Abstract
Although the rabbit is routinely used as the animal model of choice to investigate cardiac electrophysiology, the neuroanatomy of the rabbit heart is not well documented. The aim of this study was to examine the topography of the intrinsic nerve plexus located on the rabbit heart surface and interatrial septum stained histochemically for acetylcholinesterase using pressure-distended whole hearts and whole-mount preparations from 33 Californian rabbits. Mediastinal cardiac nerves entered the venous part of the heart along the root of the right cranial vein (superior caval vein) and at the bifurcation of the pulmonary trunk. The accessing nerves of the venous part of the heart passed into the nerve plexus of heart hilum at the heart base. Nerves approaching the heart extended epicardially and innervated the atria, interatrial septum and ventricles by five nerve subplexuses, i.e. left and middle dorsal, dorsal right atrial, ventral right and left atrial subplexuses. Numerous nerves accessed the arterial part of the arterial part of the heart hilum between the aorta and pulmonary trunk, and distributed onto ventricles by the left and right coronary subplexuses. Clusters of intrinsic cardiac neurons were concentrated at the heart base at the roots of pulmonary veins with some positioned on the infundibulum. The mean number of intrinsic neurons in the rabbit heart is not significantly affected by aging: 2200 ± 262 (range 1517–2788; aged) vs. 2118 ± 108 (range 1513–2822; juvenile). In conclusion, despite anatomic differences in the distribution of intrinsic cardiac neurons and the presence of well-developed nerve plexus within the heart hilum, the topography of all seven subplexuses of the intrinsic nerve plexus in rabbit heart corresponds rather well to other mammalian species, including humans.
Keywords: acetylcholinesterase, cardiac ganglia, heart, heart–brain, intrinsic cardiac nervous system, intrinsic cardiac neurons, rabbit
Introduction
The intrinsic cardiac autonomic nervous system plays a crucial role in the regulation of cardiac chronotropy, dromotropy and inotropy, as well as coronary blood flow (Tsuboi et al. 2000; Randall et al. 2003; Armour 2008; Patil et al. 2011). This system is a heterogeneous population of neural elements, including preganglionic and postganglionic nerve fibers, and cardiac ganglia containing parasympathetic, sympathetic, afferent and local circuit neurons with a wide range of neurotransmitter phenotypes (Thompson et al. 2000; Randall et al. 2003; Hoover et al. 2008).
Anatomically, the intrinsic neural plexus on the surface of the heart is generally divided into seven distinct subplexuses according to their location in the heart hilum (Pauza et al. 1997) and downstream innervation and/or physiological effects. This knowledge has been developed from the study of the intrinsic nerve plexus in a wide range of experimental mammalian species (Ardell & Randall, 1986; Yuan et al. 1994; Armour et al. 1997; Pauza et al. 2000, 2002b; Arora et al. 2003; Gray et al. 2004; Johnson et al. 2004). These data do not include the rabbit, which is a commonly used laboratory experimental animal popular to investigate the effects of the autonomic system on cardiac electrophysiology, i.e. the cholinergic influence on atrioventricular conduction (Mazgalev et al. 1986) and the vagal-release of acetylcholine in the left ventricle during ischemia (Kawada et al. 2009). More recently, a novel dual innervated rabbit heart model (Ng et al. 2001) has been developed that has reignited research to investigate autonomic effects on heart rate and left ventricular pressure (Brack et al. 2004, 2006; Winter et al. 2012), calcium and nitric oxide fluorescence levels in the cardiac ventricle (Brack et al. 2009, 2010), and inducibility of ventricular fibrillation (Brack et al. 2007, 2011; Ng et al. 2007). Understanding the intrinsic cardiac nervous system in this species is therefore of paramount importance.
To our knowledge, the intrinsic nerve plexus of the rabbit is only documented within the cardiac conduction system (Anderson, 1972a; Bojsen-Moller & Tranum-Jeånsen, 1972; Roberts et al. 1989). Therefore, the aim of the present study was twofold: (i) to examine the topography of the rabbit intrinsic nerve plexus and quantify the distribution of intrinsic cardiac nerves; and (ii) to compare juvenile rabbits with old rabbits to ascertain if aging affects the intrinsic nerve plexus.
Materials and methods
Thirty-three 2–6-month-old Californian rabbits of either sex (2.6 ± 0.4 kg) were used in accordance with local and state guidelines for the care and use of laboratory animals (Permission No. 0206). The animals were anesthetized with a lethal dose of sodium thiopental (30 mg kg−1, i.v.). In order to investigate the effect of age on the number of intrinsic cardiac neurons in the rabbit, an additional five 3–4-year-old rabbits of either sex were used (4.9 ± 0.2 kg).
Non-sectioned pressure-distended heart preparations
After death, the chest was opened and the heart was perfused with phosphate-buffered saline (PBS; 0.01 K, pH 7.4) via a cannula inserted into the left ventricle (n = 17). After the blood was flushed from the heart, the atrial walls were pressure-inflated in situ by a transmyocardial injection of 20% gelatin solution into the atria and ventricles. Once the gelatin had set, the heart was removed and immersed in a chamber filled with room temperature PBS, where the remains of the pericardium, pulmonary arteries and mediastinal fat were dissected from the heart base.
All hearts were prefixed in 4% paraformaldehyde in 0.01 m phosphate buffer (PB; pH 7.4, 4 °C, 30 min), and subsequently washed for 12 h at 4 °C in PBS containing hyaluronidase (0.5 mg/100 mL; Sigma-Aldrich, St Louis, MO, USA) to increase tissue permeability for staining. To stain the intrinsic neural plexus, whole hearts (n = 11) were placed for 4 h at 4 °C in Karnovsky–Roots medium as described previously (Pauza et al. 1999). For post-staining long-term storage, hearts were placed in 4% paraformaldehyde solution in PB (0.01 m, pH 7.4).
In order to focus on intrinsic cardiac neural elements distributed within cardiac septa, six separate pressure-distended hearts were additionally dissected to expose the interatrial and interventricular septa cutting off the walls of both atria and ventricles.
Whole-mount preparations
To perform quantitative analysis of intrinsic cardiac neurons, acetylcholinesterase staining was carried out on 16 whole-mount preparations that involved the atria, interatrial septum and infundibulum (i.e. the conus arteriosus) region to analyze ventricular neurons.
After cardiac perfusion with room temperature PBS, hearts were removed and placed into a dissecting dish containing ice-cold PBS. The walls of atria and interatrial septum were separated from ventricles with the atrial septum dissected free from the atrial walls. The atrial tissue and interatrial septum were pinned flat separately on a custom-made dissecting dish. To count ventricular neurons, the wall of the conus arteriosus was cut and pinned flat on another dissecting dish. All whole-mount preparations were stained according to the protocol described above. Once stained, preparations were fixed for 96 h in 4% paraformaldehyde solution (0.01 m PB, pH 7.4). After fixation, whole-mounts were dehydrated with 50-min washes through a graded ethanol series and immersed for 2 h in xylene (Sigma-Aldrich). Flattened whole-mount preparations were placed onto microscope slides and coverslipped using Roti®-Histokitt mounting medium (Carl Roth, Karlsruhe, Germany) for microscopic analysis.
