Abstract
Modular Cullin-RING E3 ubiquitin ligases (CRLs) use substrate binding adaptor proteins to specify target ubiquitylation. Many of the ~200 human CRL adaptor proteins remain poorly studied due to a shortage of efficient methods to identify biologically relevant substrates. Here, we report the development of Parallel Adaptor Capture (PAC) proteomics, and its use to systematically identify candidate targets for the leucine-rich repeat family of F-box proteins (FBXLs) that function with SKP1-CUL1-F-box protein (SCF) E3s. In validation experiments, we identify the unstudied F-box protein FBXL17 as a regulator of the NFR2 oxidative stress pathway. We demonstrate that FBXL17 controls the transcription of the NRF2 target HMOX1 via turnover of the transcriptional repressor BACH1 in the absence or presence of extrinsic oxidative stress. This work identifies a role for SCFFBXL17 in controlling the threshold for NRF2-dependent gene activation and provides a framework for elucidating the functions of CRL adaptor proteins.
Introduction
Signaling through the ubiquitin-proteasome system regulates a wide array of cellular processes through both degradative and non-degradative mechanisms (Behrends and Harper 2011). In this system, ubiquitin is covalently coupled to lysine residues in substrates through thioester-driven E1 ubiquitin activating enzyme-E2 ubiquitin conjugating enzyme-E3 ubiquitin ligase cascades. E3s function as specificity factors in ubiquitin transfer by physically associating with substrates, and by promoting ubiquitin transfer, either directly from the E2 to the substrate, as occurs with RING-domain E3s, or via a thioester intermediate in the E3 itself, as occurs in HECT and RBR E3s (Deshaies and Joazeiro 2009; Komander and Rape 2012).
RING-finger proteins constitute the largest class of E3s, and include simple RING E3s which directly bind substrates, and cullin-RING ligases (CRLs), which form multi-meric assemblies containing one of two RING-finger proteins (RBX1 and RBX2), and one of numerous substrate specific receptors (Deshaies and Joazeiro 2009). SKP1-CUL1-F-box protein (SCF) complexes were the first CRL to be defined (Bai et al., 1996; Feldman et al., 1997; Skowyra et al., 1997). CUL1 functions as a scaffold and associates with RBX1 via its C-terminal cullin-homology domain and with a SKP1-F-box protein substrate receptor module through its N-terminus (Zheng et al., 2002). The cullin-homology domain also contains a lysine residue that is specifically conjugated to the ubiquitin-like protein NEDD8 by the NEDD8 conjugating enzyme UBC12 (Deshaies and Joazeiro 2009), in conjunction with the bi-partite neddylation E3 DCN1-RBX1 (Monda et al., 2013; Scott et al., 2011; Scott et al., 2010). CRL neddylation dramatically activates its ubiquitin transfer activity (Duda et al., 2008; Saha and Deshaies, 2008). Importantly, rapid and global interference in the CRL pathway can be accomplished by pharmacological inhibition of the NEDD8 conjugation pathway via MLN4924, an inhibitor of the NEDD8 E1 activating enzyme (Soucy et al., 2009). Addition of MLN4924 to cells leads to dramatic accumulation of unstable CRL targets (Soucy et al., 2009) without substantially altering the constellation of SCF assemblies (Bennett et al., 2010; Lee et al., 2011).
F-box proteins contain two important domains: the F-box domain, which binds to SKP1, and C-terminal WD40 (FBXW), leucine-rich repeat (LRR) (FBXL), or other (FBXO) types of protein interaction domains that bind substrates and/or regulatory components (Bai et al., 1996; Feldman et al., 1997; Skowyra et al., 1997, Jin et al., 2004). Biochemical and structural studies have defined prominent roles for substrate phosphorylation in their recruitment to particular F-box proteins, including FBXW1(β-TRCP1), FBXW7, and FBXL1 (SKP2) (reviewed in Cardozo and Pagano, 2004; Skaar et al., 2013; Welcker and Clurman, 2008). However, it is not clear whether all F-box proteins require substrate modification for recognition, and several adaptors for other Cullin-based CRLs (e.g. SPOP) do not require post-translational modification of substrate for engagement by the relevant CRL-adaptor complex (Zhuang et al., 2009).
A major challenge in the ubiquitin field is the identification of substrates for particular E3s, due to the typically transient association between E3s and their substrates (Deshaies and Joazeiro 2009; Harper and Tan, 2012). This is especially the case for F-box proteins and CRL adaptors in general, given the large number of adaptor proteins that have been identified (~200). While numerous substrates have been identified for a small number of deeply studied F-box proteins such as β-TRCP1/2 and FBXW7 (Cardozo and Pagano, 2004; Skaar et al., 2013; Welcker and Clurman, 2008), many F-box proteins are largely or completely unstudied. The identification of substrates in mammalian cells has typically occurred on a case-by-case basis, most often using affinity enrichment methods (Yumimoto et al., 2012; Skaar et al., 2013), but more recently, global approaches using either diGLY capture or the Global Protein Stability system have been used (Benanti et al., 2007; Emanuele et al., 2011; Kim et al., 2011; Yen and Elledge, 2008). With both global approaches, secondary screens are required to identify specific adaptors for candidate substrates. An additional challenge concerns adaptor and target cell type specificity and signaling mechanisms upstream of target interaction that may be required for engagement. While some targets may be constitutively able to be recognized by cognate adaptors in many or all cell types (for example, cell cycle targets of SCFs in proliferating cells), other targets may be signal or cell type specific and may be missed in particular experimental settings.
Here we report the systematic identification of candidate substrates and binding proteins for the FBXL subfamily of SCF ubiquitin ligases using a Parallel Adaptor Capture (PAC) proteomics approach. The approach couples the CompPASS (Comparative Proteomics Analysis Software Suite) system for the identification of high-confidence candidate interacting proteins (HCIPs) (Behrends et al., 2010; Sowa et al., 2009) with brief cell treatments using either the proteasome inhibitor Bortezomib (Btz) or CRL inhibitor MLN4924. These treatments increase the abundance of CRL and proteasomal substrates, and through mass action, allow increased levels of substrates to be recovered in association with CRL adaptor protein. Dozens of candidate substrates and binding proteins were found across the 19 FBXL proteins analyzed, and extensive validation studies verified a wide cross-section of candidate interacting proteins. In order to demonstrate the utility of this resource, we analyzed the FBXL17 network, an orphan F-box protein. Among the candidate substrates for SCFFBXL17 was the BTB and CNC homology 1 protein BACH1, which functions together with MAF DNA binding proteins to repress the transcription of genes under the control of the NRF2 (Nuclear factor erythoid-derived 2 related factor 2, also called NFE2L2) oxidative stress pathway (Kitamuro et al., 2003; Sun et al., 2002). In the absence of oxidative stress, NRF2 is and unstable protein that is subject to degraded in the cytoplasm via a CUL3KEAP1 and proteasome dependent mechanism (Cullinan et al., 2004). Upon stress, KEAP1 is inactivated and NRF2 accumulates in the nucleus. BACH1 inactivation, at least in part through a proteasomal pathway, has been shown to facilitate access of NRF2-MAF complexes to promoters of genes that protect cells from oxidative stress, including the HMOX1 promoter, thereby leading to transcriptional activation (Oyake et al., 1996; Sun et al., 2002; Zenke-Kawasaki et al., 2007). We find that BACH1 turnover and HMOX1 transcription in both the absence and presence of extrinsic oxidative stress requires FBXL17, thereby revealing a key role for FBXL17 in controlling the regulatory balance between BACH1 and NRF2.
Results and Discussion
A Parallel Adaptor Capture (PAC) proteomics method for F-box target discovery
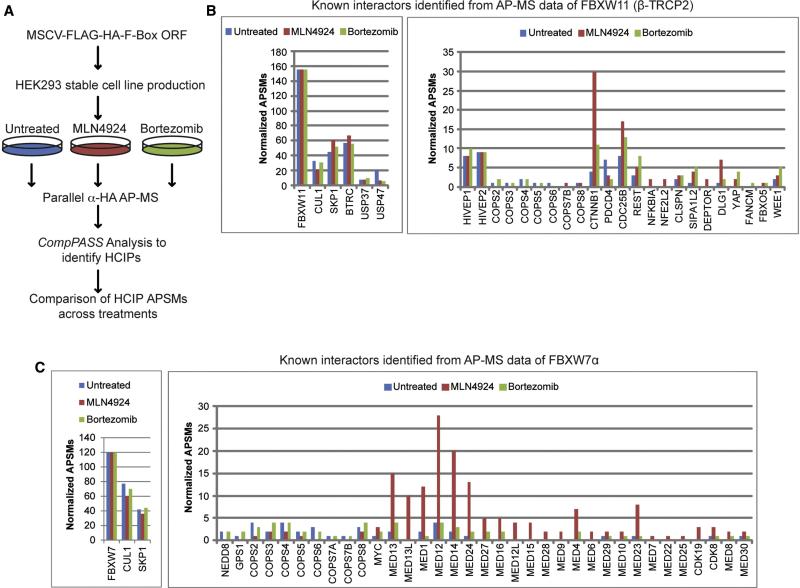
We sought to develop a methodology for the identification of candidate interacting proteins for F-box proteins that: 1) can be directed towards individual adaptor proteins, 2) allows the identification of not only substrates but also stably interacting proteins that may act in a regulatory fashion, 3) is sufficiently facile and cost-effective so as to be applied to a set of adaptor proteins in parallel, and 4) can be readily applied to different cell lines reflecting the diversity of adaptor protein/substrate expression and degradation signals. To this end, we initially generated HEK293 cell lines stably expressing two widely studied F-box proteins – β-TRCP2 (FBXW11) and FBXW7α – as FLAG-HA tagged proteins using MSCV-driven retroviruses. Triplicate cultures were either treated briefly with Btz or MLN4924, resulting in the accumulation of proteasomal or CRL substrates, respectively, or left untreated (Figure 1A). Extracts from these cells were then subjected to parallel α-HA affinity purification-mass spectrometry (AP-MS) and CompPASS analysis using a stats table of 174 unrelated bait proteins analyzed in an analogous manner. CompPASS identifies HCIPs based on the WDN-score, which incorporates the frequency with which they are identified within the stats table, the abundance (APSMs, average peptide spectral matches) when found, and the reproducibility of technical replicates (Behrends et al., 2010; Sowa et al., 2009). Proteins with WDN-scores >1.0 are considered HCIPs, although we also note that some bona fide interacting proteins may not reach the strict threshold set by a WDN-score of > 1.0. Our expectation was that we would identify 3 major classes of interacting proteins: 1) proteins such as CUL1, SKP1, and components of the CSN complex, which associate with F-box proteins independently of treatment, 2) proteins that are detected in the absence of treatment but that increase in abundance in response to treatment, and 3) proteins that only become detectable upon stabilization with either Btz and/or MLN4924. We envisioned that the later two categories would be enriched in candidate substrates, although it is also possible that some substrates that are either relatively abundant or are not proteasomal targets may be identified under all conditions and not increase in abundance upon treatment.
