (Asselin-Labat et al., 2007), whereas basal epithelial cells include CD24medCD49fhi cells that are enriched for mammary stem cells (MaSC) and CD24medCD49flo myoepithelial cells (Shackleton et al., 2006; Stingl et al., 2006). ErbB2-induced mammary cancer was suggested to be initiated by a subpopulation of parity-identified mammary epithelial cells (PI-MECs) within CD24hiCD49floL− luminal cells, whose proliferation is driven by cyclin D1. CD24medCD49f+L− basal cells that are enriched for MaSCs, however, are not regulated by cyclin D1 (Jeselsohn et al., 2010). Ablation of cyclin D1 in the mammary epithelium retards lobuloalveolar development during pregnancy and causes defective lactation (Fantl et al., 1995). A similar phenotype is exhibited by females homozygous for an IkkαAA knockin allele, in which IκB kinase α (IKKα) activation is prevented by replacement of activation loop serines with alanines (Cao et al., 2001). Furthermore, IKKα activity is required for induction of cyclin D1 upon engagement of receptor activator of NF-κB (RANK) during pregnancy (Cao et al., 2001). RANK activation by RANK ligand (RANKL) produced by PR+ luminal cells drives progesterone-induced basal MaSC expansion (Asselin-Labat et al., 2010; Joshi et al., 2010) and mammary tumorigenesis (Gonzalez-Suarez et al., 2010; Schramek et al., 2010). However, in the absence of progesterone, RANKL is produced by tumor-infiltrating FoxP3+ T cells that cause the IKKα-dependent metastatic spread of mammary cancer cells (Tan et al., 2011). Enhanced RANK expression is also associated with increased metastasis in human BCa (Palafox et al., 2012). MMTV-Erbb2-mice that are homozygote for the IkkαAA allele exhibit delayed tumorigenesis, due to defective TIC self-renewal, but this defect appears to be cyclin D1 independent (Cao et al., 2007).
Besides cyclin D1, other regulators of G1-S phase transition, including CDK2 (Ray et al., 2011), CDK4 (Yu et al., 2006), and p27/Kip1 (Hulit et al., 2006; Muraoka et al., 2002), also participate in ErbB2-induced mammary tumorigenesis. p27 binds cyclin E/A:CDK2 complexes to inhibit G1-S transition (Lu and Hunter, 2010). Kip1 is a haploinsufficient suppressor of ErbB2-induced mammary tumorigenesis; MMTV-Erbb2/Kip1+/− female mice develop mammary tumors earlier than MMTV-Erbb2/Kip1+/+ mice, but complete Kip1 deficiency prevents ErbB2-induced mammary tumorigenesis (Muraoka et al., 2002). Kip1 is rarely inactivated in human cancers, although reduced p27 expression (Catzavelos et al., 1997; Porter et al., 1997) and nuclear exclusion (Shin et al., 2002; Viglietto et al., 2002) correlate with poor prognosis. Tumor promotion by decreased p27-mediated cyclin-dependent kinase (CDK) inhibition may be due to expansion of stem/progenitor cells (Besson et al., 2007), consistent with observations that p27 plays an important role in self-renewal of human embryonic stem cells (Menchón et al., 2011). Several protein kinases that phosphorylate p27 and induce its nuclear export were previously described (Lu and Hunter, 2010).
Our previous study demonstrates that inactivation of IKKα led to lowered incidence and delayed onset, but not complete inhibition, of mammary tumorigenesis (Cao et al., 2007) in MMTV-ErbB2 Tg mice, suggesting ErbB2-induced mammary tumorigenesis may originate from IKKα-dependent and IKKα-independent TICs. In our current study, we aim to identify TICs for ErbB2-induced mammary tumorigenesis and also examine the role of IKKα and its related signaling pathway in regulating mammary TICs.
RESULTS
ErbB2-Induced TICs Form Luminal Mammary Tumors
We dissociated preneoplastic mammary glands from 5-month-old MMTV-Erbb2 mice (Guy et al., 1992), an age at which no visible tumors were detected by whole-mount mammary gland analysis (Figure S1A available online). Freshly sorted epithelial subpopulations, including enriched CD24medCD49fhi MaSC (P5), CD24medCD49flo mature myoepithelial cells (P6) (Shackleton et al., 2006; Stingl et al., 2006), CD24hiCD49floCD61− mature luminal cells (P7), and CD24hiCD49floCD61+ luminal progenitors (P8) (Asselin-Labat et al., 2007), that contained more than 90% TER119−/CD31− cells, among which the P5+P6 basal population was 87% positive for cytokeratin 5 (CK5) and the P7+P8 luminal cells were 98% positive for CK8 (Figure 1A; Figures S1B and S1C), were orthotopically transplanted into the #2 mammary fat pads of Rag1−/− FVB/N females. Palpable tumors were detected after 2–3 months in 90%–100% of mice transplanted with MaSCs and after 3 months in 60% of mice that received luminal progenitors (Figures 1B and 1C). Surprisingly, myoepithelial cells also formed tumors in 50% and 90% of the recipients after 2 or 3 months, respectively, but nearly no tumors appeared in mice transplanted with mature luminal cells (Figures 1B and 1C). MaSCs and myoepithelial cells did not exhibit a significant difference in tumorigenecity, but tumors derived from MaSCs or myoepithelial cells were significantly larger than those derived from luminal progenitors (Figure 1C). Regardless of their origin, all tumors closely resembled spontaneous tumors from MMTV-Erbb2 females in histology and were of the luminal type because they expressed the luminal marker cytokeratin 18 (CK18) and not the basal cell marker CK5 (Figure 1D). Thus, even MaSC and mature myoepithelial cells, which express basal cell markers, seem to generate luminal-type tumors. To further characterize the differentiation potential of MMTV-ErbB2 basal cells and luminal progenitors, we cultured the cells in matrigel. Whereas basal cells formed large spheroids, luminal progenitors formed fewer spheroids and also formed alveolar structures that were not observed in the basal cell cultures (Figures S1D).
Figure 1. Cellular Origins of ErbB2-Induced Mammary Cancer.
(A) Flow cytometry and cell-sorting procedures used to identify the cellular origins of ErbB2-induced mammary cancer.
(B) Mammary tumor incidence 2 and 3 months after orthotopic transplantation of 5 × 104 cells from the indicated sorted populations (n = 10 each for Erbb2/WT cells; n = 5 each for Erbb2/IkkαAA/AA cells).
(C) Tumor volumes at 3 months after transplantation as in (B).
(D) Immunohistochemical analysis of spontaneous Erbb2-induced tumors and tumors generated by transplanted cells. MaSC, mammary stem cells; Myo, mature myoepithelial cells; LP, luminal progenitors; LC, mature luminal epithelial cells; CK5, cytokeratin 5; CK18, cytokeratin 18. See also Figure S1.