Microscopic examination and quantitative analysis
Neural structures stained for acetylcholinesterase were examined stereoscopically at 6.3 × magnification using a Stemi 200 CS stereomicroscope (Zeiss, Gottingen, Germany) following illumination using fiber optic light guide (Zeiss). Images were captured using a digital camera (Axiocam HRc; Zeiss). To quantify intrinsic neuronal material, the whole-mount images were analyzed using an AxioImager M1 bright-field microscope (Zeiss, Jena, Germany). Two independent investigators performed control calculations and measurements of two nominated neuronal clusters per whole-mount from 16 hearts. The result error of both investigators was 6 ± 1%. In order to avoid an overestimation of the number of neurons, the number of neurons inside the intrinsic ganglia or neuronal clusters was estimated in whole-mounts by counting exclusively those nerve cells that contained well-stained perinuclear regions (Fig. 2c). However, some neuronal somata were poorly stained, so the number of neurons in some ganglia could have been underestimated.
Figure 2.
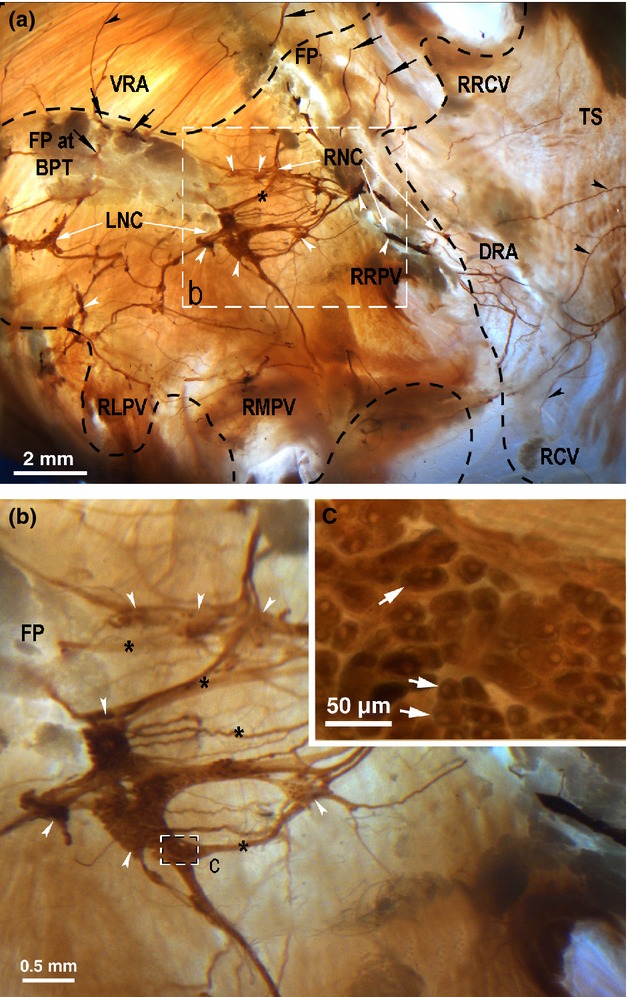
Macrophotographs of the rabbit heart base to illustrate the location and the structure of the nerve plexus of heart hilum (NPHH) in the rabbit heart stained histochemically for acetylcholinesterase. The boxed area in (a) outlines the area on the rabbit heart that is enlarged in (b). The boxed area in (b) outlines the area on the rabbit right neuronal cluster that is enlarged as the inset (c). Black arrows point the access of extrinsic cardiac nerves into the heart through the venous part of the heart hilum. White arrowheads indicate some ganglia within the neuronal clusters. Black arrowheads point to some subplexal nerves extending to the innervation regions, while the white solid arrows in the inset (c) point to some intrinsic cardiac neurons within the right neuronal cluster (RNC). Asterisks indicate some commissural nerves that connect the neuronal clusters within the venous part of the heart hilum. Dashed line demarcates the heart hilum. DRA, dorsal right atrial subplexus; FP, fat pad under interatrial septum; FP at BPT, fat pad at bifurcation of pulmonary trunk; LNC, left neuronal cluster; RCV, root of caudal vein (inferior caval vein); RLPV, root of left pulmonary vein; RMPV, root of middle pulmonary vein; RNC, right neuronal cluster; RRCV, root of right cranial vein (superior caval vein); RRPV, root of right pulmonary vein; TS, terminal groove; VRA, ventral right atrial subplexus.
Comparison of juvenile vs. aged rabbits
The calculation was performed on 11 juvenile and five old Californian rabbits of either gender.
Statistical analysis
Data analysis was performed using Origin Lab software (v.6.1; OriginLab, Northampton, MA, USA). Data are expressed as mean ± standard error of mean (SEM). Statistical analysis of the number of neuronal clusters and neuronal somata in the distinct cluster groups was performed using one-way analysis of variance (anova). Differences were considered statistically significant at P < 0.05.
Results
Distribution of intrinsic cardiac neurons in whole rabbit hearts
Intrinsic cardiac neurons were found either: (i) singular, i.e. on their own; (ii) formed into cardiac ganglia where there are a number of neuronal cell bodies collected together; or (iii) in neuronal clusters where there were large areas occupied abundantly by nerve cells. In all rabbit hearts examined, nerve cells were found within the venous part of the heart hilum as well as on the epicardial surface of the heart, including the area on the conus arteriosus (i.e. infundibulum) at the root of the pulmonary trunk (Fig. 1). Intrinsic cardiac neurons have been defined according to their location as described below.
Figure 1.
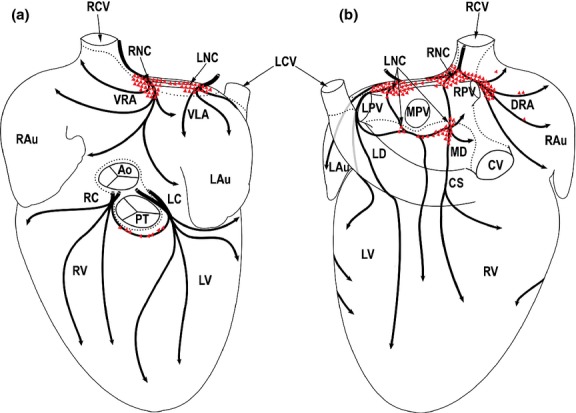
Drawings summarizing the morphological pattern of distinct epicardial nerve subplexuses from 11 rabbit hearts as seen from the ventral (a) and dorsal (b) views of the pressure-distended heart stained histochemically for acetylcholinesterase (AChE). The clusters of intrinsic cardiac neurons (ICNs) (drawn in red) were outlined from the whole-mount stained histochemically for AChE. Dotted lines demarcate limits of the heart hilum. Black arched arrows indicate the course of nerve subplexuses on the rabbit heart surface. Red polygonal triangled areas indicate the location of neuronal clusters and epicardial ganglia. Ao, ascending aorta; CS, coronary sinus; CV, caudal vein; DRA, dorsal right atrial subplexus; ICNs, intrinsic cardiac neurons; LAu, left auricle; LC, left coronary subplexus; LCV, left cranial vein; LD, left dorsal subplexus; LNC, left neuronal cluster; LPV, left pulmonary vein; LV, left ventricle; MD, middle dorsal subplexus; MPV, middle pulmonary vein; PT, pulmonary trunk; RAu, right auricle; RC, right coronary subplexus; RCV, right cranial vein (superior caval vein); RNC, right neuronal cluster; RPV, right pulmonary vein; RV, right ventricle; VLA, ventral left atrial subplexus; VRA, ventral right atrial subplexus.