Figure 1. Parallel Adaptor Capture (PAC) proteomics.
(A) Schematic representation of the Parallel Adaptor Capture (PAC) proteomics approach.
(B, C) APSMs for known interaction partners for FBXW11/β-TRCP2 (panel B) and FBXW7α (panel C) identified as HCIPs by PAC proteomics.
Application of this PAC proteomics approach for β-TRCP2 resulted in the identification of many known SCFβ-TRCP targets and regulatory proteins (Skaar et al., 2013; Sowa et al., 2009) (Figure 1B). APSMs for 8 substrates were identified in untreated cells, but for 7 of these, the number of APSMs identified was increased in the presence of Btz, MLN4924, or both. For 6 substrates, peptides were identified only in the presence of Btz and/or MLN4924. Importantly, the approach allowed the identification of substrates with 1-3 APSMs (NFKBIA, DEPTOR, NFE2L2, FANCM, FBXO5) for proteins that were only detected in the presence of either Btz and/or MLN4924 (Figure 1B). We also identified the two know regulatory proteins USP37 and USP47 in association with β-TRCP2 (Skaar et al., 2013; Sowa et al., 2009) (Figure 1B). Likewise, with FBXW7α, we identified the known substrates c-MYC (Welcker and Clurman, 2008) and MED13 (Davis et al., 2013), a subunit of the Mediator complex with increased APSMs in the presence of Btz and/or MLN4924, and identified the related protein MED13L only in the presence of MLN4924 (Figure 1C). We also identified 22 additional Mediator subunits, 14 of which were only identified in the presence of Btz and/or MLN4924. Of these 14, 9 were identified with 4 normalized APSMs or less (Figure 1C). Thus, PAC proteomics may provide a facile method to identify targets and regulators of CRL adaptors.
Systematic analysis of FBXL proteins using PAC proteomics
We employed PAC proteomics to systematically survey FBXL proteins for candidate substrates. Twenty-one FBXL proteins have been identified based on the presence of an N-terminal F-box domain and C-terminal LRRs (Jin et al., 2004), and the majority are expressed in HEK293 cells based on deep sequencing (Sultan et al., 2008) (Figure 2A, Table S1), suggesting that these cells provide a reasonable setting in which to begin to identify targets. With the exception of SKP2, which has been widely studied, little is known concerning the targets of the majority of FBXL proteins (Skaar et al., 2013). Focused studies or high throughput yeast 2-hybrid screens have revealed candidate targets for a subset of FBXLs (Table S1), but the full repertoire of substrates for most FBXLs are unknown and many proposed interactions remain unvalidated (Cui et al., 2011; Kuchay et al., 2013; Vashisht et al., 2009; Yam et al., 1999; reviewed in Skaar et al., 2013). While there may be particular settings in which an extrinsic stimulus is required to engage an F-box protein with substrates, many such signals are cell intrinsic and constitutive, as indicated by our ability to identify known substrates for β-TRCP and FBXWα in unstimulated HEK293 cells.
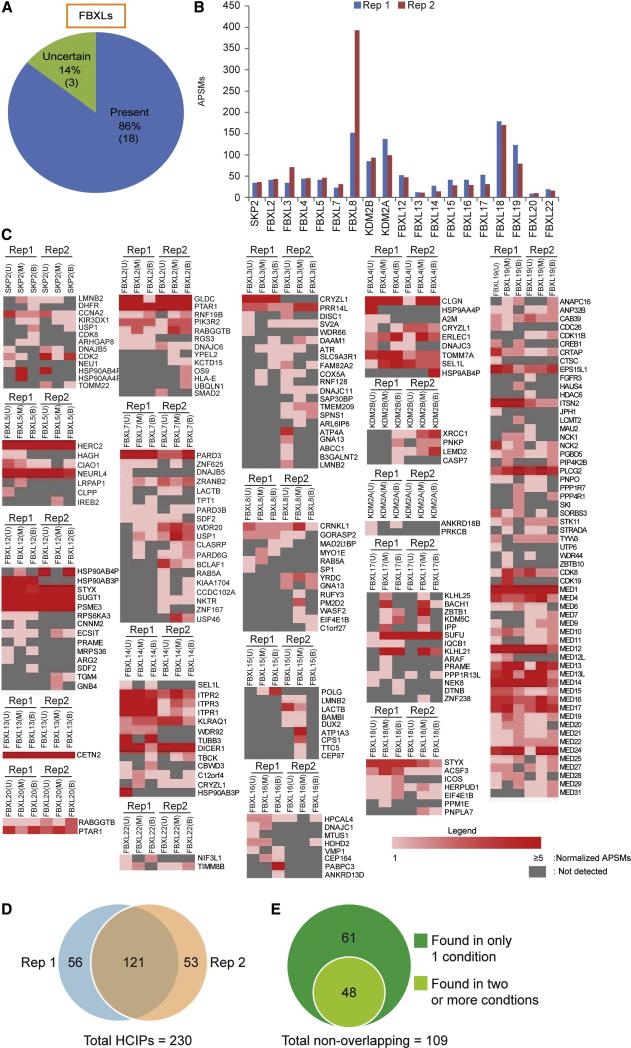
Figure 2. Application of PAC proteomics to the FBXL family of SCF adaptors.
(A) Expression of FBXL proteins in HEK293 cells, as determined by analysis of RNA-Seq data from (Sultan et al., 2008).
(B) Heat map representation of APSMs for HCIPs from each FLAG-HA-FBXL in untreated (U), MLN4924-treated (M), and Btz-treated (B) treated cells in biological replicates 1 and 2.
(C) Venn diagram comparing the overlap in HCIPs identified in biological replicate experiments, independent of the treatment in which the HCIP was identified.
(D) Venn diagram of the 97 HCIPs found in only one PAC proteomics replicate experiments for HCIPs that were identified under only one treatment condition and those found with 2 or 3 treatment conditions.
All human FBXLs (with the exception of FBXL6 and FBXL21 which resisted cloning) were expressed in HEK293 cells using a doxycycline-inducible retroviral system, or in some cases constitutive expression (Table S1) and subjected to PAC proteomics in biological duplicate. Bait abundance (APSMs) was typically within a factor of 2 for biological duplicates but varied greatly between FBXLs (>150 for FBXL8 to <20 for FBXL13, FBLX20, and FBXL22) (Figure 2B). As expected, FBXLs broadly associated with SKP1, but we did not identify CUL1 or COP9 Signalosome peptides with KDM2A (FBXL11) or KDM2B (FBXL10) (Table S1), consistent with the absence of CUL1 binding (Frescas et al., 2007). We also did not observe CUL1 or CSN with FBXL13 or FBXL16, despite the presence of readily detectable SKP1 peptides. Taken together, these data suggest that the majority of FBXLs examined here form intact CRL complexes.
Across the 19 FBXLs examined, we identified 177 HCIPs in replicate 1 and 174 HCIPs in replicate 2 under at least one of the 3 conditions examined (Figure 2C,D), with an overall validation rate of ~80% as described below. Of these, 121 were found in both biological replicates in at least one condition (Figure 2D), and will be referred to as Class A HCIPs. Among the 109 proteins that were only identified in one of the two biological replicates, 48 were found in 2 or 3 conditions (Class B), while 61 were “singlets”, being found in only one condition (Class C) (Figure 2E). Of these 61 proteins, 52 were found with only 1 or 2 APSMs.