IKKα and NIK Promote Basal Cell Expansion and Mammary Tumorigenesis
IKKα is a critical mediator of ErbB2-, but not Wnt- or Ras-induced, mammary tumorigenesis, and its activity is required for TIC self-renewal (Cao et al., 2007). Similar results were obtained using the immortalized ErbB2-induced mammary cancer cell line MT2 (Tan et al., 2011). To identify cellular targets dependent on IKKα, we analyzed preneoplastic mammary glands of 5-month-old MMTV-Erbb2/IkkaAA/+ and MMTV-Erbb2/IkkaAA/AA females by flow cytometry of enriched Ter119−CD45−CD31− (L−) mammary epithelial cells (Figure S1E). IKKα inactivation markedly decreased the CD24medCD49f+L− basal cell population, whereas there was no significant change in the CD24hiCD49floL−total luminal population and a marginal change in CD24hi CD49floCD61+L− luminal progenitors (Figure S1F). Correspondingly, IKKα inactivation prevented spheroid formation by basal cells but had little effect, if any, on spheroid formation by luminal progenitors, although it did reduce their ability to form alveolar structures (Figure S1D). MMTV-Erbb2/IkkaAA/AA mammary glands contained a single layer of CK5+ basal cells but were devoid of p63-positive basal cells and nuclear IKKα-positive cells (Figure S1G), suggesting a heterogeneity of the basal cell population. This effect of IKKα inactivation on basal cell number was absent in FVB/N virgins not carrying the MMTV-Erbb2 transgene (data not shown), explaining why virgin IkkaAA/AA females do not exhibit mammary gland defects (Cao et al., 2001). Thus, IKKα is involved in mammary cell proliferation only in response to pregnancy-associated signals, such as RANKL (Asselin-Labat et al., 2010), or upon elevated ErbB2 activity.
We also transplanted purified MaSCs, mature myoepithelial cells, luminal progenitors, and mature luminal cells from MMTV-Erbb2/IkkaAA/AA females into mammary fat pads of Rag1−/− females (Figure 1B). Consistent with a requirement for IKKα activity in basal cell maintenance, MaSCs or mature myoepithelial cells from MMTV-Erbb2/IkkaAA/AA mice did not give rise to tumors (Figure 1B). In contrast, MMTV-Erbb2/IkkaAA/AA luminal progenitors retained tumorigenic potential and gave rise to tumors in 40% and 60% of recipients after 2 or 3 months, respectively (Figures 1B and 1C). These results suggest that IKKα kinase activity is critical for maintaining basal TICs but is dispensable for expansion of luminal progenitors.
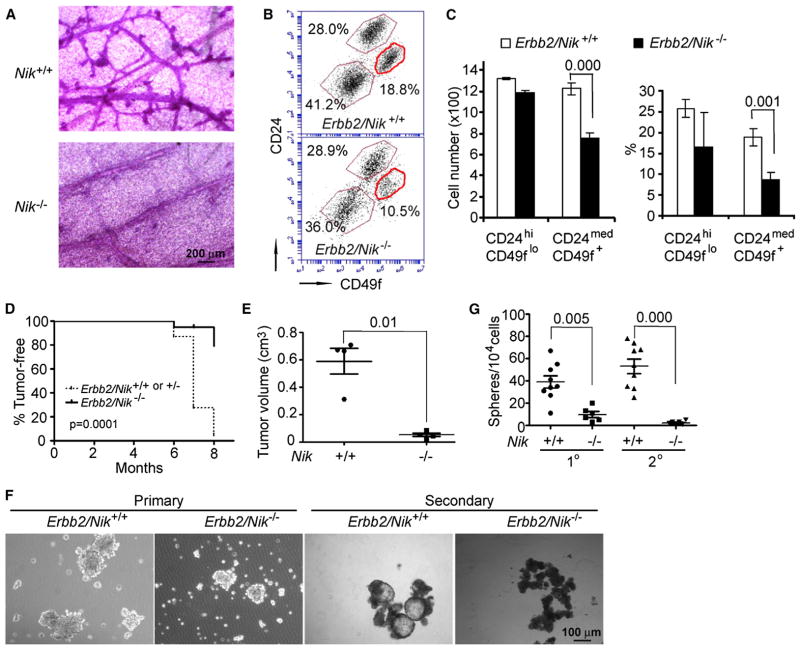
To examine the role of the IKKα-activating kinase NF-κB-inducing kinase (NIK) (Senftleben et al., 2001), we backcrossed C57B/6 Nik−/− mice to the FVB/N background for eight generations and crossed the offspring with FVB/N MMTV-Erbb2 mice to generate MMTV-Erbb2/Nik+/+, MMTV-Erbb2/Nik+/−, or MMTV-Erbb2/Nik−/− females. Nik ablation dramatically reduced the number of preneoplastic mammary gland lesions (Figure 2A) and the number of Ki67-positive mammary epithelial cells (Figures S2A and S2B). Nik ablation also reduced the CD24medCD49f+L− and CK5+ basal cell populations, while having little effect, if any, on the CD24hiCD49floL− luminal cell population (Figures 2B and 2C; Figure S2C). MMTV-Erbb2/Nik−/− females exhibited delayed tumor onset relative to MMTV-Erbb2/Nik+/+ or MMTV-Erbb2/Nik+/− females (Figure 2D). By 8 months of age, only 20% MMTV-Erbb2/Nik−/− females developed mammary tumors, whereas 90% of the MMTV-Erbb2/Nik+/+ and MMTV-Erbb2/Nik+/− cohorts exhibited mammary tumors. Notably, Nik−/− mice in the FVB/N genetic background developed multi-organ granulocytic infiltration and died between 5 to 9 months (data not shown), thus excluding long-term analysis. To circumvent this complication, we isolated primary epithelial cells from mammary tumors of MMTV-Erbb2/Nik−/− and MMTV-Erbb2/Nik+/+ littermate females and transplanted them into FVB/N mammary glands. NIK-deficient cancer cells exhibited markedly reduced tumorigenic potential (Figure 2E) and formed smaller and fewer mammospheres relative to NIK-expressing cells and failed to form secondary mammospheres after passage (Figures 2F and 2G). Likewise, NIK-deficient primary tumors gave rise to fewer secondary tumors and almost no tertiary tumors by comparison to NIK-expressing tumors (Figure S2D). We also examined the frequency of TICs in MMTV-Erbb2/Nik+/+ and MMTV-Erbb2/Nik−/− tumors using serial dilution. As few as 100 MMTV-Erbb2/Nik+/+ primary cancer cells formed tumors in transplanted mice, but it took 104–105 MMTV-Erbb2/Nik−/− cancer cells to form an equivalent number of tumors (Figure S2E). NIK was required for activation and nuclear translocation of IKKα (Figures S2F and S2G).
Figure 2. NIK Is Required for ErbB2-Induced Mammary Tumorigenesis and TIC Self-Renewal.
(A) Stained whole mounts of preneoplastic mammary glands from 5-month-old MMTV-Erbb2/Nik+/+ and MMTV-Erbb2/Nik−/− females.
(B) Flow cytometry of CD45−, CD31−, and Ter119− (lineage-negative, L−) epithelial cells from preneoplastic mammary glands of the indicated mice.
(C) Quantitation of cell numbers (left panel) and percentages (right panel) of different mammary epithelial cell populations from (B). Mean ± SEM (n = 4).
(D) Occurrence of mammary tumors in MMTV-Erbb2/Nik+/+ or MMTV-Erbb2/Nik+/− (n = 23) and MMTV-Erbb2/Nik−/− (n = 21) females.
(E) Volumes of secondary tumors formed by CD45−, CD31−, Ter119−, and CD140a− (L−) tumor cells of the indicated Nik genotypes. Data represent mean ± SEM (n = 4).
(F) Left panels: primary L− mammary tumor cells from indicated mice were grown as mammospheres. Right panels: secondary mammosphere formation by single cell suspensions derived from primary mammospheres of the indicated genotypes.
(G) Quantitation of sphere numbers from (F). Mean ± SEM (n = 6–9). (C), (D), (E), and (G): exact p values are indicated in the panels. See also Figure S2.