An illustration of the rabbit heart hilum with neural structures primarily on the left atrial walls is shown in Figs 2, 3 and 4. As shown, three localities of ganglionic cells were regularly identified at: (i) the dorso-cranial groove above the interatrial septum; and (ii) on the ventral right atrial region (Figs 2, 3 and 4a). The right neuronal cluster of the venous part of heart hilum was distributed at the cranial aspect of the interatrial groove, and this was found in every heart. The right atrial ganglia were identified epicardially along the nerves extending from the right neuronal cluster on the anterior wall of the root of the right cranial vein (i.e. superior caval vein), and were present in 50% of hearts as indicated by the white arrowheads in Fig. 3. Because the neurons within the latter sites merged frequently with the right neuronal cluster, they were considered as nerve cells belonging to the right neuronal cluster. Occasionally, certain minute epicardial ganglia on the dorsal right atrium were identified along the nerves coursing toward the sinus node region by the side of the root of the right pulmonary vein (Figs 2a and 4a).
Figure 3.
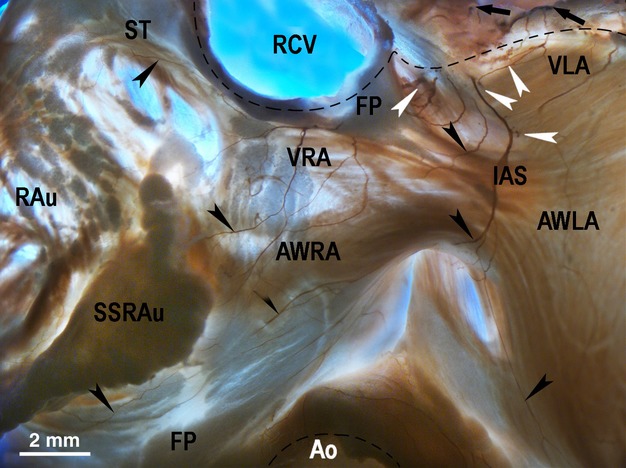
Macrophotograph demonstrating the location and the morphological pattern of the ventral right (VRA) and left (VLA) atrial nerve subplexuses in the rabbit heart stained histochemically for acetylcholinesterase. Black arrows point to the access of extrinsic cardiac nerves into the heart through the venous part of the heart hilum (at the bifurcation of the pulmonary trunk). Black arrowheads point to some subplexal nerves extending to the innervation regions. White arrowheads point to the epicardial ganglia on the anterior wall of the root of the right cranial veins. Dashed line demarcates the heart hilum. Ao, aorta; AWLA, anterior wall of left atrium; AWRA, anterior wall of right atrium; IAS, projection of the interatrial septum; RAu, right auricle; RCV, right cranial vein; SSRAu, superior surface of right auricle; ST, sulcus terminalis; VLA, ventral left atrial subplexus; VRA, ventral right atrial subplexus.
Figure 4.
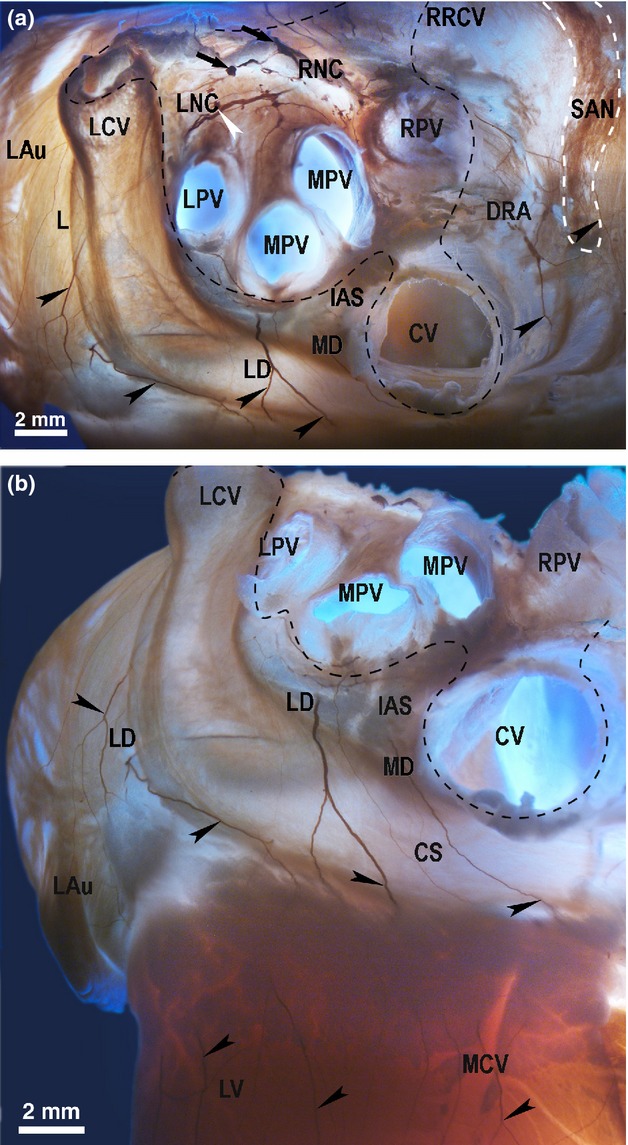
The location of the left dorsal (LD) and the dorsal right atrial (DRA) neural subplexuses in the rabbit heart stained histochemically for acetylcholinesterase. A dorso-caudal view (a) and a caudal view (b). Black arrows point to the access of extrinsic cardiac nerves into the heart through the venous part of the heart hilum (at the bifurcation of the pulmonary trunk). White arrowhead indicates the ganglion of the left neuronal cluster (LNC). Black arrowheads point to some epicardial nerves of the LD, MD and DRA subplexuses extending to the innervation regions. Dashed line demarcates the venous part of the heart hilum. CS, coronary sinus; CV, orifice of caudal vein (inferior caval vein); DRA, dorsal right atrial subplexus; IAS, projection of the interatrial septum; LAu, left auricle; LCV, left cranial vein; LD, left dorsal subplexus; LNC, left neuronal cluster; LPV, left pulmonary vein; LV, left ventricle; MCV, middle cardiac vein; MD, middle dorsal nerve subplexus; MPV, middle pulmonary vein; RNC, right neuronal cluster; RPV, right pulmonary vein; RRCV, root of the right cranial vein (superior caval vein); SAN, region of sinus node (outlined by dashed white line).
Intrinsic cardiac nerve cells were mainly located within the left neuronal cluster of the venous part of the heart hilum at the roots of the left and middle pulmonary veins, and were identified in every heart (Figs 1, 2 and 4a). On the dorsal left atrial surface, some epicardial ganglia were identified below the root of the middle pulmonary vein, and between the roots of the middle pulmonary vein and caudal vein (i.e. inferior caval vein) in every heart (Figs 1 and 4).
In all hearts examined, there were solitary neuronal cells and small ventricular ganglia that were scattered nearby the pulmonary trunk and distributed on the most upper part of the conus arteriosus, as indicated by the white arrowheads in Fig. 4a,b and shown schematically in Fig. 1.
Quantification of rabbit intrinsic cardiac ganglia
High-magnification micrographs of individual intrinsic cardiac neuronal cell bodies are illustrated in Fig. 2c. Intrinsic ganglia varied in shape and size, but most of them were unilayered and had irregular appearances because of their extensions at the sites where nerves connected to a ganglion. The smallest ganglia were epicardial and involved three–five neuronal somata, whereas the largest ones contained up to 728 neurons and were identified within the venous part of heart hilum beneath the pulmonary arteries (Figs 1, 2 and 4; Table 1). Ventricular epicardial ganglia contained a significantly (P < 0.01) smaller number of neurons compared with the atrial ganglia (Table 1).
Table 1.