Overview and topology of the FBXL interaction landscape
Across the entire FBXL landscape, we identified 62 proteins (27%) with either Btz or MLN4924 treatment that were not identified in untreated samples, and 56 proteins (24%) whose abundance increased in response to Btz or MLN4924, consistent with the possibility that these proteins are ubiquitylation and proteasome targets (Figure 2C, 3A, 3B). Only 23 proteins (10%) were identified uniquely in FBXL complexes from untreated cells. We identified known interactors for 3 FBXL proteins (Figure 2C, 3A): the CCNA2-CDK2 complex in association with SKP2, the recently reported phosphoinositide-3-kinase regulatory subunit p85β PIK3R2 in association with FBXL2, and IREB2 in association with FBXL5 (Kuchay et al., 2013; Vashisht et al., 2009; Yam et al., 1999). In addition, we identified the β-subunit of the RAB geranylgeranyltransferase RABGGTB and the β-subunit of the prenyl transferase PTAR1 in association with both FBXL2 and FBXL20. Both FBXL2 and FBXL20, which are closely related (Jin et al., 2004), contain C-terminal CAAX motifs, and FBXL2 has been shown to be geranylgeranylated (Wang et al., 2005), consistent with the observed interactions.
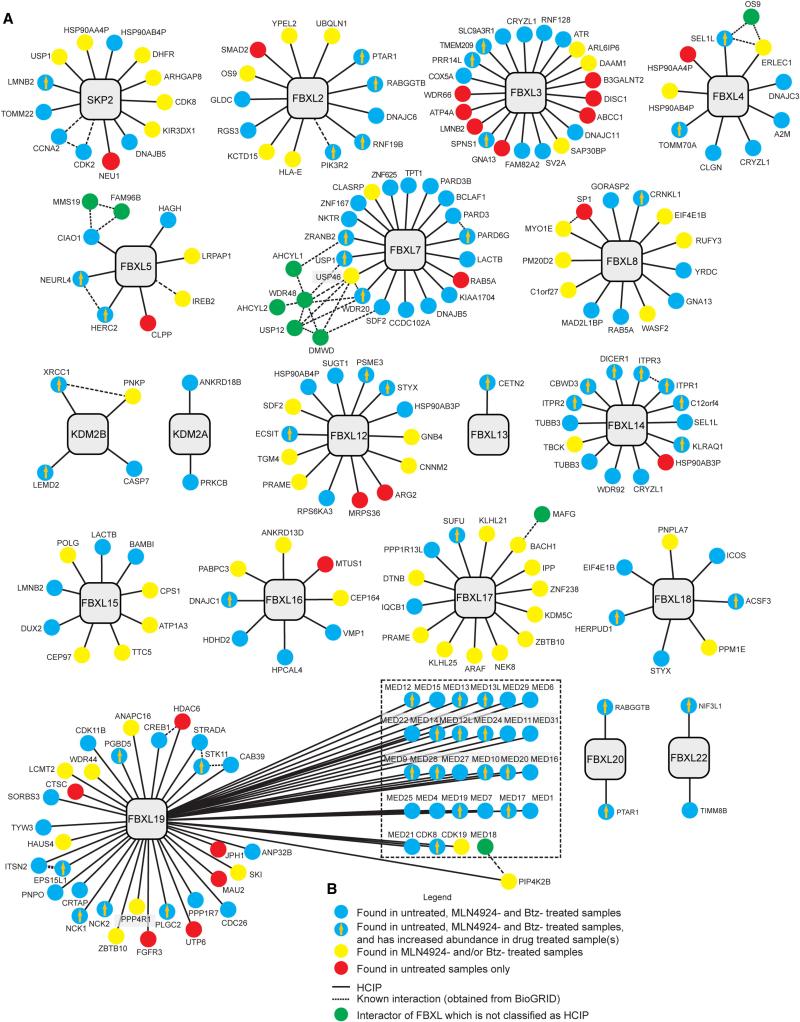
Figure 3. Interaction landscape of the FBXL adaptor family.
(A) Interaction maps for FBXLs examined by PAC proteomics. The interactions shown represent the union between biological replicates 1 and 2.
(B) Legend depicting symbols used in panel A.
HCIPs identified across FBXLs ranged from 2 to 59, with a median of 10 HCIPs per bait (Figure 2C, 3A,B). Six FBXL proteins (FBXL2, 3, 5, 12, 16, and 17) associated with E3 ubiquitin ligases, including FBXL12 and FBXL17 which both bound the CUL2 adaptor PRAME (Figure 3A). Cross-regulation of CRLs is anticipated based on the results of Global Protein Stability studies (Emanuele et al., 2011; Yen and Elledge, 2008). SKP2, KDM2A, FBXL2, 3, 17, 18, and 19 associated with protein/lipid kinases, or protein phosphatases (Figure 3A). Several FBXLs have multiple interacting proteins that are functionally or structurally related. FBXL17 was found to associate with several BTB-domain containing proteins, including a group involved in transcriptional control (BACH1, ZNF238, ZBTB1) and a group that act as CUL3 substrate adaptors (KLHL25 and KLHL21). Functional dissection of the BACH1-FBXL17 interaction will be addressed below. FBXL14 associated with 3 members of the type II inositol 1,4,5-trisphosphate receptor family of proteins, suggesting roles in inositol signaling. FBXL13, FBXL15, and FBXL16 were linked to centrosome function through their association with CETN2, CEP97, and CEP164 (Figure 3). FBXL7 associated with three related partitioning defective (PAR) proteins – PARD3, PARD3L, and PARD6G – suggesting a role in cell polarity control. PARD3 is known to interact with PARD6G, and the finding that PARD6G increases in response to either Btz or MLN4924 suggests that it may be a ubiquitylation substrate (see below). In C. elegans, par-6 is regulated by a CUL2FEM1 ubiquitin ligase (Pacquelet et al., 2008). In addition, FBXL7 associated with 3 related deubiquitylating (Dub) enzymes (USP1, USP12 and USP46) as well as their regulatory components WDR20, WDR48, and DMWD. The WDR20 and WDR48 regulatory subunits are common to all three Dubs (Cohn et al., 2009; Sowa et al., 2009). USP1, USP46, and WDR20 are candidate ubiquitylation targets (Figure 3A), as they are either only detected or APSMs increase in the presence of MLN4924 and/or Btz.
FBXL5 associated with its known target, the iron responsive element binding protein 2 (IREB2), which is degraded in the presence of excess iron (Vashisht et al., 2009). Interestingly, we also identified the cytosolic Fe-S cluster assembly complex (CIA) composed of CIAO, MMS19, and FAM96B (MIP18) (Gari et al., 2012; Stehling et al., 2012). FBXL5 contains an N-terminal Hemerythrin domain that binds 2 iron atoms and O2, and is intimately involved in iron homeostasis (Chollangi et al., 2012; Ruiz et al., 2013; Salahudeen et al., 2009). We speculate that the CIA complex is involved in loading iron in the Hemerythrin domain of FBXL5. FBXL19 contains the largest number of HCIPs (59), but approximately 50% of these are components of the Mediator complex. Strong association with the Mediator complex implies distinct roles for FBXL19 and FBXW7α in controlling Mediator function, possibly by acting on distinct subunits or in distinct contexts.
FBXL Interactome Validation
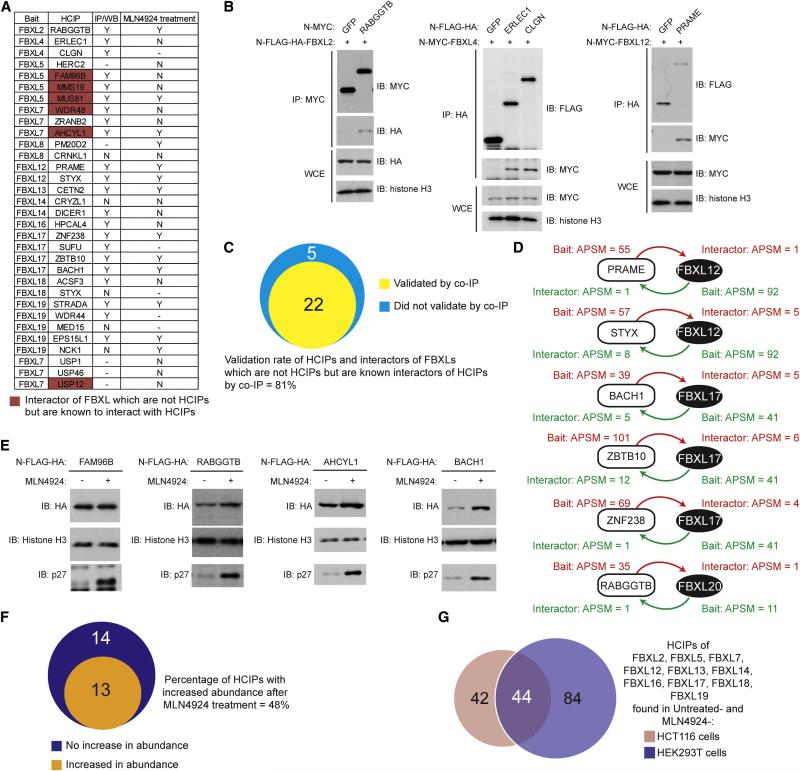
To test for reciprocal interactions, 22 HCIP and 5 sub-threshold interacting proteins were cloned into epitope-tagged vectors and tested for co-immunoprecipitation with one of 12 cognate FBXL proteins via transient transfection in HEK293T cells (Figure 4A, S1). Twenty-two of 27 FBXL interacting proteins (81%) were found to associate with their cognate FBXLs, including RABGGTB in association with FBXL2, ERLEC1 and CLGN in association with FBXL4, and PRAME in association with FBXL12 (Figure 4B). In addition, the 5 sub-threshold interactors, which are known to associate with HCIPs within the individual immune complexes, were also found to interact with their cognate FBXL proteins (Figure 4A). This may reflect the stringent threshold (99% confidence level) used for HCIP identification. To further corroborate these studies, we performed proteomics on 2 HCIPs for FBXL12, 3 HCIPs for FBXL17, and 1 HCIP for FBXL20, and identified peptides for the corresponding endogenous FBXL protein (Figure 4D).