The NIK-IKKα Module Induces p27 Nuclear Exclusion
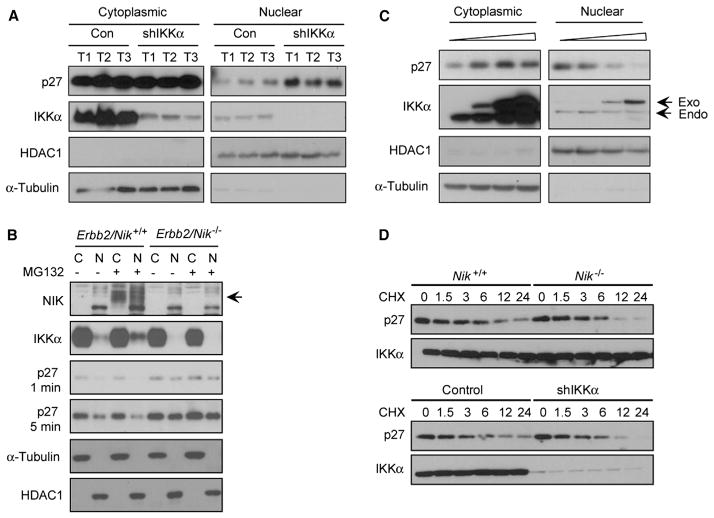
IKKα controls expansion of lobuloalveolar cells during pregnancy through its effect on cyclin D1 expression, and an MMTV-cyclin D1 transgene rescues the lactation defect caused by IKKα inactivation (Cao et al., 2001). However, when we compared cyclin D1 expression in lysates of MMTV-Erbb2/Ikkα+/+ or MMTV-Erbb2/IkkαAA/AA mammary cancer cells, we failed to detect significant differences (Cao et al., 2007). Cyclin D1 and its associated kinase activity, as well as other proteins involved in cell cycle and apoptosis, including p16, p19, E2F1, and Bcl2, were also not significantly altered upon IKKα silencing in MT2 cells (Figures S3A and S3B). Interestingly, the presence of nuclear cyclin D1 was partially correlated with Ki67 nuclear staining in tumors from MMTV-Erbb2/Ikkα+/+ female mice, but no significant correlation was between the two was found in MMTV-Erbb2/IkkαAA/AA tumors or in preneoplastic mammary glands of either MMTV-Erbb2/Ikkα+/+ or MMTV-Erbb2/IkkαAA/AA females (Figure S3C), suggesting that cyclin D1 may be dispensable for expansion of basal TICs. Skp2, whose messenger RNA (mRNA) expression was reported to be controlled by IKKα-mediated NF-κB activation (Schneider et al., 2006), was also not altered in tumors from IKKα-silenced cells (Figure S3D). However, the total amount of p27 was occasionally elevated in some of the tumors derived from IKKα-silenced cells. Although this effect was variable, further analysis revealed that the amount of nuclear p27, a negative regulator of G1-S cell-cycle transition and ErbB2-induced mammary tumorigenesis (Muraoka et al., 2002), was consistently elevated in IKKα-silenced MT2 cells, whereas cytoplasmic p27 was barely affected (Figure 3A). We also purified L− primary cancer cells from Erbb2/Nik+/+ and Erbb2/Nik−/− tumors and treated them with the proteasome inhibitor MG-132 to prevent constitutive NIK turnover. This resulted in NIK accumulation and enhanced IKKα nuclear translocation in MMTV-Erbb2/Nik+/+ cells, as well as reduced nuclear p27 (Figure 3B). p27 levels were higher in MMTV-Erbb2/Nik−/− cells regardless of MG-132 treatment. We also found that spontaneous ErbB2-induced tumors contained many cells with nuclear IKKα, and in most of these cells, p27 was excluded from the nucleus (Figure S3E). These results suggest that the NIK-IKKα module is a negative regulator of nuclear p27 accumulation. Reduced nuclear p27 was also found in MCF7 human breast cancer cells that were transiently transfected with an IKKα expression vector; increased IKKα expression resulted in a dose-dependent decrease in nuclear p27 and increased cytoplasmic p27 (Figure 3C). Silencing of IKKα expression in MCF7 or T47D cells led to nuclear accumulation of p27, without significantly influencing IKKβ expression and nuclear RelA/p65 (Figure S3F). Notably, neither NIK nor IKKα had an effect on the turnover of p27 (Figure 3D).
Figure 3. IKKα Controls the Subcellular Localization of p27.
(A) IKKα controls nuclear p27 in mammary tumors. Mammary tumors were formed by MT2 cells expressing either control or IKKα shRNAs. Cytoplasmic and nuclear fractions were prepared from three individual tumors of each group and analyzed by immunoblotting with the indicated antibodies.
(B) L− primary mammary tumor cells were isolated from the indicated mice and incubated with MG132, a proteasome inhibitor, for 6 hr. Cytoplasmic and nuclear fractions were prepared, separated by SDS-PAGE, and immunoblotted with the indicated antibodies.
(C) Overexpression of IKKα in MCF7 cells decreases nuclear p27 accumulation. MCF7 cells were transfected with increasing amounts of an IKKα expression vector (0–2 μg per plate). After 36 hr, nuclear and cytoplasmic fractions were analyzed by immunoblotting.
(D) NIK and IKKα do not affect p27 turnover. L− mammary tumor cells from the indicated mice or MT2 cells expressing control or IKKα shRNA were treated with cycloheximide (CHX) for the indicated times (hr). Gel-separated cell lysates were immunoblotted with the indicated antibodies. See also Figure S3.
IKKα Phosphorylates p27 and Promotes Its Nuclear Exclusion
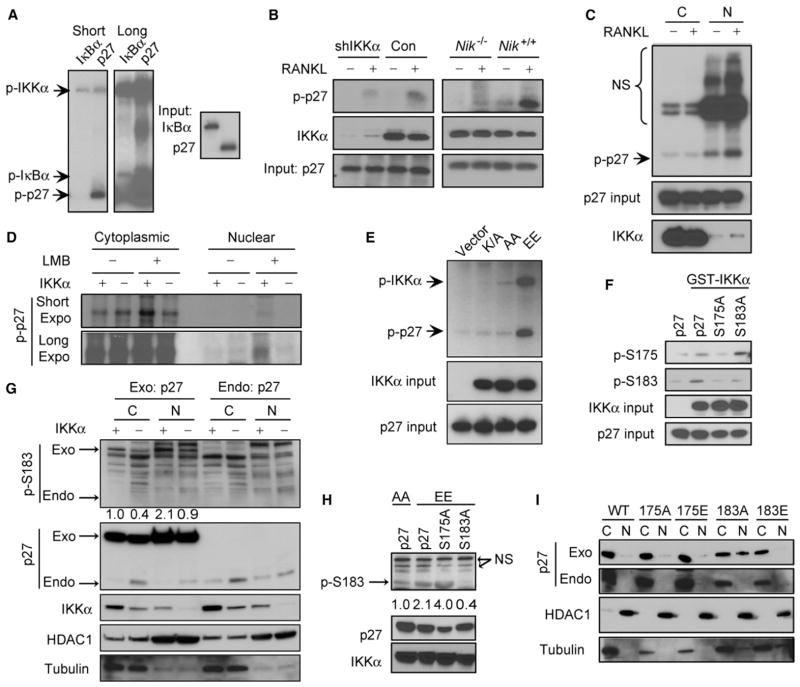
To examine if p27 is a substrate for IKKα, we carried out an in vitro kinase assay. IKKα purified from baculovirus-infected insect cells phosphorylated p27 better than IκBα, the original IKKα substrate (Figure 4A). Endogenous IKKα from RANKL-stimulated mammary cancer cells, whose activation was NIK-dependent, also phosphorylated p27 (Figure 4B). Only the nuclear form of IKKα from RANKL-stimulated cells phosphorylated p27, whereas the cytoplasmic form was inactive (Figure 4C). IKKα kinase activity was required for p27 phosphorylation as only immunoprecipitated constitutively active IKKα(EE), in which activation loop serines were replaced with glutamates, phosphorylated p27, whereas kinase-dead IKKα(K/A) or inactive IKKα(AA) mutants failed to do so (Figure 4D).