The mean number and the range of the intrinsic neuronal somata in the rabbit whole-mount preparations.
| Neurons | Juvenile (n = 11) |
Old (n = 5) |
Overall (n = 16) |
|||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |
| RNC of VHH | 643 ± 81 | 141–977 | 794 ± 125 | 669–918 | 698 ± 60 | 141–977 |
| LNC of VHH | 1222 ± 129* | 562–1930* | 1168 ± 164* | 934–1483* | 1161 ± 100* | 562–1930* |
| Singular per VHH | 304 ± 58 | 133–576 | 203 ± 43 | 128–280 | 278 ± 48 | 128–576 |
| Interatrial septum | 19 ± 19 | 0–97 | 2 ± 2 | 0–8 | 11 ± 10 | 0–97 |
| On average per atria | 2033 ± 137 | 1409–2723 | 1971 ± 265 | 1441–2256 | 2020 ± 117 | 1409–2723 |
| Ganglionic ventricular at CA | 31 ± 5 | 6–69 | 22 ± 10 | 8–42 | 27 ± 7 | 6–69 |
| Single ventricular at CA | 36 ± 7 | 6–55 | 23 ± 7 | 11–36 | 32 ± 5 | 6–55 |
| On average per ventricles | 65 ± 22 | 12–124 | 45 ± 17 | 19–78 | 91 ± 18 | 12–169 |
| On average per heart | 2146 ± 125 | 1650–2822 | 2012 ± 249 | 1513–2276 | 2118 ± 108 | 1513–2822 |
P < 0.05 RNC vs. LNC.
CA, conus arteriosus; LNC, left neuronal cluster; RNC, right neuronal cluster; VHH, venous part of heart hilum.
A full breakdown of intrinsic neural cells contained within distinct cardiac locations is presented in Table 1. Overall, we estimate that there are, in total, 2118 ± 108 intrinsic cardiac neurons contained within the rabbit heart, ranging between 1513 and 2822. Although rabbit intrinsic cardiac neurons were mostly concentrated into cardiac neuronal clusters within the venous part of the heart hilum or epicardial ganglia, there were abundant single neurons that were identified within the dorsal aspect of the venous part of the heart hilum and scattered epicardially on the conus arteriosus as well as endocardially in the rabbit interatrial septum (Table 1; Figs 1, 2, 5 and 6).
Figure 5.
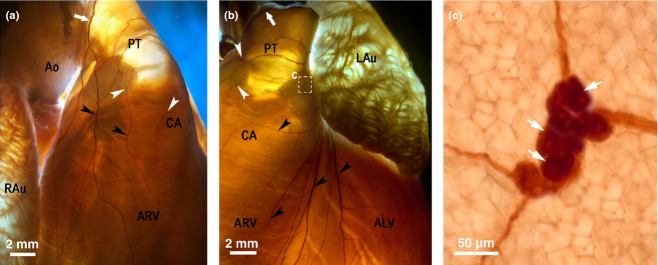
Macrophotographs of the right (a) and the left (b) coronary subplexuses (RC and LC) in the rabbit heart stained histochemically for acetylcholinesterase. White arrows indicate the access of extrinsic cardiac nerves into the heart through the arterial part of the heart hilum. The boxed area ‘c’ in the panel (b) is enlarged as the panel (c). Black arrowheads point to some subplexal nerves extending to the innervation regions, the white ones point to some small epicardial ganglia on the conus arteriosus (CA) and the root of pulmonary trunk (PT). ALV, anterior wall of the left ventricle; Ao, root of the aorta; ARV, anterior wall of the right ventricle; LAu, left auricle; RAu, right auricle.
Figure 6.
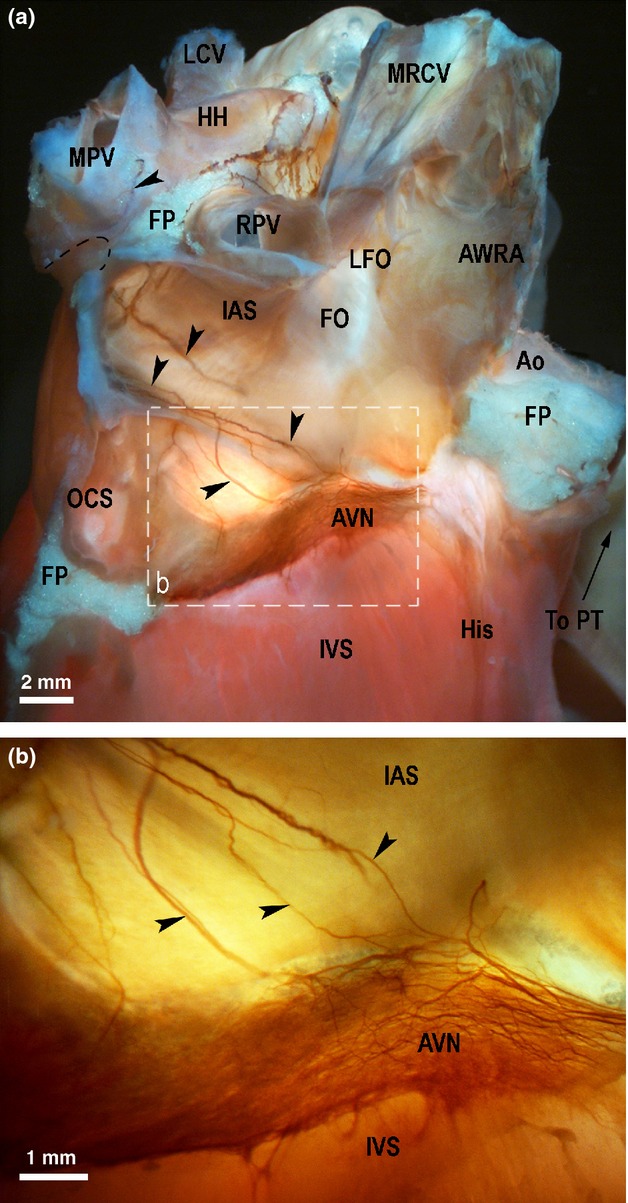
Macrophotographs of the right side of the rabbit cardiac septa to illustrate the endocardial extension of the middle dorsal (MD) subplexal nerves (black arrowheads) to the AVN region. The right atrium and right ventricle were cut off to expose the septum from the right side. The boxed area in the panel (a) outlines the area on the rabbit heart that is enlarged in (b). Ao, aorta; AVN, atrioventricular nodal area; AWRA, remainder of anterior wall of the right atrium; FO, fossa ovalis; FP, fat pad; HH, heart hilum; His, bundle of His; IAS, interatrial septum; IVS, interventricular septum; LCV, left pulmonary vein; LFO, limbus of fossa ovalis; MPV, middle pulmonary vein; MRCV, medial wall of the right cranial vein; OCS, opening of coronary sinus; PT, orifice of pulmonary trunk; RPV, right pulmonary vein.
Architecture of the rabbit intrinsic cardiac nerve plexus
Access of extrinsic cardiac nerves
Extrinsic cardiac nerves access the rabbit heart through arterial and venous parts of the heart hilum. In general terms, nerves accessing the arterial part of the heart hilum were readily identified between the ascending aorta and the pulmonary trunk. These nerves appear to selectively extend towards the anterior surface of the left and right ventricle (Figs 1, 2a, 3 and 4). In 10 hearts (out of 11 examined), numerous nerves were situated around the pulmonary trunk, i.e. in the front, left and right edges of the pulmonary trunk. Solitary nerves were identified in front of the ascending aorta (in two hearts examined, out of 11), behind the ascending aorta (in seven hearts examined, out of 11) and on the left of the ascending aorta (in three hearts examined, out of 11).