Figure 4. Experimental validation of candidate FBXL-interacting protein pairs.
(A) FBXL interacting proteins selected for validation and a summary of validation results for reverse immunoprecipitation-western blotting, and stabilization with MLN4924. Supporting data is provided in Figure S1 and S2.
(B) Selected examples of reciprocal interaction validation by immunoprecipitation-western blot analysis. N-terminally MYC or FLAG-HA tagged interacting proteins were transiently co-expressed in HEK293T cells, using GFP as a control. After 48 h, the indicated immune complexes or whole cell extracts (WCE) were immunoblotted with the indicated antibody.
(C) Venn diagram showing the results of validation experiments examining the reciprocal association of FBXLs and their interacting proteins tested. Supporting data is provided in Figure S1.
(E, F) Validation of substrates as CRL targets via stabilization by MLN4924 treatment. (E) HEK293 cells stably expressing N-terminally FLAG-HA tagged FBXL interacting proteins were incubated with or without MLN4924 (1 μM, 4 h), and extracts subjected to immunoblotting with the indicated antibodies. Histone H3 was used as a loading control and p27 was used as a positive control. (F) Venn diagram showing the extent of validation as a CRL target based on increased steady-state abundance in the presence of MLN4924. Supporting data is provided in Figure S2.
(G) FBXL2, FBXL5, FBXL7, FBXL12, FBXL13, FBXL14, FBXL16, FBXL17, FBXL18, FBXL19 were N-terminally tagged with FLAG-HA and stably expressed in HCT116 cells. PAC proteomics was performed on untreated cells and on cells treated with MLN4924 (1 μM, 4 h). The Venn diagram displays overlap between HCIPS found for these FBXLs in HCT116 and HEK293 cells under these 2 conditions. Supporting data is provided in Figure S3 and Table S5.
Inhibition of CRL neddylation by MLN4924 results in a cohort of inactive SCF complexes and would imply the involvement of CRLs in turnover of specific proteins (Soucy et al., 2009; Bennett et al., 2010; Lee et al., 2011). However, given that our method also identifies proteins that are not themselves ubiquitylation targets but are associated with a ubiquitylation substrate or are stable components of SCF complexes, we envisioned that only a subset of proteins identified by PAC proteomics would represent unstable ubiquitylation and proteasome targets. To examine CRL dependent stability, 27 FBXL interacting proteins were stably expressed as FLAG-HA fusion proteins in HEK293 cells using an MSCV-based retroviral system and tested for stabilization by MLN4924 (4 h). In this setting, indirect transcriptional effects of MLN4924 on endogenous candidate target genes are eliminated. In total, 13 of 27 proteins tested (48%) - including RABBGTB, AHCYL1, and BACH1, but not FAM96B - displayed an increase in abundance in response to MLN4924 treatment analogous to that of p27 used as a positive control, consistent with their turnover by the CRL system (Figure 4E, F, S2). Eleven of the 12 tested were found to associate with their cognate FBXLs (Figure 4A, S1), consistent with the involvement of the identified FBXLs in controlling turnover.
Some CRL adaptors likely target proteins in a cell type or signal dependent manner. Thus, as an alternative approach for validation, 10 FBXL proteins (Figure 4G) were stably expressed in the colon cancer cell line HCT116 in the presence and absence of MLN4924 and subjected to AP-MS. This resulted in the identification of 86 HCIPs, of which 44 were identified in HEK293 cells (Figure 4G, S3, Table S3). This overlapping set of proteins contained targets for all 10 FBXLs examined. Thus, a wide cross-section of SCF-FBXL targets found in HEK293 cells were validated in HCT116 cells. In the absence of detailed validation, the HCIPs identified here should be considered as candidate targets or regulatory proteins, but our validation experiments suggest that a substantial fraction of targets reciprocally interact with the cognate F-box proteins and can be identified in an independent cell line. We also cannot rule out the possibility that false negatives are present in the validation results by reciprocal AP-MS or immunoprecipitation-immunoblotting due to interference by epitope tags employed.
The SCFFBXL17-BACH1 interaction network
To demonstrate the utility of the SCF-FBXL interactome, we selected BACH1, which was a candidate target of the previously unstudied adaptor FBXL17. BACH1 is a member of the “cap'n'collar”/basic region leucine zipper protein family (CNC-bZip) and also contains a BTB domain that typically serves as the site of homo- or hetero-dimerization. Some but not all BTB domain-containing proteins function as CUL3 substrate adaptors, but BACH1 does not associate with CUL3 (Figure 4A) and lacks the 3-box critical for CUL3 binding (Zhuang et al., 2009). BACH1 binds MAFK, MAFG, MAFF, which interact with MAF Recognition Elements (MARE) located in the promoters of genes in the oxidative response pathway (Sun et al., 2002; Sun et al., 2004; Warnatz et al., 2011). BACH1-MAF complexes repress MARE-dependent genes by blocking access to activating NRF2-MAF complexes, the primary transcriptional activators in the cellular response to oxidative stress. Previous studies suggest that BACH1 is regulated through at least 2 mechanisms. First, BACH1 was proposed to be sequestered in the cytoplasm by the HMMR (hyaluronan-mediated motility receptor) protein (Yamasaki et al., 2005). Second, a pool of BACH1 was proposed to be regulated by the ubiquitin-proteasome system (Zenke-Kawasaki et al., 2007), and we speculated that FBXL17 could be involved in this later pathway.
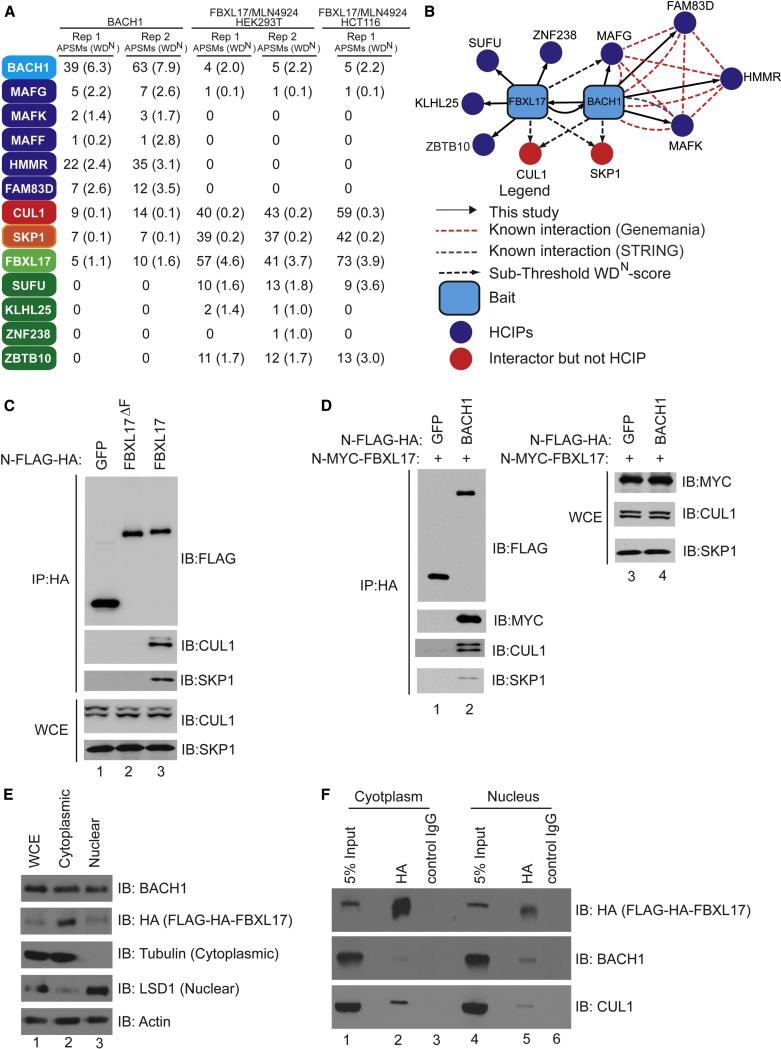
Initially, we subjected BACH1 to CompPASS-based interaction proteomics and identified several HCIPs, including the known BACH1-associated proteins MAFF, MAFG and MAFK, HMMR, and FAM83D (Figure 5A,B). In addition, we also identified FBXL17 as an HCIP as well as CUL1 and SKP1 (Figure 5A, B). Moreover, while FLAG-HA-FBXL17 immune complexes from both HEK293T and HCT116 contained MAFG, suggesting that SCFFBXL17 recognizes the heterodimeric BACH1-MAF complex, we did not detect either HMMR or FAM83D, suggesting that FBXL17 recognizes the repressive form of the complex (Figure 5A). In addition, several proteins including SUFU, KLHL25, and ZBTB10 that association with FBXL17 were not identified in BACH1 immune complexes, indicating that FBXL17 interacts independently with BACH1. As expected, FBXL17 uses its F-box to assemble into an SCF complex (Figure 5C), and BACH1 associates with FBXL17, CUL1, and SKP1 by immunoprecipitation-western analysis (Figure 5D). Previous studies indicated that BACH1 is localized in both the nucleus and the cytoplasm (Suzuki et al., 2004; Suzuki et al., 2003), a result that was confirmed here with α-BACH1 antibodies. As antibodies that detect endogenous FBXL17 in crude cell extracts or in immunopreciptiation assays were not available, we examined localization in HEK293 cells stably expressing FLAG-HA-FBXL17 via a retrovirus and found that it is present in both compartments, although more abundant in the cytoplasm (Figure 5E). FBXL17 associated with BACH1 in both compartments, albeit with an ~2-fold enrichment in the nucleus (Figure 5F). These data suggested a regulatory connection between FBXL17 and BACH1.