Figure 4. p27 Is a Direct Substrate for Nuclear IKKα.
(A) In vitro kinase assays using GST-IKKα purified from insect cells and HIS-p27 or HIS-IκBα purified from Escherichia coli.
(B) In vitro kinase assays using endogenous IKKα immunoprecipitated from control or IKKα-silenced MT2 cells or primary mammary tumor cells of the indicated genotypes incubated with or without RANKL for 2 hr.
(C) Nuclear IKKα is a potent p27 kinase. MT2 cells were treated with or without RANKL. After 2 hr, cytoplasmic and nuclear lysates were immunoprecipitated with an IKKα antibody. The immune complexes were incubated with HIS-p27, and kinase reactions were conducted.
(D) HEK293T cells were transiently transfected with vectors encoding HA-tagged kinase dead (K/A), inactive (AA), or activated (EE) IKKα. Thirty-six hours later, lysates were prepared and immunoprecipitated with HA antibody. Immunecomplex kinase assays with HIS-p27 were performed as above.
(E) 32P metabolic labeling of MT2 cells expressing control or IKKα shRNA with or without leptomycin B (LMB) treatment. After 4 hr, endogenous p27 was immunoprecipitated, separated by SDS-PAGE, and autoradiographed.
(F) Kinase assays using purified GST-IKKα and HIS-p27 or the indicated p27 mutants. Kinase reactions were separated by SDS-PAGE and immunoblotted with the indicated antibodies.
(G) SKBR3 cells expressing control (+) or IKKα (−) shRNAs were transiently transfected with YFP-p27 (exo) or left untransfected (endo) for 16 hr. Cytoplasmic and nuclear lysates were separated by SDS-PAGE and immunoblotted with the indicated antibodies.
(H) SKBR3 cells were transiently transfected with vectors encoding the indicated p27 variants, together with IKKα(AA) or IKKα(EE) expression vectors. After 36 hr, cell lysates were gel analyzed by immunoblotting.
(I) S183 phosphorylation regulates p27 nuclear accumulation. SKBR3 cells were transiently transfected with the indicated p27 variants. After 36 hr, cytoplasmic and nuclear lysates were prepared and analyzed by immunoblotting. See also Figure S4.
To determine if endogenous p27 is phosphorylated by IKKα, MT2 cells transduced with control or IKKα small hairpin RNAs (shRNAs) were labeled with 32P-orthophosphate in the presence or absence of the nuclear export inhibitor leptomycin B (LMB). Neither IKKα expression nor LMB treatment affected phosphorylation of cytoplasmic p27, but LMB treatment enhanced nuclear p27 phosphorylation in control cells, but not in IKKα-silenced cells (Figures 4E and S4A). Mass spectrometric analysis of p27 that was phosphorylated in vitro by IKKα indicated that the main phosphoacceptors were four serines (S) and one threonine (T): S12, T42, S175, S178, and S183 (Figure S4B). The sequences around these phosphoacceptor sites exhibit similarity to known and consensus IKK phosphorylation sites (Figure S4C) and are conserved across mammalian species (Figure S4D). Alignment of p21/Cip1 and p57/Kip2 with the p27 sequence revealed that T42 and S183 are also conserved in p57/Kip2, but not in p21 (data not shown). Replacement of S175 and S183 with alanines reduced the extent of IKKα-mediated p27 phosphorylation (Figure S4E). Wild-type (WT) p27 and the S175A and S183A mutants were in vitro phosphorylated by IKKα and subjected to two-dimensional tryptic phosphopeptide mapping. Replacement of either S175 or S183, which are located within a single tryptic peptide (Figure S4B, blue color), with alanines reduced the phosphorylation of the corresponding peptide (Figure S4F). We also detected p27 phosphorylation at S175 and S183 using phospho-S175- or phospho-S183-specific antibodies. Interestingly, whereas the S183A mutation had no effect on S175 phosphorylation, the S175A substitution affected S183 phosphorylation by recombinant IKKα, suggesting that p27 may be sequentially phosphorylated, at least in vitro (Figure 4F). However, in MDA-MB-231 breast cancer cells, IKKα silencing reduced S183 phosphorylation, while having no significant effect on S175 phosphorylation (Figure S4G). Although phosphorylation of endogenous p27 at S183 was barely detectable, IKKα silencing caused increased nuclear accumulation of endogenous p27 and a significant reduction in both cytoplasmic and nuclear S183 phosphorylation of transiently expressed exogenous p27 in ErbB2-positive SKBR3 cells (Figure 4G). Overexpression of activated IKKα(EE), but not inactive IKKα(AA), induced S183 phosphorylation in breast cancer cells that was abolished by the S183A mutation, but not by the S175A mutation (Figure 4H). Consistent with a role for IKKα in regulating p27 subcellular localization, silencing of IKKα in MDA-MD-231 or MT2 cells reduced CDK2 kinase activity measured with p27 as a substrate (Figure S4H) and overexpression of IKKα(EE), but not IKKα(AA), increased CDK2 kinase activity (Figure S4I). We examined the effect of the two mutations on the nuclear localization of p27 and found that the S183A substitution enhanced p27 nuclear localization in human BCa cells, whereas a phosphomimetic S183E substitution interfered with nuclear localization (Figures 4I and S4J–S4L). Ectopic expression of different p27 variants had no effect on the subcellular localization of endogenous p27 (Figures 4I and S4L). Consistent with these observations, IKKα silencing resulted in decreased cell proliferation as indicated by fewer cells at the S/G2/M phases (Figure S4M) or fewer BrdU-positive cells in MT2-generated tumors (Figure S4N). These data suggest that IKKα-mediated phosphorylation promotes the nuclear export of p27, resulting in enhanced CDK2 activity.