Within the venous part of the heart hilum, i.e. around the roots of the right cranial (superior caval vein), caudal (inferior caval vein) and pulmonary veins, nerves were consistently identified at the medial side of the root of the right cranial vein and in the fat pad located at the bifurcation of the pulmonary trunk (in every heart examined). A few preparations contained thin accessing nerves on the root of the left pulmonary vein (in two hearts examined, out of 11) as well as on the root of the medial pulmonary vein (in three hearts examined, out of 11). The nerves accessing the venous part of heart hilum appeared to proceed forward to the majority of the atria and posterior surface of the left and right ventricles (Fig. 1). Nerves accessing the heart at the bifurcation of pulmonary trunk passed into the left neuronal cluster in every heart examined. In all hearts, nerves accessing at the root of the right cranial vein entered into the right neuronal cluster of the venous part of the heart hilum (Figs 1, 2 and 3a).
Ganglia to right atrial myocardium
The intrinsic cardiac nerves extending from both the neuronal clusters of the venous part of the heart hilum (100% of hearts) spread epicardially by two right atrial neural subplexuses – the ventral and dorsal right atrial subplexuses (Figs 1, 2, 3 and 4a). The ventral right atrial subplexal nerves coursed within the epicardium at the medial surface of the root of the right cranial vein and extended onto the ventral surface of the right atrial appendage as well as onto the ventral surface of the right and left atria (in 100% of hearts; Figs 3 and 4a). The dorsal right atrial subplexal nerves passed between the roots of the right cranial vein and right pulmonary vein, and extended widely into the dorsal and lateral surfaces of the right atrium, including the region of sinus node and right atrial appendage (Figs 1, 2, 3 and 4a).
Ganglia to left atrial myocardium
Intrinsic cardiac nerves passing from the left neuronal cluster (100% of hearts) distributed epicardially by the left dorsal (Figs 1 and 4b) and the ventral left atrial subplexuses (Figs 1 and 3). The left dorsal subplexal nerves passed intramurally between the root of the left cranial vein and the dorsal wall of the left atrium, crossing the coronary groove and extended onto the posterior surface of the left ventricle (100% of hearts; Figs 1 and 4b). In all hearts, there were some solitary nerves of the left dorsal subplexus that coursed superficially over the root of the left cranial vein onto the posterior left ventricle surface (Figs 1 and 4b). A small group of the left dorsal subplexal nerves also branched out from the left neuronal cluster spread to the lateral surface of the left atrial appendage (100% of hearts).
In four hearts (28%), thin left dorsal subplexal nerves pass the left neuronal cluster and cross the coronary groove. The ventral left atrial subplexal nerves coursed ventrally from the left neuronal cluster, and spread only on the ventral surface of the left atrium and atrial appendage (100% of hearts; Fig. 3). In all hearts, the nerves of the left and medial dorsal subplexus regularly originated from the left neuronal cluster, and either: (i) crossed over the coronary groove onto the dorsal surface of the left ventricle; (ii) passed along the coronary groove and bypassed the root of the caudal vein distributed in the caudal surface of the right atrium (in every heart examined except three, 27% of hearts); (iii) penetrated into dorsal aspects of the interatrial groove, reached the interatrial septum and spread dorso-ventrally as endocardial nerves on the right side of the interatrial septum, where they gradually became thinner and ramified at the region of the atrio-ventricular node (100% of hearts; Figs 4 and 6).
Ganglia to ventricular myocardium
In all hearts, nerves accessing the arterial part of the heart hilum, i.e. between the aorta and pulmonary trunk, extended widely into the anterior walls of the right and left ventricle through the right coronary and left coronary subplexuses, respectively (Figs 1 and 5). A characteristic feature of these subplexuses is that numerous subplexal nerves proceeded directly to the ventricles. Sparse and comparatively thin nerves did enter the ventricular ganglia near the pulmonary trunk, on the wall of the conus arteriosus. In all hearts, numerous right coronary subplexal nerves spread onto the ventral and lateral surfaces of the right ventricle and right side of the conus arteriosus. In several hearts, left coronary subplexal nerves extended onto the dorsal surface of the right ventricle (in three hearts, 27%), as well as spreading widely along the ventral interventricular groove (in two hearts, 18%). In all hearts, left coronary subplexal nerves supplied the ventral, lateral and dorsal walls surfaces of the left ventricle, as well as the left side of the conus arteriosus (Figs 1 and 5).
Inter-ganglionic connectivity
In 100% of hearts examined, there were numerous thin commissural nerves that are recognizable to connect between left and right neuronal clusters within the venous part of the heart hilum (Figs 1 and 2). Similar in thickness, commissural nerves interconnected both the right and left coronary subplexuses on the conus arteriosus (in 100% of hearts examined; Figs 1 and 5).
Comparison of juvenile vs. aged rabbits
The comparison of the intrinsic nerve plexus between juvenile and aged rabbits is illustrated in Table 1. In juvenile whole rabbit hearts, the total number of the intrinsic cardiac neurons from both atrial and ventricular regions was 2146 ± 125, and ranged from 1650 to 2822. Within the rabbit heart hilum, 643 ± 81 neurons were found in the right neuronal cluster, and 1222 ± 129 neurons were identified in the left neuronal cluster (P < 0.05). The total number of the intrinsic cardiac neurons in juvenile rabbits compared with aged animals was not significantly different (P > 0.05) in any region studied.
Discussion
To the best of our knowledge, this study is the first to detail the neuroanatomy of the rabbit heart intrinsic nerve plexus using standard histochemical acetylcholinesterase staining. The main results of this study are summarized below.
There are three main routes through which extrinsic cardiac nerves access the heart: the medial side of the right cranial vein (superior caval vein); in fat pads located at the bifurcation of the pulmonary trunk; and between the ascending aorta and the pulmonary trunk.
Intrinsic cardiac nerves innervate the heart through seven epicardial subplexuses. The left atrium was supplied by nerves extending from the left dorsal, medial dorsal, ventral left atrial and ventral right atrial subplexuses. The right atrium, sinus and atrioventricular nodal areas are supplied by the left dorsal and ventral right atrial and medial dorsal subplexuses. The right ventricle is supplied by the right coronary and medial dorsal subplexuses, whilst the left ventricle was supplied by the left coronary, left dorsal and medial dorsal subplexuses.
The distribution sites of intrinsic cardiac neurons and their relation with epicardial subplexuses suggest that the right neuronal cluster supplies the dorsal right atrial and ventral right atrial subplexuses, whilst the left neuronal cluster supplies the medial dorsal, ventral left atrial and left dorsal subplexuses. Epicardial ganglia on the conus arteriosus supply both coronary subplexuses.
Architecture and relation to other species
In general, the morphological pattern of the rabbit intrinsic nerve plexus corresponds to those of larger mammals, but it is somewhat less complex and less dense. Approximately 95% of intrinsic cardiac neurons are concentrated within the heart hilum and approximately 5% on the ventricle, which is similar to other species investigated earlier by us (Pauza et al. 2002b, 2013; Saburkina et al. 2010; Rysevaite et al. 2011a,2011b). The finding that the rabbit nerve plexus of the heart hilum supplies five atrial, i.e. the dorsal right atrial, ventral right atrial, medial dorsal, left dorsal and ventral left atrial, subplexuses is also a similar finding to our previously published work from human, canine, porcine and sheep hearts that also had five discrete ganglionated nerve subplexuses occupying specific and discrete locations originated from the heart hilum (Pauza et al. 2000, 2002b; Batulevicius et al. 2003, 2005, 2008; Saburkina et al. 2010).