Figure 5. The SCFFBXL17-BACH1 interaction network.
(A) Proteomic analysis of FBXL17 and BACH1 complexes. APSMs and WDN-scores are provided for HA-BACH1 and HA-FBXL17 complexes isolated from HEK293 cells and for HA-FBXL17 complexes isolated from HCT116 cells.
(B) Interaction map for FBXL17 and BACH1 complexes. Blue edges, this study; Red edges, Genemania; Green edges, STRING (threshold = 900).
(C) Association of FBXL17 with SKP1 requires the F-box motif. N-FLAG-HA tagged GFP, FBXL17 and FBXL17ΔF were co-expressed in HEK293T cells. Forty-eight hours after transfection, the cells were harvested and immunoprecipitated with anti-HA resin and immnunoblotted with indicated antibodies.
(D) N-FLAG-HA tagged GFP or FBXL17 were transiently co-transfected with N-MYC-BACH1 into HEK293T cells. Forty-eight hours after transfection, the cells were harvested and immunoprecipitated with α-HA resin and immnunoblotted with indicated antibodies.
(E, F) FBXL17 associates with BACH1 preferentially in the nucleus at steady-state. (E) Nuclear and cytoplasmic fractions from HeLa cells stably expressing N-FLAG-HA-FBXL17 were immunoblotted with the indicated antibodies. (F) Nuclear and cytoplasmic fractions from cells in panel E treated with MLN4924 (1 μM, 4 h) were subjected to immunoprecipitation with anti-HA resin and immunoblotted with the indicated antibodies.
SCFFBXL17 regulates the NRF2-HMOX1 pathway via BACH1 degradation
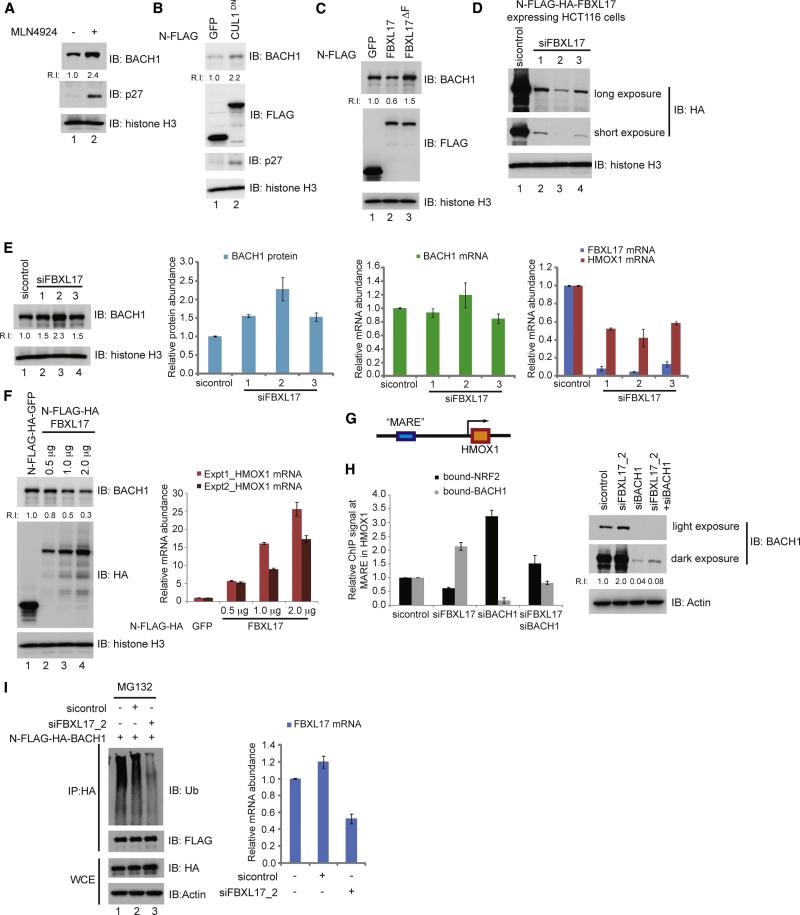
Having found that FLAG-HA-BACH1 is stabilized by MLN4924 (Figure 4E), we next asked whether endogenous BACH1 is similarly regulated. We found a 2.4-fold increase in BACH1 levels upon a 4 h MLN4924 treatment (Figure 6A) and a 2.2 fold increase upon overexpression of dominant negative CUL1 (CUL1DN) (Skaar et al., 2013) (Figure 6B), consistent with the idea that endogenous BACH1 is regulated by the SCF system. Overexpression of FBXL17 reduced BACH1 levels by ~2-fold while FBXL17ΔF stabilized BACH1 (Figure 6C). Of note, the relatively small 2.4-fold increase in BACH1 in response to MLN4924 compared with that of p27 (~10-fold) assayed in parallel indicated that BACH1 levels are tightly regulated, a point that is amplified below.
Figure 6. Regulation of the NRF2-HMOX1 pathway via SCFFBXL17-dependent control of BACH1 abundance.
(A) MLN4924 (1 μM, 4 h) increases the abundance of endogenous BACH1 in HEK293T cells as determined by immunoblotting with the indicated antibodies. p27 was used as a positive control.
(B) Expression of CUL1DN but not GFP as a control increases the abundance of BACH1 in HEK293T cells, as revealed by immunoblotting of cell extracts. p27 was used as a positive control.
(C) Overexpression of FBXL17 reduces steady-state BACH1 levels while overexpression of FBXL17ΔF increases BACH1 abundance in HEK293T cells.
(D) Validation of FBXL17 siRNAs. HCT116 cells stably expressing N-FLAG-HA-FBXL17 were transiently transfected with either control siRNA or independent FBXL17 siRNAs. Seventy-two hours later, cells were harvested, lysed and immnublotted with the indicated antibodies.
(E) HeLa cells were transfected with control or independent FBXL17 siRNAs. Seventy-two hours later, cells were either lysed and extracts immunoblotted with the indicated antibodies, mRNA isolated and used for quantitative PCR (qPCR) to assess the levels of FBXL17, BACH1 and HMOX1 transcripts. GAPDH used in the normalization of all qPCR analyses. Error bars represent standard error from three independent analyses.
(F) Increased HMOX1 transcription correlates with decreased BACH1 upon ectopic FBXL17 expression. HeLa cells, transfected with the indicated plasmids, were harvested and process for either immunobloting with the indicated antibodies or for qPCR to assess HMOX1 levels. Error bars represent standard deviation from technical triplicates.
(G) Schematic diagram showing the “MARE” enhancer region of the HMOX1 gene where both BACH1 and NRF2 have been shown to bind.
(H) FBXL17 regulates the balance between NRF2 and BACH occupancy on the HMOX1 promoter. HeLa cells were transiently transfected with control siRNA, FBXL17_2 siRNA, BACH1 siRNA or both BACH1 and FBXL17_2 siRNAs. Seventy-two hours post transfection, cells were either lysed for immunoblotting (right panel) or cross-linked and subjected to ChIP-qPCR analysis using NRF2 or BACH1 antibodies. Error bars represent standard error from three independent analyses.
(I) FBXL17-dependent ubiquitylation of BACH1. HEK293T cells were transfected with the indicated constructs. Forty hours after transfection, the cells were treated with MG132 (6 h) prior to either lysis for protein analysis or qPCR to determine FBXL17 mRNA levels. Protein samples were lysed in 2% SDS, and used for immunoprecipitation with α-HA antibodies followed by immunoblotting with α-Ub or α-FLAG. Error bars represent standard deviation from technical triplicates.
In principle, a balance between NRF2 and BACH1 proteins may exist in order to maintain functional levels of HMOX1 transcription under basal conditions (intrinsic oxidative stress) and to also be able to rapidly respond rapidly upon extrinsic oxidative stress. In this scenario, the relative turnover rates of BACH1 and NRF2 are expected to play important roles in determining both the balance of proteins and there respective responses to upstream signals. To examine this relationship, we first tested whether BACH1 levels and HMOX1 transcription in the absence of extrinsic oxidative stress are altered upon FBXL17 depletion. After defining the relative potency of 5 FBXL17 siRNAs using cells stably expressing FLAG-HA-FBXL17 or qPCR (Figure 6D, S4B), we depleted FBXL17 in HeLa cells and determined BACH1 protein abundance by immunoblotting and HMOX1, BACH1 and FBXL17 mRNA abundance by qPCR. We observed a 1.5-2.3 fold increase in BACH1 protein abundance, which correlated with siRNA efficiency (Figure 6D, E, S4B). Increased BACH1 levels resulted in a 40-60% decrease in basal HMOX1 transcript levels, which also correlated with the potency of RNAi (Figure 6E). In parallel, we transiently transfected BACH1 into HeLa cells under conditions that result in a 5.5-fold increase in BACH1 levels, and observed a 40% reduction in HMOX1 transcription by qPCR (Figure S4C), suggesting a maximum repression of ~2-fold upon elevated BACH1 abundance. In contrast, overexpression of FBXL17 in HeLa cells reduced BACH1 abundance and resulted in a dose-dependent increase in HMOX1 mRNA (up to 20-fold) when compared to GFP expression employed as a control (Figure 6F). FBXL17 may also function to independently control the abundance of other proteins with which it associates, as FBXL17 RNAi stabilized ZBTB10 but not ZBTB33 (Figure S4A).