Reduced p27 Expression Restores Basal Cell Expansion and Tumorigenesis in IkkaAA/AA Mice
Kip1 is a haploinsufficient suppressor of ErbB2-induced mammary tumorigenesis (Muraoka et al., 2002). To evaluate the effect of reduced Kip1 dosage on ErbB2-stimulated basal cell expansion, we crossed MMTV-Erbb2 mice with Kip1+/− mice. The preneoplastic mammary glands of 4-month-old MMTV-Erbb2/Kip1+/− mice contained more pronounced side and terminal buds than those of MMTV-Erbb2/Kip1+/+ mice, but there were hardly any differences in appearance of Kip1+/− and Kip1+/+ mammary glands without the MMTV-Erbb2 transgene (Figure S5A). Flow cytometry revealed more CD24medCD49f+L−basal cells in mammary glands of MMTV-Erbb2/Kip1+/− mice (Figures S5B and S5C). To place p27 as a downstream effector of the NIK-IKKα module, we generated MMTV-Erbb2/IkkαAA/+/Kip1+/+, MMTV-Erbb2/IkkαAA/+/Kip1+/−, MMTV-Erbb2/IkkαAA/AA/Kip1+/+, and MMTV-Erbb2/IkkαAA/AA/Kip1+/− mice. As described above, reduced Kip1 dosage enhanced mammary ductal branching and expansion (Figure 5A). The mammary glands of 5-month-old MMTV-Erbb2/IkkαAA/AA females were nearly devoid of side and terminal buds relative to MMTV-Erbb2/IkkαAA/+ females, but this defect was reversed upon deletion of one Kip1 allele (Figure 5A). IKKα inactivation reduced the number of CD24medCD49f+L− (Figures 5B and 5C) and CK5-positive (Figures 5D and S5D) basal cells in MMTV-Erbb2 mammary glands without significantly affecting the number of CD24hiCD49flo L−(Figures 5B and 5C) and CK18-positive luminal cells (Figures 5D and S5D). All of these defects were reversed by monoallelic Kip1 deletion (Figures 5B–5D and S5D). These data suggest that IKKα and p27 function in the same pathway to regulate basal cell expansion. Inactivation of IKKα also inhibited the proliferation of mammary epithelial cells assessed by Ki67 staining (Figures S5E and S5F). This defect was also reversed by ablation of one Kip1 allele (Figures S5E and S5F). We examined the self-renewal ability of L− TICs isolated from tumors of the above four genotypes using the mammosphere formation assay. MMTV-Erbb2/IkkαAA/AA cells formed small primary spheres that could not be successfully propagated upon in vitro passage (Figures 5E and S5G). Kip1 heterozygocity rescued this defect and led to efficient formation of both primary and secondary mammospheres by MMTV-Erbb2/IkkαAA/AA/Kip1+/− TICs. Silencing of p27 expression restored tumorigenic growth in IKKα-silenced MT2 cells (Figures 5F and 5G). Most importantly, MMTV-Erbb2/IkkαAA/AA females exhibited reduced tumor multiplicity relative to MMTV-Erbb2/IkkαAA/+ females, which was completely rescued by ablating one Kip1 allele (Figure 5H).
Figure 5. Reduced p27 Expression Rescues Proliferation and Tumorigenic Defects Caused by IKKα Inactivation.
(A) Preneoplastic mammary glands of 5-month-old MMTV-Erbb2 females of the indicated genotypes were subjected to whole-mount analysis.
(B) Flow cytometric analysis of L− mammary epithelial cells from preneoplastic mammary glands of 5-month-old females of the indicated genotypes.
(C) Quantitation of the different cell populations analyzed in (B).
(D) Quantitation of CK5- and CK18-positive L− mammary epithelial cells in preneoplastic mammary glands from 5-month-old females of the indicated genotypes. Mean ± SEM (n = 3).
(E) Quantitation of mammosphere numbers formed by L− mammary cancer cells of the indicated genotypes or by trypsinized primary mammospheres. Mean ± SEM (n = 4–6).
(F) MT2 cells infected with lentiviruses expressing control, IKKα, or IKKα+p27 shRNAs were analyzed for IKKα and p27 expression by immunoblotting.
(G) Mammary tumor formation by orthotopically transplanted MT2 cells from (F). Tumor volume = length × width2 × 0.52. Values represent mean ± SEM (n = 5).
(H) Quantitation of tumor multiplicity in 7- to 8-month-old females of the indicated genotypes. Tumor multiplicity was determined by counting all visible tumors in each mouse. Mean ± SEM (n = 6–12). (C)–(E), (G), and (H): exact p values are indicated. See also Figure S5.
Nuclear IKKα Correlates with Reduced Nuclear p27 and Increased Metastasis in BCa
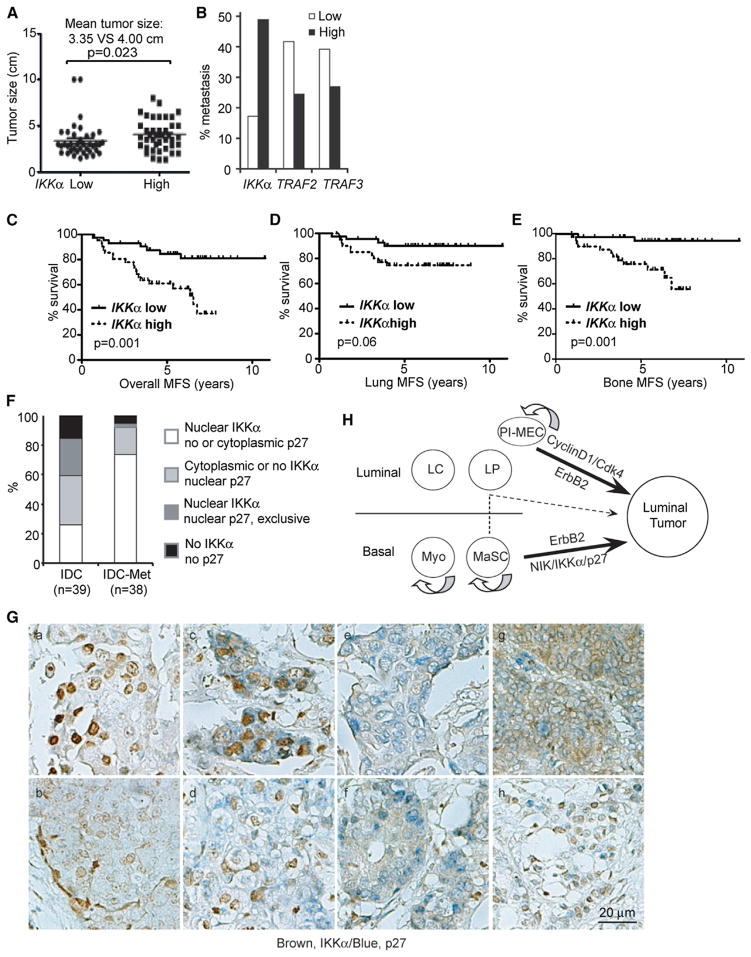
Analysis of the expression array data set GSE2603, which is based on 121 human BCa specimens, including 81 with full prognostic records (Minn et al., 2005), revealed a significant positive correlation between IKKα mRNA amounts and tumor size (Figure 6A). IKKα mRNA abundance also exhibited a positive correlation with the incidence of metastasis (Figure 6B) and a negative correlation with metastasis-free survival (Figures 6C–6E). Curiously, expression of TRAF2 and TRAF3, negative regulators of NIK-IKKα signaling (Vallabhapurapu et al., 2008), exhibited inverse correlation with metastatic events (Figure 6B). In agreement with IKKα’s role in control of mouse mammary epithelial cell proliferation, gene set enrichment analysis (GSEA), a computational method that determines whether a defined set of genes shows statistically significant and concordant differences between two biological states (Subramanian et al., 2005), revealed that 16 gene sets related to cell-cycle progression were significantly upregulated in tumors with high IKKα mRNA content (Figure S6A). Other pathways that were upregulated in the high IKKα group included genes involved in proteasome-mediated proteolysis, DNA replication and synthesis, RNA processing, and mRNA translation (Figure S6A), all of which are critical for cell-cycle progression. A significant enrichment of mRNAs encoding cell-cycle regulators, including CCNB1, CCNB2, CCND3, CCNE2, CCNA2, CDK2, and CDK7, was also observed in the high IKKα group (Figure S6B). Based on IKKα mRNA expression, the above cell-cycle regulators formed three distinct clusters: 51% of the cancers expressed the lowest amounts of cell-cycle regulators clustered in the low IKKα group, and 32% of BCa expressed intermediate amounts of cell-cycle regulators and clustered in the medium IKKα group, whereas 21% expressed the highest amounts of cell-cycle regulators and were in the high IKKα group (Figure S6B).