In rabbits, rats, guinea pigs and mice, ganglionic cells of the cardiac hilum are distributed at approximately the same locations, i.e. at the cranial aspect of the interatrial groove, medially and below the roots of the left and right cranial veins and epicardially of the left and right pulmonary veins with few neurons spread endocardially within the interatrial septum (Anderson, 1972b; Batulevicius et al. 2003, 2005; Rysevaite et al. 2011a).
Atrioventricular nodal region innervation appears to originate from one subplexus; namely the middle dorsal subplexus. This subplexal route may, however, also contain some fibers from the left dorsal subplexus, as these two subplexuses communicate by thin epicardial nerves on the dorso-caudal side of the left atrium above the sinus of the left cranial vein. Data from the current study suggest that these nerves appear to innervate the node from the superior dorsal axis, which is not in accord with other studies in the rabbit. Several studies demonstrate an additional innervation route from the inferior dorsal axis (Anderson, 1972a; Bojsen-Moller & Tranum-Jeånsen, 1972; Roberts et al. 1989). We currently cannot offer any concrete reasons for these differences, expect that there is some unknown methodological explanation (see Limitations). This inferior dorsal route of innervation is, however, present in other species studied by us, i.e. the dog (Pauza et al. 2002b) and humans (Pauza et al. 2000), and also studied by others, i.e. guinea pig (Anderson, 1972b) and humans (Anderson & Taylor, 1972).
In the rabbit, there are prominent subplexal nerves proceeding from the left and right coronary subplexuses that extend toward the anterior or ventral portion of both ventricles. These observations are in accord with the rat where abundant nerves extend to the ventral surface of both ventricles from the arterial part of the heart hilum (Batulevicius et al. 2003). In contrast to mice, the ventral surface of both ventricles received nerves from the right ventral subplexal (Rysevaite et al. 2011a).
Quantifiable neuronal data
We estimate that approximately 1500–3000 intrinsic cardiac neurons are involved in the rabbit intrinsic nerve plexus. The reports by Pardini et al. (1987) and Batulevicius et al. (2003), who counted neurons in tissue sections, indicate that about 4000–7000 neurons reside within the rat heart. This is in contrast to the studies of de Souza et al. (1996) and Akamatsu et al. (2007) that reported 1000–2000 intrinsic cardiac neurons in rat heart preparations. These discrepancies may reflect methodological using cardiac sections vs. whole-mount preparations. However, whole-mount preparations are thought to allow an approximate estimation of neuronal number because many intrinsic cardiac neurons in rats are densely packed one above another, and neurons within these clusters are poorly discernible even by a conventional upright (non-stereoscopic) light microscope.
Our data also demonstrate that rabbit intrinsic cardiac neurons are generally congregated within unilayered neuronal clusters. This is in contrast to humans, canine, porcine, sheep and other large animal cardiac neurons that are mostly arranged into compact ganglia (Pauza et al. 1999, 2000, 2002a,2002b; Batulevicius et al. 2008; Saburkina et al. 2010). Serial confocal sections through those ganglia as well as routine histology revealed that neuronal somata were distributed in the periphery adjacent to the well-defined fibrous capsule (Arora et al. 2003). The interior of those ganglia was comprised of neuropil, with numerous dendrites of the peripherally located neurons or small nerves (Arora et al. 2003; Pauziene & Pauza, 2003).
Functional importance
Physiological studies have shown that some of the cardiac ganglia may affect parasympathetic control of cardiac rate (at the cranial aspect of the coronary groove) and atrioventricular conduction (at the junction of the inferior caval vein and left atrium) modulating the actions of the sinus node and the atrioventricular node, respectively (Gatti et al. 1995; Gray et al. 2004). The fact that our study again demonstrates that there are commissural nerves connecting different neuronal ganglionic regions provided further support of strong connectivity and communicative nature of the intrinsic nerve plexus in the heart. This is supported by recent data that demonstrated that neuronal regions surrounding the pulmonary veins on the left atrium are capable of modulating heart rate that is initiated in the right atrium (Zarzoso et al. 2013). Perhaps more importantly, the intrinsic nerve plexus around the pulmonary veins has received much attention as a target for therapeutic intervention to control atrial tachyarrhythmias, particularly atrial fibrillation (reviewed by Shen et al. 2012).
The presence of neurons and ganglia on cardiac ventricles below the coronary groove is an established fact (Davies et al. 1952; Mitchell et al. 1953; Gagliardi et al. 1988), but the function of these neurons is unknown. Taking into account their narrow distribution on the rabbit cardiac ventricles (region of the conus arteriosus adjacent to the root of the pulmonary trunk), their role is seemingly related to function of pulmonary valve and pulmonary circulation. Moreover, dissemination of a certain amount of intrinsic epicardial ganglia in this site of conus arteriosus is acknowledged from a number of mammalian species, including humans (Smith, 1970a,1970b, 1971; Pauza et al. 2000, 2002a,2002b; Saburkina & Pauza, 2006; Batulevicius et al. 2008; Saburkina et al. 2010; Rysevaite et al. 2011a,2011b).
Together, the findings of the current study highlight that further work is very much needed to delineate the functional role of ganglionic structures located both on the atria and ventricles.
Neurochemical phenotype
In the current study, we have not performed any immunohistochemical investigation of the intrinsic nerve plexus as this is subject to a more detailed and separate investigation. Notwithstanding this, we hypothesize that the neuronal material stained using Karnovsky & Roots (1964) acetylcholinesterase histochemical staining will contain both the cholinergic and adrenergic nerve structures, as suggested by Koelle et al. (1987). This is supported by previous work of ours that we have studied in other species (Rysevaite et al. 2011a; Pauza et al. 2013). This, however, is in dispute with some investigators who show evidence that the acetylcholinesterase staining method reflects cholinergic inputs. This is owing to studies that have illustrated that selective cholinergic denervation using vinblastine leads to a loss of acetylcholinesterase staining in the heart (reviewed in more detail by Coote, 2013). Clearly, further experimental investigations are warranted to confirm what exactly is being stained using the Karnovsky and Roots staining medium within this species, and others.
Juvenile vs. aged rabbits
The present study demonstrates that aging does not affect the mean number of intrinsic neurons in the rabbit heart. This is in contrast to rat and guinea pigs where there are marked age-related changes in the neuronal number (Batulevicius et al. 2003, 2005). Despite this, our data are in accord with previous reports on humans and canines (Pauza et al. 2002a,2002b). In general, the age-related differences of the neuron number in the rabbit and guinea-pig hearts might indicate that the hearts of rodents and lagomorphs, unlike the human and canine hearts, do not undergo a decrease in neuronal number with aging (Batulevicius et al. 2005). Regarding the age-related degeneration of the nerve structures in the rodent heart, the significant reduction of the total numbers of vagal efferent cardiac axons and basket endings was reported in the aged rats (Ai et al. 2007).
Limitations
Acetylcholinesterase staining has been used to provide a basis and to characterize the intrinsic nerve network in the rabbit, which has not previously been carried out. This methodology may, however, have two potential drawbacks.
This stain might, however, also stain non-cholinergic nerve fibers. Despite the convincing historical studies showing that acetylcholinesterase staining was abolished following vagal denervation and specific chemical cholinergic ablation in the dog (reviewed by Coote, 2013), questions remain as to what is being revealed using the acetylcholinesterase staining methodology employed in this species.