Increased HMOX1 transcription upon reduced BACH1 levels via FBXL17 overexpression suggested that BACH1-MAF protects MARE sequences from activation by NRF2 that escapes the CUL3KEAP1 system in the absence of extrinsic oxidative stress. To test this, we performed ChIP-qPCR analyses of MARE sequences in the enhancer region of HMOX1 using antibodies against NRF2 or BACH1 (Figure 6G, H). As anticipated, depletion of BACH1 led to a dramatic reduction (~10-fold) in MAREHMOX1 associated with BACH1 and a 3-fold increase in MAREHMOX1 associated with NRF2 (Figure 6H). In contrast, depletion of FBXL17 resulted in a ~2-fold reduction in NRF2-associated MAREHMOX1 and a 2.1-fold increase in BACH1-associated MAREHMOX1. Consistent with this affect occurring via BACH1, co-depletion of BACH1 and FBXL17 resulted in a 1.5-fold increase in NRF2-associated MAREHMOX1 (Figure 6H). The smaller changes seen with double depletion may reflect residual BACH1 present in these cells relative to single siRNA depletion (Figure 6H).
These data suggest that SCFFBXL17 and CUL3KEAP1 control the balance of BACH1 and NRF2 available for binding to the HMOX1 promoter in cells without extrinsic oxidative stress. If this is the case, the expectation is that ongoing BACH1 ubiquitylation would be reduced upon depletion of FBXL17. Indeed, FBXL17 RNAi reduced the extent of ubiquitin conjugates observable on FLAG-HA-BACH1 purified from HEK293 cells under denaturing conditions (Figure 6I, lane 3) relative to cells transfected with control siRNA (lane 2).
BACH1 turnover via SCFFBXL17 regulates the response of the HMOX1 promoter to oxidative stress
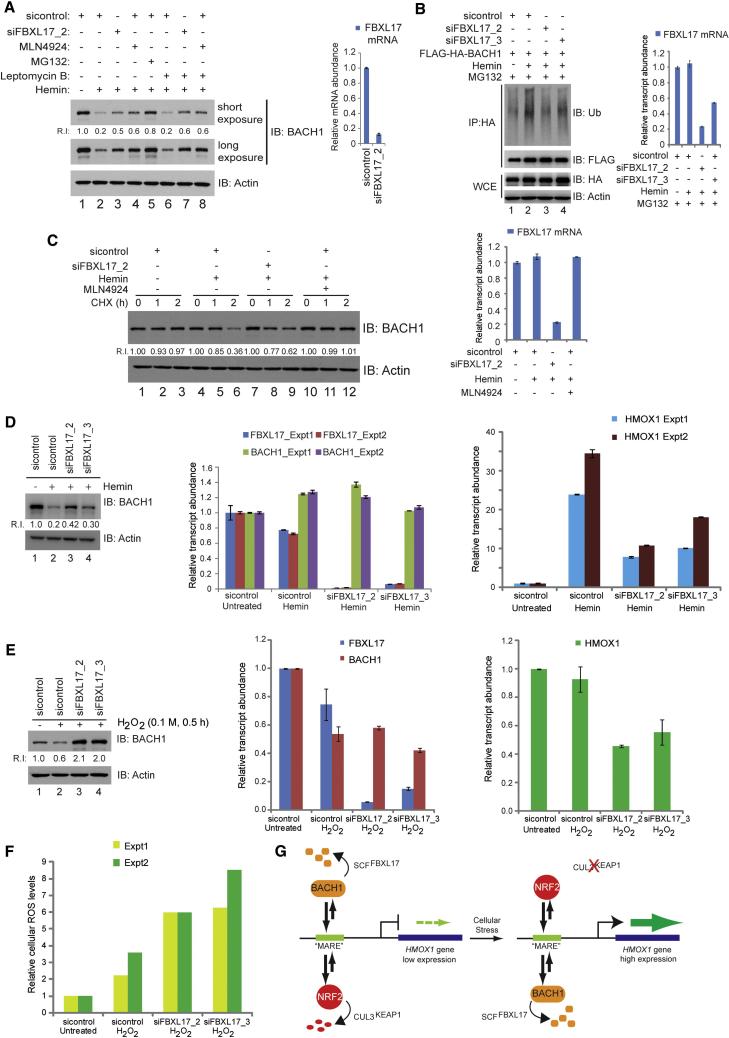
While Heme is an important co-factor in cellular processes, high Heme levels lead to ROS, and subsequently to activation of HMOX1 transcription (Igarashi and Sun, 2006). HMOX1 catalyzes Heme breakdown, thereby relieving the ROS burden in cells. Thus, this transcriptional feedback loop is important for cellular homeostasis. Previous studies revealed that Heme inhibits DNA binding by the BACH1-MAF complex (Ogawa et al., 2001), thereby directly promoting ubiquitin and proteasome-dependent turnover of BACH1 (Zenke-Kawasaki et al., 2007). To examine the potential role of FBXL17 in this pathway, we initially exposed HeLa cells to 10 μM Hemin (Fe+3-bound Heme, 4h) in the presence or absence of proteasome inhibitor or MLN4924 (Figure 7A). As expected, Hemin reduced BACH1 levels but this was blunted by both MG132 and MLN4924 (Lanes 2, 4, 5). We also examined whether degradation could occur in the nucleus. Addition of leptomycin to block nuclear export followed by Hemin addition resulted in loss of BACH1 levels to an extent similar to that found in the absence of leptomycin (Figure 7A, lane 6), indicating that BACH1 turnover can occur exclusively in the nucleus, and this was blunted by MLN4924 to an extent similar to that found in the absence of leptomycin (lane 8). Consistent with this, FLAG-HA-BACH1 ubiquitylation in response to Hemin was reduced by depletion of FBXL17, with the most potent siRNA (siFBXL17_2) having the strongest effect (Figure 7B, lane 2). We next examined turnover of BACH1 in response to Hemin addition after FBXL17 depletion. FBXL17 depletion increased the abundance of BACH1 in response to Hemin (Figure 7A, lane 3), and reduced the rate of BACH1 turnover (Figure 7C), albeit not as potently as MLN4924, possibly reflecting incomplete FBXL17 depletion (20% FBXL17 mRNA remained). The effect of FBXL17 depletion on Hemin-dependent BACH1 levels was not affected by leptomycin (Figure 7A, lane 7). We then examined the effect of FBXL17 depletion on HMOX1 transcription. While Hemin addition resulted in a 25 to 35-fold increase in HMOX1 transcripts as measured by qPCR in 2 independent experiments, FBXL17 depletion with the most potent siRNA reduced this activation to <10-fold with little effect on BACH1 mRNA levels (Figure 7D). Thus, FBXL17 provides one mechanism for controlling HMOX1 activation in response to Hemin, although we cannot exclude the participation of other mechanisms such as sequestration of BACH1 by HMMR, or possibly other E3s.
Figure 7. BACH1 turnover via SCFFBXL17 regulates the response of the HMOX1 promoter to cellular stress.
(A) Proteasome and CRL inhibition blocks BACH1 loss in response to Hemin (4 h), as determined by immunoblotting of HeLa cell extracts with the indicated antibodies. Error bars represent standard deviation from technical triplicates.
(B) FBXL17 depletion reduces Hemin-dependent ubiquitylation of BACH1 in response to Hemin. HEK293T cells were transfected with the indicated constructs. Forty hours after transfection, the cells were treated with MG132 (6 h) prior to either lysis for protein analysis or qPCR to determine FBXL17 mRNA levels. Protein samples were lysed in 2% SDS, and used for immunoprecipitation with α-HA antibodies followed by immunoblotting with α-Ub or α-FLAG. Error bars represent standard deviation from technical triplicates.
(C) FBXL17 depletion stabilizes BACH1 turnover in response to Hemin. Sixty hours post-transfection with the indicated siRNAs, HEK293T cells were either treated with Hemin and CHX or Hemin and MLN4924 as indicated prior to immunoblotting of cell extracts. Parallel cultures were used for qPCR analysis to determine efficiency of FBXL17 depletion. Error bars represent standard deviation from technical triplicates.
(D) FBXL17 depletion blocks HMOX1 transcriptional activation in response to Hemin. Sixty hours post-transfection of HeLa cells with control of FBXL17 siRNAs, cells were treated with or without 10 μM Hemin (4 h). A portion of cells were used for immunoblotting for BACH1 and the remainder used for FBXL17 and HMOX1 mRNA analysis by qPCR. Error bars represent standard deviation from technical triplicates.
(E, F) FBXL17 depletion reduces HMOX1 induction in response to H2O2. HeLa cells were transfected with either control or two different FBXL17 siRNAs. Sixty hours post-transfection of HeLa cells with the indicated siRNAs, cells were labeled with CMH2DCCFDA for subsequent analysis of oxidative stress, and then treated with H202 for 30 min. (E) Cells extracts were subjected to analysis of BACH1 levels by immunoblotted or mRNA was subjected to qPCR for FBXL17, BACH1, and HMOX1. (F) An aliquot of cells was analyzed using flow cytometry to monitor oxidative stress via CM-H2DCCFDA. Error bars represent standard error from duplicate analyses (Experiments 1 and 2).
(G) Schematic representation of the relationship between BACH1 and NRF2 in the control of HMOX1 expression and the role of SCFFBXL17 in controlling the balance between activating and repressive transcriptional control.