Figure 6. IKKα Expression and Nuclear Presence Correlate with Tumor Size, Metastasis, and p27 Distribution in BCa.
(A–E) The Affymetrix data set GSE2603, which includes xenografted BCa cells and 81 BCa specimens, was downloaded and analyzed. Clinical breast cancer specimens were divided into two groups based on mRNA expression. (A) Correlation of IKKα mRNA with primary tumor size. (B) Percentage of metastatic tumors in the high and low IKKα, TRAF2, and TRAF3 groups. (C–E) Correlation between IKKα mRNA and overall metastasis-free survival (C), lung metastasis-free survival (D), and bone metastasis-free survival (E).
(F and G) The subcellular distribution of IKKα and p27 in human invasive ductal carcinomas (n = 39 IDCs without metastasis and n = 38 IDCs with metastasis) was determined by immunohistochemistry staining of a BCa tissue array. (a) and (b) represent specimens with nuclear IKKα/no p27; (c) and (d) represent specimens with nuclear IKKα/cytoplasmic p27; (e) and (f) represent specimens with IKKα-negative/nuclear p27; (g) represents specimens with cytoplasmic IKKα/nuclear p27; and (h) represents specimens with nuclear IKKα/nuclear p27. Brown, IKKα; blue, p27.
(H) Schematic summary of cellular origins of ErbB2-induced mammary tumors. NIK-IKKα-p27 signaling targets basal cells to promote ErbB2-induced tumori-genesis. It is unknown if differentiation of basal cells into luminal progenitors is required for luminal tumor formation (dotted line and arrow). Cyclin D1, on the other hand, targets a subpopulation of PI-MECs. See also Figure S6.
We stained an array of human BCa specimens with antibodies to IKKα and p27 (Figure S6C). The majority of invasive ductal carcinomas (IDCs) showed a mutually exclusive relationship between IKKα and p27 such that 25.6% (10/39) of IDCs without metastasis and 73.7% (28/38) of IDCs with metastasis exhibited high nuclear IKKα and low nuclear p27 (Figures 6F and 6G; Figure S6C). Among IDCs with valid ER, PR, and ERBB2 status, 45% ER/PR+ (18/40), 84% ERBB2+ (14/17), and 62% triple-negative (11/17) IDCs exhibited high nuclear IKKα and low nuclear p27. We confirmed that RANK, a potential upstream activator of NIK/IKKα during mammary basal cell expansion (Asselin-Labat et al., 2010) and tumorigenesis (Schramek et al., 2010), is expressed in 22% of IDCs (16/72), among which 87.5% (14/16) exhibit nuclear IKKα expression (Figure S6D). Our analysis plus the known correlation between p27 subcellular distribution and breast cancer prognosis (Shin et al., 2002; Viglietto et al., 2002) underscores the importance of this pathway in initiation and progression of human BCa and suggests that the role of IKKα in p27 regulation may be conserved across different mammalian species.
In summary, we identified different TICs for ErbB2-induced mammary tumorigenesis. The NIK/IKKα module is critical for basal TIC expansion during tumor initiation primarily by phosphorylating p27 and driving its nuclear export (Figure 6H). However, basal TICs-formed tumors have a final luminal appearance, suggesting a conversion from basal to luminal lineage. Indeed, we observed that purified basal cells cultured in matrigel formed three major types of spheroid structures: (1) hollow spheres with one or two layers of cells (data not shown), (2) hollow spheres with multiple cell layers at some regions that contained cells that were double positive for CK5 and CK8 (Figure S6E), suggesting a transition from basal TICs to early progenitors (Liu et al., 2008; Wang et al., 2008), and (3) solid spheres in which most cells were CK8-positive with hardly any CK5 expression (Figure S6F), mimicking the phenotype of basal TIC-derived tumors (Figure 1).
DISCUSSION
Early observations that ErbB2-induced mammary cancer is of the luminal type (Guy et al., 1992) led to the suggestion that ErbB2-induced tumors are derived from luminal progenitors (Visvader, 2011). A previous study also revealed that unlike the Wnt oncogene, the Erbb2 oncogene does not cause basal cell expansion in preneoplastic mammary glands (Shackleton et al., 2006), suggesting that ErbB2-induced tumors may originate from luminal progenitors. Our study, however, suggests that ErbB2-induced mammary cancer can arise from both basal cells and luminal progenitors. Although our current purification procedures do not yield 100% pure populations, one can exclude the possibility that the tumors formed by transplanted basal cells originate from contaminating luminal cells. It is quite unlikely that the ~10% luminal cell contamination in the basal cell population (Figure S1C) can give rise to tumors that are larger and more numerous than those formed by 90% pure luminal progenitors. Furthermore, inactivation of IKKα had no obvious effect on the luminal population, both in preneoplastic mammary glands and in tumors, but it had a clear effect on cells that express basal cell markers and on the tumorigenic potential and mammosphere-forming ability of isolated basal cells. These results suggest that a small portion of the basal cell population may serve as the TICs for ErbB2-induced tumors and that the expansion of such cells that are capable of transdifferentiation into luminal cells is IKKα-dependent. These findings are supported by previous identification of PI-MEC in parous and nulliparous mammary glands (Wagner et al., 2002). These cells express CD49f (Matulka et al., 2007), the same basal cell marker used to identify multipotent MaSC (Shackleton et al., 2006), and were proposed to be the tumorigenic target for ErbB2 (Henry et al., 2004). However, another study has pointed out a different PI-MEC population present within the CD24hiCD49flo luminal cell fraction as an alternative target for ErbB2 (Jeselsohn et al., 2010). Our results suggest that ErbB2 can target both basal cells and luminal progenitors in nulliparous mammary glands. In addition to ErbB2, Notch activation (Bouras et al., 2008) or loss of BRCA1 (Molyneux et al., 2010) can target both basal and luminal cells. The final luminal appearance of all ErbB2-induced tumors may be explained by the commitment of ErbB2-transformed basal cells to luminal differentiation. Furthermore, IKKα-dependent and ErbB2-transformed basal cells may become luminal progenitors (Figure 6H, dotted arrow) that are distinct from luminal PI-MECs, the proposed target of cyclin-D1-associated CDK activity (Jeselsohn et al., 2010). Conversion of MaSC to luminal progenitors was also seen upon Notch activation (Bouras et al., 2008). Conversely, basal mammary tumors initiated by BRCA1 loss are derived from a subset of luminal progenitors (Lim et al., 2009; Molyneux et al., 2010), suggesting that different oncogenic events can cause TIC transdifferentiation. Indeed, we observed that purified basal cells cultured in matrigel formed hollow spheres with multiple cell layers at some regions in which double CK5- and CK8-positive cells were identified (Figure S6E), suggesting a transition from basal TICs to early progenitors (Liu et al., 2008; Wang et al., 2008).