This histochemical staining will only reveal neuronal structures if they are located less superficially, i.e. no deeper than 1–2 mm. If structures are deeper, they will not be revealed by the acetylcholinesterase method. This discrepancy may go some way to help explain the difference in findings of the current study to others in rabbit. We do not deny the possibility of intrinsic nerves accessing the atrioventricular node via other routes aside from the dorso-cranial that were not revealed or seen by the methods utilized in the present investigation. Clearly, further experimental work is needed to answer these questions and confirm what is being stained, and to reveal the fullest extent of cardiac innervation. Nevertheless, the results of the current study provide a sound basis for further work to be carried out.
Summary
These findings suggest that both the distribution of the intrinsic cardiac neurons and the architecture of the rabbit intrinsic nerve plexus correspond to the mouse and rat hearts, although, in general the topography of all seven rabbit nerve subplexuses completely corresponds to the human subplexuses, and partially to that of the mouse and rat subplexuses. In addition, the quantity and regionalization of ganglionic plexus are not affected by age. Our data support the use of the rabbit heart as a model for experimental neurocardiology, and provide gross anatomical basis for further immunohistochemical, electron microscopic investigations and physiological experiments.
Acknowledgments
The authors sincerely thank Mr Sarunas Janavicius, Lithuanian Association of Rabbit Breeders, for providing material for this study. This study was supported by Grants MIP-11184 and MIP-13037 from the Research Council of Lithuania. K.E.B. is supported by a British Heart Foundation Intermediate Basic Science Research Fellowship (FS 12/2/29300).
References
- Ai J, Gozal D, Li L, et al. Degeneration of vagal efferent axons and terminals in cardiac ganglia of aged rats. J Comp Neurol. 2007;50:74–88. doi: 10.1002/cne.21431. [DOI] [PubMed] [Google Scholar]
- Akamatsu FE, De-Souza RR, Liberti EA. Fall in the number of intracardiac neurons in aging rats. Mech Ageing Dev. 1999;109:153–161. doi: 10.1016/s0047-6374(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Anderson RH. Histologic and histochemical evidence concerning the presence of morphologically distinct cellular zones within the rabbit atrioventricular node. Anat Rec. 1972a;173:7–23. doi: 10.1002/ar.1091730102. [DOI] [PubMed] [Google Scholar]
- Anderson RH. The disposition, morphology and innervation of cardiac specialized tissues in the guinea pig. J Anat. 1972b;111:453–468. [PMC free article] [PubMed] [Google Scholar]
- Anderson RH, Taylor IM. Development of atrioventricular specialised tissue in human heart. Br Heart J. 1972;34:1205–1214. doi: 10.1136/hrt.34.12.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol. 1986;251:H764–H773. doi: 10.1152/ajpheart.1986.251.4.H764. [DOI] [PubMed] [Google Scholar]
- Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol. 2008;93:165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- Armour JA, Murphy DA, Yuan BX, et al. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Arora RC, Waldmann M, Hopkins DA, et al. Porcine intrinsic cardiac ganglia. Anat Rec. 2003;271:249–258. doi: 10.1002/ar.a.10030. [DOI] [PubMed] [Google Scholar]
- Batulevicius D, Pauziene N, Pauza DH. Topographic morphology and age-related analysis of the neuronal number of the rat intracardiac nerve plexus. Ann Anat. 2003;185:449–459. doi: 10.1016/S0940-9602(03)80105-X. [DOI] [PubMed] [Google Scholar]
- Batulevicius D, Pauziene N, Pauza DH. Architecture and age-related analysis of the neuronal number of the guinea pig intrinsic cardiac nerve plexus. Ann Anat. 2005;187:225–243. doi: 10.1016/j.aanat.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Batulevicius D, Skripka V, Pauziene N, et al. Topography of the porcine epicardiac nerve plexus as revealed by histochemistry for acetylcholinesterase. Auton Neurosci. 2008;138:64–75. doi: 10.1016/j.autneu.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Bojsen-Moller F, Tranum-Jeånsen J. Rabbit heart nodal tissue, sinuatrial ring bundle and atrioventricular connexions indentified as a neuromuscular system. J Anat. 1972;112:367–382. [PMC free article] [PubMed] [Google Scholar]
- Brack KE, Coote JH, Ng GA. Interaction between direct sympathetic and vagus nerve stimulation on heart rate in the isolated Langendorff perfused rabbit heart. Exp Physiol. 2004;89:128–139. doi: 10.1113/expphysiol.2003.002654. [DOI] [PubMed] [Google Scholar]
- Brack KE, Coote JH, Ng GA. The effect of direct autonomic nerve stimulation on left ventricular force in the isolated innervated Langendorff perfused rabbit heart. Auton Neurosci. 2006;124:69–80. doi: 10.1016/j.autneu.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Brack KE, Patel VH, Coote JH, et al. Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the isolated rabbit heart. J Physiol. 2007;583:695–704. doi: 10.1113/jphysiol.2007.138461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack KE, Patel VH, Mantravardi R, et al. Direct evidence of nitric oxide release from neuronal nitric oxide synthase activation in the left ventricle as a result of cervical vagus nerve stimulation. J Physiol. 2009;587:3045–3054. doi: 10.1113/jphysiol.2009.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack KE, Coote JH, Ng GA. Vagus nerve stimulation inhibits the increase in Ca2+ transient and left ventricular force caused by sympathetic nerve stimulation but has no direct effects alone – epicardial Ca2+ fluorescence studies using fura-2 AM in the isolated innervated beating rabbit heart. Exp Physiol. 2010;95:80–92. doi: 10.1113/expphysiol.2009.048215. [DOI] [PubMed] [Google Scholar]
- Brack KE, Coote JH, Ng GA. Vagus nerve stimulation protects against ventricular fibrillation independent of muscarinic receptor blockade. Cardiovasc Res. 2011;91:437–446. doi: 10.1093/cvr/cvr105. [DOI] [PubMed] [Google Scholar]
- Coote JH. Myths and realities of the cardiac vagus. J Physiol. 2013;59:4073–4085. doi: 10.1113/jphysiol.2013.257758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies F, Francis ETB, King TS. Neurological studies of the cardiac ventricles of mammals. J Anat. 1952;86:130–143. [PMC free article] [PubMed] [Google Scholar]
- Gagliardi M, Randall WC, Bieger D, et al. Activity of neurons located on the in situ canine heart. Am J Physiol. 1988;255:789–800. doi: 10.1152/ajpheart.1988.255.4.H789. [DOI] [PubMed] [Google Scholar]
- Gatti PJ, Johnson TA, Phan P, et al. The physiological and anatomical demonstration of functionally selective parasympathetic ganglia located in discrete fat pads on the feline myocardium. J Auton Nerv Syst. 1995;51:255–259. doi: 10.1016/0165-1838(94)00139-b. [DOI] [PubMed] [Google Scholar]
- Gray AL, Johnson TA, Ardell JL, et al. Parasympathetic control of the heart. II. A novel interganglionic intrinsic cardiac circuit mediates neural control of heart rate. J Appl Physiol. 2004;96:2273–2278. doi: 10.1152/japplphysiol.00616.2003. [DOI] [PubMed] [Google Scholar]