We also examined H2O2-dependent regulation of BACH1 via FBXL17. Thirty minutes after H2O2 addition, BACH1 abundance was reduced by 40% in sicontrol-treated cells, but was increased by 2-fold in cells depleted of FBXL17 (Figure 7E). This correlated with a ~2-fold reduction in the extent of HMOX1 transcript abundance after a brief treatment with H2O2 (Figure 7E). At this time point, little increase in HMOX1 transcription was found, indicating that the effects likely reflect HMOX1-repression due to accumulated BACH1 levels upon FBXL17 depletion. Nevertheless, FBXL17 depletion resulted in a ~3-fold increase in cellular ROS levels upon priming with H2O2 in two independent experiments (Figure 7F), consistent with a defect in ROS control via an inability to fully activate HMOX1 transcription.
Concluding Remarks
The identification of E3 targets represents a major challenge for the field. Many substrates interact with E3s transiently and in some cases, targets or signals may be cell type specific. Moreover, the ability to identify E3 targets may be limited by their cellular abundance. The PAC proteomics technology described here provides a facile methodology for identification of proteins that associate with SCF adaptors and are possible substrates or regulatory proteins. In principle, this approach could be applied to other CRL adaptor families and can employ multiple cell lineages or extracellular stimuli, as done here for FBXLs. PAC proteomics is complementary to global approaches such as diGLY capture proteomics and the GPS system, and has the advantage of identifying targets of a specific adaptor rather than a family of adaptors or CRLs, as has been done previously using these technologies (Emanuele et al., 2011; Kim et al., 2011; Yen and Elledge, 2008). The approach is highly sensitive in that candidate substrates may be identified with only a single peptide, thereby providing access to substrates of low abundance. However, this sensitivity will likely be a function of the levels of adaptor protein expression achieved and in some cases (e.g. FBXL13), only low levels of expression was achieved using either constitutive or inducible expression (Figure 2B). Several proteins identified as candidate substrates for FBXLs were also identified as candidate CRL substrates either by diGLY capture or using the GPS system, including FAM96B, IREB2, MED6, HDAC6, ZNF238, PRAME, ITPR1, and NEURL4 (Emanuele et al., 2011; Kim et al., 2011). In addition, IPP, ITPR3 and SLC7A11 were also identified by diGLY capture or as rapidly turned over proteasome targets (Kim et al., 2011). The presence of validatable targets such as BACH1 identified by PAC proteomics for FBXL targets but not seen previously in high throughput methods points to the value of directed analyses of individual adaptor proteins.
Our analysis of SCFFBXL17 and its target BACH1 provides new insight into how the ubiquitin system regulates the transcriptional response to oxidative stress (Figure 7G). Previous overexpression studies suggested a role for the HOIP E3 in BACH1 ubiquitylation (Zenke-Kawasaki et al., 2007) but the subsequent finding that HOIP catalyzes linear ubiquitylation (Kirisako et al., 2006) makes it unlikely that this E3 controls BACH1 turnover via the proteasome. We propose that SCFFBXL17 and CUL3KEAP1 maintain a dynamic balance in the abundance of BACH1 and NRF2 in the absence of extrinsic oxidative stress, and the relative levels of BACH1 and NRF2 under these conditions controls the set-point for HMOX1 transcription (Figure 7G). This balance may allow the cell to protect NRF2-regulated genes from NRF2 that escapes KEAP1-dependent turnover. Rapid inactivation of CUL3KEAP1 in response to extrinsic stress would promote accumulation of NRF2 in the face of constitutive BACH1 turnover via FBXL17, thereby shifting the balance of the transcriptional machinery on the HMOX1 promoter. Interestingly, the dynamic range of BACH1 abundance observed using either FBXL17 depletion, proteasome inhibition or CRL inhibition (<3-fold) indicates that BACH1 is tightly regulated, thereby providing a highly tunable system for regulating HMOX1 expression. In addition, we found that overexpression of BACH1 by ~5-fold reduces HMOX1 expression by only ~2-fold, comparable to the repression seen with BACH1 stabilization via FBXL17 depletion, suggesting a mode of HMOX1 transcription that is insensitive to BACH1-mediated repression. The precise mechanism of FBXL17-BACH1 recognition requires further study. We detected MAFG in association with FBXL17 in the absence of extrinsic stress, indicating that the E3 can bind to the BACH1-MAFG complex, but it is not clear whether it recognizes and ubiquitylates BACH1 while bound to DNA or only upon its dissociation (either spontaneously or due to intrinsic stress mechanisms). Moreover, previous studies indicate that Hemin promotes direct dissociation of BACH1 from DNA (Ogawa et al., 2001). Thus, it is possible that SCFFBXL17 acts constitutively on BACH1-MAF complexes only after their dissociation from DNA, rather than extrinsic stress inducing a specific degradation signal on BACH1. We note however, that depletion of FBXL17 does not completely block BACH1 turnover in response to Hemin, and it may be possible that multiple mechanisms, including the previously proposed sequestration mechanism via HMMR, may also be involved. However, it seems likely that these mechanisms are primarily functioning in the nucleus, given that leptomycin had no effect on BACH1 turnover in response to Hemin. Interestingly, FBXL17 also interacts with several other BTB proteins, including ZBTB10, ZNF238/ZBTB22, ZBTB33, and several CUL3 adaptor proteins including KLHL25, KLHL13, KLHL17 (Table S2). Thus, FBXL17 likely has other substrates, possibly including ZBTB10, which we also found to be stabilized upon FBXL17 depletion. Further studies are required to understand the relationship between FBXL17 and BTB proteins generally, but we speculate that a common element(s) in BTB proteins may be targeted by FBXL17.
EXPERIMENTAL PROCEDURES
Cell culture, transfection and reagents
All cell lines were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FBS) and maintained at 5% CO2/37°C. Plasmid or siRNAs (30 nM) were transfected using lipid-based reagents. siRNA sequences, specific lipid reagents employed, transfection times, and antibodies employed are provided in Supplemental Experimental Methods. The following reagent concentrations were typically used: MLN4924 (1 μM); cycloheximide (100 μg/ml); MG132 (10 μM); Btz (500 nM); doxycycline (4 μg/ml).
Immunoprecipitation, PAC Proteomic Analysis, and in vivo ubiquitylation
Cell lysis [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% NP-40 and supplemented with protease inhibitors (Roche)], immunoprecipitation, and mass spectrometry were performed essentially as described (Behrends et al., 2010; Sowa et al., 2009), as described in detail in Supplemental Experimental Procedures. Briefly, cells (107) were treated with Btz, MLN4924, or DMSO (as control) for 4 h and cell pellets lysed for AP-MS using α-HA beads, in parallel. After elution with HA peptide, trypsinized samples were analyzed on an LTQ Velos (Thermo) mass spectrometer. Spectra were search with Sequest prior to CompPASS analysis [peptide false discovery rate <1.0% and protein false discovery rate <4.85% (Table S1)]. Detailed methods for in vivo ubiquitylation are provided in Supplemental Experimental Procedures. Briefly, siRNA transfected HEK293T cells were transfected with a plasmid expressing HA-BACH1 and prior to harvesting, cells were treated with MG132 (4h). Denaturing cell lysates were subjected to α-HA immunoprecipitation prior to immunoblotting with the indicated antibody.