The NIK-IKKα module is only required for maintenance of CD24medCD49f+L− basal cells in ErbB2-induced mammary tumors and is dispensable for maintenance of luminal progenitors. Given that IKKα activity is needed for ErbB2-induced mammary tumorigenesis and for the propagation of such tumors upon transplantation (Cao et al., 2007), our results suggest that IKKα-dependent basal cells are the major TIC type in ErbB2-induced mammary cancer. This connection between ErbB2 and IKKα does not seem to occur during normal development, because ErbB2 is important for virgin ductal morphogenesis (Jackson-Fisher et al., 2004), but IKKα is only needed for pregnancy-induced lobuloalveolar expansion (Cao et al., 2001). Furthermore, whereas IKKα controls lobuloalveolar expansion through cyclin D1 (Cao et al., 2001), the NIK-IKKα module does not regulate cyclin D1 expression during ErbB2-induced tumorigenesis. Instead, the NIK-IKKα cascade acts, at least in part, by decreasing the amount of nuclear p27. The role of p27 in normal mammary gland development is controversial (Davison et al., 2003; Muraoka et al., 2001), but it is a well-established haploinsufficient suppressor of ErbB2-induced mammary cancer (Muraoka et al., 2002). We found that IKKα phosphorylates p27 at S183 to cause its nuclear export. In human BCa, an inverse correlation between nuclear IKKα and nuclear p27 was found in about 73.7% of metastatic IDCs but only in 25% of non-metastatic IDCs, suggesting that the prometastatic activity of IKKα (Tan et al., 2011) correlates with its ability to control p27 distribution and function. It remains to be seen, however, whether the human TICs, within which IKKα induces nuclear exclusion of p27, are of basal origin. Phosphorylation-induced p27 nuclear export is a common mechanism in human cancers that relieves the inhibitory effect of p27 on cyclin:CDK2 complexes (Shin et al., 2002; Viglietto et al., 2002). p27 is phosphorylated at several different sites by distinct kinases. S10 phosphorylation was linked to ERK1/2 (Foster et al., 2003), CDK5 (Kawauchi et al., 2006), or nuclear kinase-interacting stathmin (KIS) (Boehm et al., 2002), and it controls the nuclear export or stability of p27. AKT phosphorylates p27 at T157, which is located within its nuclear localization signal (NLS), and causes its cytoplasmic retention in advanced human BCa (Shin et al., 2002; Viglietto et al., 2002). Other oncogenic kinases also phosphorylate p27 and regulate its subcellular localization and/or turnover (Lu and Hunter, 2010). We now add the NIK-IKKα module as another important regulator of p27 subcellular localization. At this stage, our results suggest that IKKα-mediated p27 phosphorylation has no effect on p27 turnover. However, we did observe that some human BCa specimens with positive nuclear IKKα exhibit reduced or no p27 expression.
IKKα is involved in several signaling pathways that regulate tumor initiation, progression, or metastasis. Besides its role in suppression of maspin, an inhibitor of metastasis in prostate (Luo et al., 2007) and breast (Tan et al., 2011) cancers, IKKα was also reported to regulate cancer cell invasion through classical NF-αB activation (Merkhofer et al., 2010). In hepatocellular carcinoma, IKKα activates Notch signaling by phosphorylating FOXA2 (Liu et al., 2012). Notch activation is critical for commitment of MaSC to luminal differentiation and leads to mammary tumorigenesis (Bouras et al., 2008). Although Notch activation cannot explain the role of IKKα in expansion of basal TICs, it will be interesting to determine if Notch is activated by IKKα-mediated phosphorylation of Foxa2 during conversion of basal TICs into luminal progenitors.
The upstream signal responsible for activation of the NIK-IKKα module during ErbB2-induced tumorigenesis remains elusive. In human IDCs, IKKα nuclear staining is not tightly linked to the ER, PR, or ERBB2 status, suggesting an ERBB2-independent mechanism of IKKα activation. It is reasonable to speculate that RANK signaling may be responsible for basal cell expansion in ErbB2-induced tumors, as it does in normal mammary glands stimulated with progesterone (Asselin-Labat et al., 2010; Joshi et al., 2010). Indeed, a recent study found that elevated RANK expression is linked to metastasis in human BCa (Palafox et al., 2012). However, RANKL, which is required for RANK activation, is produced either by progesterone-stimulated normal luminal epithelial cells or tumor-infiltrating FoxP3+ T cells in advanced ErbB2-induced tumors, and it is not present during early stages of ErbB2 tumorigenesis (Gonzalez-Suarez et al., 2010; Tan et al., 2011). If RANK signaling is responsible for IKKα activation in ErbB2-induced cancer, it should take place during a narrow window when progesterone increases at diestrus, resulting in expansion of CD24medCD49f+L− basal cells (Joshi et al., 2010). However, other signals may maintain NIK-dependent IKKα activation once progesterone-induced RANKL expression had subsided. Perhaps, the upregulation of RANK results in its synergistic interaction with other signaling molecules, thereby generating a signal that leads to NIK stabilization and IKKα activation. However, in those human IDCs with nuclear IKKα staining that are RANK negative, other signaling pathways are likely to activate the NIK-IKKα module.
EXPERIMENTAL PROCEDURES
Mice and Human BCa Tissue Array
Mice were maintained under specific pathogen-free conditions, and experimental protocols were approved by the University of California, San Diego Animal Care Program, following National Institutes of Health (NIH) guidelines. MMTV-Erbb2-tg and Nik−/− (Yin et al., 2001) mice were intercrossed for eight generations to generate mice of the expected genotypes in the FVB/N background. MMTV-Erbb2/IkkαAA/AA male mice were further crossed with Kip1+/− females (Kiyokawa et al., 1996) of the C57BL/6 background. Preneoplastic mammary glands were harvested at 5 months of age unless otherwise indicated. Tumors were considered established when they became palpable for two consecutive weeks. One million cancer cells were injected into the #2 mammary fat pad for orthotopic transplantation unless otherwise indicated. Tumor size was measured using a caliper and volume was calculated as length × width2 × 0.52.
Human BCa tissue array was kindly provided by Dr. Jin Q. Cheng at the H. Lee Moffitt Cancer Center. All primary BCa specimens were obtained from patients who underwent surgery at the H. Lee Moffitt Cancer Center, and samples were deidentified prior to our analysis. The Affymetrix data set GSE2603 was previously published, and all samples were deidentified prior to our analysis (Minn et al., 2005).
Harvesting Mammary Epithelial Cells and Flow Cytometry
Single cell suspensions of preneoplastic mammary glands or tumors were prepared (Shackleton et al., 2006) with slight modification. Briefly, tissues were harvested and cut into small pieces, followed with 300 U/ml collagenase and 100 U/ml hyaluronidase (Stemcell, Vancouver, BC, Canada) digestion for 6 hr in 2% FCS-containing HBSS. Organoids were sequentially resuspended in 0.25% trypsin-EDTA (Mediatech, Corning, NY, USA) for 3 min, 5 μg/ml dispase I (Stemcell), and 0.1 μg/ml DNase (Worthington, Lakewood, NJ, USA) for 1 min before filtration through a 40 μm mesh and antibody staining. Mammary or tumor epithelial cells were magnetically purified using a Mammary Epithelial Cell Enrichment Kit (Stemcell) to remove lineage-positive (CD45-, CD31-, and Ter119-positive) cells, followed by surface-labeling with CD24, Cd49f, and CD61 antibodies and 7-AAD for cell viability before flow cytometric analysis.
Statistics
All results wherever necessary were subjected to statistical analyses. A two-tailed Mann-Whitney test, nonparametric without assuming Gaussian distribution, was performed for most studies. A Kaplan-Meier curve (for tumorigenesis study and metastasis-free survival of human patients) was generated by Prism software and analyzed with a log rank (Mantel-Cox) test.
Supplementary Material
Significance.