- Hoover DB, Shepherd AV, Southerland EM, et al. Neurochemical diversity of afferent neurons that transduce sensory signals from dog ventricular myocardium. Auton Neurosci. 2008;141:38–45. doi: 10.1016/j.autneu.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TA, Gray AL, Lauenstein JM, et al. Parasympathetic control of the heart. I. An interventriculo-septal ganglion is the major source of the vagal intracardiac innervation of the ventricles. J Appl Physiol. 2004;96:2265–2272. doi: 10.1152/japplphysiol.00620.2003. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ, Roots L. A “direct-coloring” thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kawada T, Akiyama T, Shimizu S, et al. Detection of endogenous acetylcholine release during brief ischemia in the rabbit ventricle: a possible trigger for ischemic preconditioning. Life Sci. 2009;85:597–601. doi: 10.1016/j.lfs.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Koelle GB, Massoulie J, Eugene D, et al. Distributions of molecular forms of acetylcholinesterase and butyrylcholinesterase in nervous tissue of the cat. Proc Natl Acad Sci USA. 1987;84:7749–7752. doi: 10.1073/pnas.84.21.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazgalev T, Dreifus LS, Michelson EL, et al. Effect of postganglionic vagal stimulation on the organization of atrioventricular nodal conduction in isolated rabbit heart tissue. Circulation. 1986;74:869–880. doi: 10.1161/01.cir.74.4.869. [DOI] [PubMed] [Google Scholar]
- Mitchell GA, Brown R, Cookson FB. Ventricular nerve cells in mammals. Nature. 1953;172:812. doi: 10.1038/172812a0. [DOI] [PubMed] [Google Scholar]
- Ng GA, Brack KE, Coote JH. Effects of direct sympathetic and vagus nerve stimulation on the physiology of the whole heart – a novel model of isolated Langendorff perfused rabbit heart with intact dual autonomic innervation. Exp Physiol. 2001;86:319–329. doi: 10.1113/eph8602146. [DOI] [PubMed] [Google Scholar]
- Ng GA, Brack KE, Patel VH, et al. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res. 2007;73:750–760. doi: 10.1016/j.cardiores.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Pardini BJ, Patel KP, Schmid PG, et al. Location, distribution and projections of intracardiac ganglion cells in the rat. J Auton Nerv Syst. 1987;20:91–101. doi: 10.1016/0165-1838(87)90106-8. [DOI] [PubMed] [Google Scholar]
- Patil J, Stucki S, Nussberger J, et al. Angiotensinergic and noradrenergic neurons in the rat and human heart. Regul Pept. 2011;167:31–41. doi: 10.1016/j.regpep.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Pauziene N, Tamasauskas KA, et al. Hilum of the heart. Anat Rec. 1997;248:322–324. doi: 10.1002/(SICI)1097-0185(199707)248:3<322::AID-AR3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Skripka V, Pauziene N, et al. Anatomical study of the neural ganglionated plexus in the canine right atrium: implications for selective denervation and electrophysiology of the sinoatrial node in dog. Anat Rec. 1999;255:271–294. doi: 10.1002/(SICI)1097-0185(19990701)255:3<271::AID-AR4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Skripka V, Pauziene N, et al. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259:353–382. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Pauziene N, Pakeltyte G, et al. Comparative quantitative study of the intrinsic cardiac ganglia and neurons in the rat, guinea pig, dog and human as revealed by histochemical staining for acetylcholinesterase. Ann Anat. 2002a;184:125–136. doi: 10.1016/S0940-9602(02)80005-X. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Skripka V, Pauziene N. Morphology of the intrinsic cardiac nervous system in the dog: a whole-mount study employing histochemical staining with acetylcholinesterase. Cells Tissues Organs. 2002b;172:297–320. doi: 10.1159/000067198. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Saburkina I, Rysevaite K, et al. Neuroanatomy of the murine cardiac conduction system: a combined stereomicroscopic and fluorescence immunohistochemical study. Auton Neurosci. 2013;176:32–47. doi: 10.1016/j.autneu.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Pauziene N, Pauza DH. Electron microscopic study of intrinsic cardiac ganglia in the adult human. Ann Anat. 2003;185:135–148. doi: 10.1016/S0940-9602(03)80077-8. [DOI] [PubMed] [Google Scholar]
- Randall DC, Brown DR, McGuirt AS, et al. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1066–R1075. doi: 10.1152/ajpregu.00167.2003. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Slocum GR, Riley DA. Morphological study of the innervation pattern of the rabbit sinoatrial node. Am J Anat. 1989;185:74–88. doi: 10.1002/aja.1001850108. [DOI] [PubMed] [Google Scholar]
- Rysevaite K, Saburkina I, Pauziene N, et al. Morphologic pattern of the intrinsic ganglionated nerve plexus in mouse heart. Heart Rhythm. 2011a;8:448–454. doi: 10.1016/j.hrthm.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysevaite K, Saburkina I, Pauziene N, et al. Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations. Heart Rhythm. 2011b;8:731–738. doi: 10.1016/j.hrthm.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saburkina I, Pauza DH. Location and variability of epicardiac ganglia in human fetuses. Anat Embryol (Berl) 2006;211:585–594. doi: 10.1007/s00429-006-0110-4. [DOI] [PubMed] [Google Scholar]
- Saburkina I, Rysevaite K, Pauziene N, et al. Epicardial neural ganglionated plexus of ovine heart: anatomic basis for experimental cardiac electrophysiology and nerve protective cardiac surgery. Heart Rhythm. 2010;7:942–950. doi: 10.1016/j.hrthm.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MJ, Choi E-K, Tan AY, et al. Neural mechanisms of atrial arrhythmia. Nat Rev. 2012;9:30–39. doi: 10.1038/nrcardio.2011.139. [DOI] [PubMed] [Google Scholar]
- Smith RB. The development of the intrinsic innervation of the human heart between the 10 and 70 mm stages. J Anat. 1970a;107:271–279. [PMC free article] [PubMed] [Google Scholar]
- Smith RB. The occurrence and location of intrinsic cardiac ganglia and nerve plexuses in the human neonate. Anat Rec. 1970b;166:33–40. doi: 10.1002/ar.1091690104. [DOI] [PubMed] [Google Scholar]
- Smith RB. Intrinsic innervation of the human heart in foetuses between 70 mm and 420 mm crown – rump length. Acta Anat. 1971;78:200–209. doi: 10.1159/000143588. [DOI] [PubMed] [Google Scholar]
- de Souza RR, Gama EF, de Carvalho CA, et al. Quantitative study and architecture of nerves and ganglia of the rat heart. Acta Anat. 1996;156:53–60. doi: 10.1159/000147828. [DOI] [PubMed] [Google Scholar]
- Thompson GW, Collier K, Ardell JL, et al. Functional interdependence of neurons in a single canine intrinsic cardiac ganglionated plexus. J Physiol. 2000;528:561–571. doi: 10.1111/j.1469-7793.2000.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi M, Furukawa Y, Nakajima K, et al. Inotropic, chronotropic, and dromotropic effects mediated via parasympathetic ganglia in the dog heart. Am J Physiol. 2000;279:H1201–H1207. doi: 10.1152/ajpheart.2000.279.3.H1201. [DOI] [PubMed] [Google Scholar]
- Winter J, Tanko SA, Brack KE, et al. Differential cardiac responses to unilateral sympathetic nerve stimulation in the isolated innervated rabbit heart. Auton Neurosci. 2012;166:4–14. doi: 10.1016/j.autneu.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Yuan BX, Ardell JL, Hopkins DA, et al. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec. 1994;239:75–87. doi: 10.1002/ar.1092390109. [DOI] [PubMed] [Google Scholar]
- Zarzoso M, Rysevaite K, Milstein ML, et al. Nerves projecting from the intrinsic cardiac ganglia of the pulmonary veins modulate sinoatrial node pacemaker function. Cardiovasc Res. 2013;99:566–575. doi: 10.1093/cvr/cvt081. [DOI] [PMC free article] [PubMed] [Google Scholar]