Chromatin Immunoprecipitation (ChIP), qPCR, and ROS analysis
ChIP was performed after formaldehyde crosslinking of cells using 2 μg of ChIP-grade α-BACH1 (Santa Cruz, sc-271211) or α-NRF2 (Santa Cruz, sc-13032) and q-PCR performed using SYBR-green (Roche) in combination with primers specific for the HMOX1 or MCM5 (control) promoters (sequences provided in Supplemental Experimental Procedures). FBXL17 and BACH1 mRNA expression was also performed by SYBR-green qPCR using specific primer pairs (see Supplemental Experimental Procedures). For ROS analysis, HeLa cells post-siRNA transfection were incubated with 5 μM of CM-H2DCFDA (Invitrogen) at 37°C for 30 minutes (see Supplemental Experimental Procedures). Cells were treated with 100 μM H2O2 (30 min) prior to flow cytometry (10,000 events) and analysis using FACSDiva.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank members of the Harper lab for useful discussions and Aria Olumi for providing the FBXL10 construct. This work was supported by NIH grant AG011085 to J.W.H, and NIH grants CA118487 and GM071004 to Y.S. M-K.M.T. and H-J.L. were supported by Agency for Science, Technology and Research (A*STAR) Predoctoral Fellowships.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes 3 figures, 3 tables, Supplemental Experimental Procedures, and Supplemental References, and can be found with this article at http://
References
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benanti JA, Cheung SK, Brady MC, Toczyski DP. A proteomic screen reveals SCFGrr1 targets that regulate the glycolytic-gluconeogenic switch. Nat. Cell Biol. 2007;9:1184–1191. doi: 10.1038/ncb1639. [DOI] [PubMed] [Google Scholar]
- Bennett EJ, Rush J, Gygi SP, Harper JW. Dynamics of cullin-RING ubiquitin ligase network revealed by systematic quantitative proteomics. Cell. 2010;143:951–965. doi: 10.1016/j.cell.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nature Reviews. Molecular Cell Biology. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- Chollangi S, Thompson JW, Ruiz JC, Gardner KH, Bruick RK. Hemerythrin-like domain within F-box and leucine-rich repeat protein 5 (FBXL5) communicates cellular iron and oxygen availability by distinct mechanisms. J. Biol. Chem. 2012;287:23710–23717. doi: 10.1074/jbc.M112.360404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn MA, Kee Y, Haas W, Gygi SP, D'Andrea AD. UAF1 is a subunit of multiple deubiquitinating enzyme complexes. J. Biol. Chem. 2009;284:5343–5351. doi: 10.1074/jbc.M808430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, He S, Xing C, Lu K, Wang J, Xing G, Meng A, Jia S, He F, Zhang L. SCFFBXL(1)(5) regulates BMP signalling by directing the degradation of HECT-type ubiquitin ligase Smurf1. EMBO J. 2011;30:2675–2689. doi: 10.1038/emboj.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Larimore EA, Fissel BM, Swanger J, Taatjes DJ, Clurman BE. The SCF-Fbw7 ubiquitin ligase degrades MED13 and MED13L and regulates CDK8 module association with Mediator. Genes Dev. 2013;27:151–156. doi: 10.1101/gad.207720.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell. 2008;134:995–1006. doi: 10.1016/j.cell.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele MJ, Elia AE, Xu Q, Thoma CR, Izhar L, Leng Y, Guo A, Chen YN, Rush J, Hsu PW, et al. Global identification of modular cullin-RING ligase substrates. Cell. 2011;147:459–474. doi: 10.1016/j.cell.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RM, Correll CC, Kaplan KB, Deshaies RJ. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell. 1997;91:221–230. doi: 10.1016/s0092-8674(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Frescas D, Guardavaccaro D, Bassermann F, Koyama-Nasu R, Pagano M. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature. 2007;450:309–313. doi: 10.1038/nature06255. [DOI] [PubMed] [Google Scholar]
- Gari K, Leon Ortiz AM, Borel V, Flynn H, Skehel JM, Boulton SJ. MMS19 links cytoplasmic iron-sulfur cluster assembly to DNA metabolism. Science. 2012;337:243–245. doi: 10.1126/science.1219664. [DOI] [PubMed] [Google Scholar]
- Harper JW, Tan MK. Understanding cullin-RING E3 biology through proteomics-based substrate identification. Mol. Cell. Prot. 2012;11:1541–1550. doi: 10.1074/mcp.R112.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxidants & redox signaling. 2006;8:107–118. doi: 10.1089/ars.2006.8.107. [DOI] [PubMed] [Google Scholar]
- Jin J, Cardozo T, Lovering RC, Elledge SJ, Pagano M, Harper JW. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–2580. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, Sano S, Tokunaga F, Tanaka K, Iwai K. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25:4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamuro T, Takahashi K, Ogawa K, Udono-Fujimori R, Takeda K, Furuyama K, Nakayama M, Sun J, Fujita H, Hida W, et al. Bach1 functions as a hypoxiainducible repressor for the heme oxygenase-1 gene in human cells. J. Biol. Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- Kuchay S, Duan S, Schenkein E, Peschiaroli A, Saraf A, Florens L, Washburn MP, Pagano M. FBXL2- and PTPL1-mediated degradation of p110-free p85beta regulatory subunit controls the PI(3)K signalling cascade. Nat. Cell Biol. 2013;15:472–480. doi: 10.1038/ncb2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Sweredoski MJ, Graham RL, Kolawa NJ, Smith GT, Hess S, Deshaies RJ. The steady-state repertoire of human SCF ubiquitin ligase complexes does not require ongoing Nedd8 conjugation. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.006460. M110.006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monda JK, Scott DC, Miller DJ, Lydeard J, King D, Harper JW, Bennett EJ, Schulman BA. Structural conservation of distinctive N-terminal acetylation-dependent interactions across a family of mammalian NEDD8 ligation enzymes. Structure. 2013;21:42–53. doi: 10.1016/j.str.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Sun J, Taketani S, Nakajima O, Nishitani C, Sassa S, Hayashi N, Yamamoto M, Shibahara S, Fujita H, Igarashi K. Heme mediates derepression of Maf recognition element through direct binding to the transcriptional repressor Bach1. EMBO J. 2001;20:2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol. Cell. Biol. 1996;16:6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacquelet A, Zanin E, Ashiono C, Gotta M. PAR-6 levels are regulated by NOS-3 in a CUL-2 dependent manner in Caenorhabditiselegans. Dev. Biol. 2008;319:267–272. doi: 10.1016/j.ydbio.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Ruiz JC, Walker SD, Anderson SA, Eisenstein RS, Bruick RK. F-box and leucine-rich repeat protein 5 (FBXL5) is required for maintenance of cellular and systemic iron homeostasis. J. Biol. Chem. 2013;288:552–560. doi: 10.1074/jbc.M112.426171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A, Deshaies RJ. Multimodal activation of the ubiquitin ligase SCF by Nedd8 conjugation. Mol. Cell. 2008;32:21–31. doi: 10.1016/j.molcel.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahudeen AA, Thompson JW, Ruiz JC, Ma HW, Kinch LN, Li Q, Grishin NV, Bruick RK. An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science. 2009;326:722–726. doi: 10.1126/science.1176326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DC, Monda JK, Bennett EJ, Harper JW, Schulman BA. N-terminal acetylation acts as an avidity enhancer within an interconnected multiprotein complex. Science. 2011;334:674–678. doi: 10.1126/science.1209307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DC, Monda JK, Grace CR, Duda DM, Kriwacki RW, Kurz T, Schulman BA. A dual E3 mechanism for Rub1 ligation to Cdc53. Mol. Cell. 2010;39:784–796. doi: 10.1016/j.molcel.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar JR, Pagan JK, Pagano M. Mechanisms and function of substrate recruitment by F-box proteins. Nature Reviews. Mol. Cell Biol. 2013;14:369–381. doi: 10.1038/nrm3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehling O, Vashisht AA, Mascarenhas J, Jonsson ZO, Sharma T, Netz DJ, Pierik AJ, Wohlschlegel JA, Lill R. MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science. 2012;337:195–199. doi: 10.1126/science.1219723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, Seifert M, Borodina T, Soldatov A, Parkhomchuk D, et al. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science. 2008;321:956–960. doi: 10.1126/science.1160342. [DOI] [PubMed] [Google Scholar]
- Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc. Nat. Acad. Sci. 2004;101:1461–1466. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Tashiro S, Hira S, Sun J, Yamazaki C, Zenke Y, Ikeda-Saito M, Yoshida M, Igarashi K. Heme regulates gene expression by triggering Crm1-dependent nuclear export of Bach1. EMBO J. 2004;23:2544–2553. doi: 10.1038/sj.emboj.7600248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Tashiro S, Sun J, Doi H, Satomi S, Igarashi K. Cadmium induces nuclear export of Bach1, a transcriptional repressor of heme oxygenase-1 gene. J. Biol. Chem. 2003;278:49246–49253. doi: 10.1074/jbc.M306764200. [DOI] [PubMed] [Google Scholar]
- Vashisht AA, Zumbrennen KB, Huang X, Powers DN, Durazo A, Sun D, Bhaskaran N, Persson A, Uhlen M, Sangfelt O, et al. Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science. 2009;326:718–721. doi: 10.1126/science.1176333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Gale M, Jr., Keller BC, Huang H, Brown MS, Goldstein JL, Ye J. Identification of FBL2 as a geranylgeranylated cellular protein required for hepatitis C virus RNA replication. Mol. Cell. 2005;18:425–434. doi: 10.1016/j.molcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Warnatz HJ, Schmidt D, Manke T, Piccini I, Sultan M, Borodina T, Balzereit D, Wruck W, Soldatov A, Vingron M, et al. The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in control of the cell cycle. J. Biol. Chem. 2011;286:23521–23532. doi: 10.1074/jbc.M111.220178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nature Reviews. Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- Yam CH, Ng RW, Siu WY, Lau AW, Poon RY. Regulation of cyclin A-Cdk2 by SCF component Skp1 and F-box protein Skp2. Mol. Cell. Biol. 1999;19:635–645. doi: 10.1128/mcb.19.1.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki C, Tashiro S, Nishito Y, Sueda T, Igarashi K. Dynamic cytoplasmic anchoring of the transcription factor Bach1 by intracellular hyaluronic acid binding protein IHABP. J. Biochem. 2005;137:287–296. doi: 10.1093/jb/mvi031. [DOI] [PubMed] [Google Scholar]
- Yen HC, Elledge SJ. Identification of SCF ubiquitin ligase substrates by global protein stability profiling. Science. 2008;322:923–929. doi: 10.1126/science.1160462. [DOI] [PubMed] [Google Scholar]
- Yumimoto K, Matsumoto M, Oyamada K, Moroishi T, Nakayama KI. Comprehensive Identification of Substrates for F-box Proteins by Differential Proteomics Analysis. J. Prot. Res. 2012;11:3175–3185. doi: 10.1021/pr201216u. [DOI] [PubMed] [Google Scholar]
- Zenke-Kawasaki Y, Dohi Y, Katoh Y, Ikura T, Ikura M, Asahara T, Tokunaga F, Iwai K, Igarashi K. Heme induces ubiquitination and degradation of the transcription factor Bach1. Mol. Cell. Biol. 2007;27:6962–6971. doi: 10.1128/MCB.02415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- Zhuang M, Calabrese MF, Liu J, Waddell MB, Nourse A, Hammel M, Miller DJ, Walden H, Duda DM, Seyedin SN, et al. Structures of SPOP-substrate complexes: insights into molecular architectures of BTB-Cul3 ubiquitin ligases. Mol. Cell. 2009;36:39–50. doi: 10.1016/j.molcel.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.