Our results indicate that the NIK-IKKα module is an important regulator of ErbB2-induced mammary tumorigenesis by virtue of its ability to promote the nuclear exclusion of p27/Kip1, thereby supporting the proliferation and expansion of TICs. Although the IKKα-dependent TICs copurify with a cell population that expresses basal cell markers, these cells give rise to luminal tumors. The presence of nuclear IKKα in human breast cancer and its correlation with the nuclear exclusion of p27 suggest that targeting the NIK-IKKα module offers an opportunity to prevent the expansion of breast cancer TICs and thereby control tumor recurrence and metastatic spread.
Acknowledgments
We thank Santa Cruz Biotechnology, Cell Signaling, and GeneTex for providing antibodies, T. Hunter and J. Meisenhelder for discussions and technical support, J. Cheng for providing human BCa tissue array, S. Dowdy and C. Denicourt for sharing reagents, and A. Koff for sharing Kip1+/− mice. W.Z. was supported by a postdoctoral fellowship from Susan G. Komen for the Cure (KG080649) and a K99/R00 award from the National Institutes of Health (NIH) (CA158055). X.W. was supported by a postdoctoral fellowship from Susan G. Komen for the Cure (KG111506). A.S. was supported by an NIH training grant (T32CA121938). Work was also supported by an NIH grant (CA127923) to M.K., who is an American Cancer Society Research Professor.
Footnotes
Supplemental Information includes six figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.ccr.2013.03.012.
References
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- Besson A, Hwang HC, Cicero S, Donovan SL, Gurian-West M, Johnson D, Clurman BE, Dyer MA, Roberts JM. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev. 2007;21:1731–1746. doi: 10.1101/gad.1556607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ, Nabel EG. A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J. 2002;21:3390–3401. doi: 10.1093/emboj/cdf343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg A, Linell F, Idvall I, Johansson S, Sigurdsson H, Fernö M, Killander D. HER2/neu amplification and comedo type breast carcinoma. Lancet. 1989;1:1268–1269. doi: 10.1016/s0140-6736(89)92365-9. [DOI] [PubMed] [Google Scholar]
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Cao Y, Luo JL, Karin M. IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc Natl Acad Sci USA. 2007;104:15852–15857. doi: 10.1073/pnas.0706728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, et al. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997;3:227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- Davison EA, Lee CS, Naylor MJ, Oakes SR, Sutherland RL, Hennighausen L, Ormandy CJ, Musgrove EA. The cyclin-dependent kinase inhibitor p27 (Kip1) regulates both DNA synthesis and apoptosis in mammary epithelium but is not required for its functional development during pregnancy. Mol Endocrinol. 2003;17:2436–2447. doi: 10.1210/me.2003-0199. [DOI] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- Foster JS, Fernando RI, Ishida N, Nakayama KI, Wimalasena J. Estrogens down-regulate p27Kip1 in breast cancer cells through Skp2 and through nuclear export mediated by the ERK pathway. J Biol Chem. 2003;278:41355–41366. doi: 10.1074/jbc.M302830200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980–6985. doi: 10.1038/sj.onc.1207827. [DOI] [PubMed] [Google Scholar]
- Hulit J, Lee RJ, Li Z, Wang C, Katiyar S, Yang J, Quong AA, Wu K, Albanese C, Russell R, et al. p27Kip1 repression of ErbB2-induced mammary tumor growth in transgenic mice involves Skp2 and Wnt/beta-catenin signaling. Cancer Res. 2006;66:8529–8541. doi: 10.1158/0008-5472.CAN-06-0149. [DOI] [PubMed] [Google Scholar]
- Jackson-Fisher AJ, Bellinger G, Ramabhadran R, Morris JK, Lee KF, Stern DF. ErbB2 is required for ductal morphogenesis of the mammary gland. Proc Natl Acad Sci USA. 2004;101:17138–17143. doi: 10.1073/pnas.0407057101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C, Hinds PW. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell. 2010;17:65–76. doi: 10.1016/j.ccr.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, et al. kConFab. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci USA. 2008;105:1680–1685. doi: 10.1073/pnas.0711613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Lee DF, Chen CT, Yen CJ, Li LY, Lee HJ, Chang CJ, Chang WC, Hsu JM, Kuo HP, et al. IKKα activation of NOTCH links tumorigenesis via FOXA2 suppression. Mol Cell. 2012;45:171–184. doi: 10.1016/j.molcel.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle. 2010;9:2342–2352. doi: 10.4161/cc.9.12.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Menchón C, Edel MJ, Izpisua Belmonte JC. The cell cycle inhibitor p27Kip1 controls self-renewal and pluripotency of human embryonic stem cells by regulating the cell cycle, Brachyury and Twist. Cell Cycle. 2011;10:1435–1447. doi: 10.4161/cc.10.9.15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkhofer EC, Cogswell P, Baldwin AS. Her2 activates NF-kappaB and induces invasion through the canonical pathway involving IKKalpha. Oncogene. 2010;29:1238–1248. doi: 10.1038/onc.2009.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R, Mackay A, Grigoriadis A, Tutt A, Ashworth A, et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell. 2010;7:403–417. doi: 10.1016/j.stem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Muraoka RS, Lenferink AE, Law B, Hamilton E, Brantley DM, Roebuck LR, Arteaga CL. ErbB2/Neu-induced, cyclin D1-dependent transformation is accelerated in p27-haploinsufficient mammary epithelial cells but impaired in p27-null cells. Mol Cell Biol. 2002;22:2204–2219. doi: 10.1128/MCB.22.7.2204-2219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka RS, Lenferink AE, Simpson J, Brantley DM, Roebuck LR, Yakes FM, Arteaga CL. Cyclin-dependent kinase inhibitor p27(Kip1) is required for mouse mammary gland morphogenesis and function. J Cell Biol. 2001;153:917–932. doi: 10.1083/jcb.153.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palafox M, Ferrer I, Pellegrini P, Vila S, Hernandez-Ortega S, Urruticoechea A, Climent F, Soler MT, Muñoz P, Viñals F, et al. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 2012;72:2879–2888. doi: 10.1158/0008-5472.CAN-12-0044. [DOI] [PubMed] [Google Scholar]
- Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- Ray D, Terao Y, Christov K, Kaldis P, Kiyokawa H. Cdk2-null mice are resistant to ErbB-2-induced mammary tumorigenesis. Neoplasia. 2011;13:439–444. doi: 10.1593/neo.101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G, Saur D, Siveke JT, Fritsch R, Greten FR, Schmid RM. IKKalpha controls p52/RelB at the skp2 gene promoter to regulate G1- to S-phase progression. EMBO J. 2006;25:3801–3812. doi: 10.1038/sj.emboj.7601259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krähn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietto G, Motti ML, Bruni P, Melillo RM, D’Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- Wang XY, Yin Y, Yuan H, Sakamaki T, Okano H, Glazer RI. Musashi1 modulates mammary progenitor cell expansion through proliferin-mediated activation of the Wnt and Notch pathways. Mol Cell Biol. 2008;28:3589–3599. doi: 10.1128/MCB.00040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wu L, Wesche H, Arthur CD, White JM, Goeddel DV, Schreiber RD. Defective lymphotoxin-beta receptor-induced NF-kappaB transcriptional activity in NIK-deficient mice. Science. 2001;291:2162–2165. doi: 10.1126/science.1058453. [DOI] [PubMed] [Google Scholar]
- Yu Q, Sicinska E, Geng Y, Ahnström M, Zagozdzon A, Kong Y, Gardner H, Kiyokawa H, Harris LN, Stål O, Sicinski P. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9:23–32. doi: 10.1016/j.ccr.2005.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.