Abstract
Studies in a variety of model organisms indicate that nutrient signaling is tightly coupled to longevity. In nutrient replete conditions, organisms develop, grow, and age quickly. When nutrients become sparse as with dietary restriction, growth and development decline, stress response pathways become induced and organisms live longer. Considerable effort has been devoted to understanding the molecular events mediating lifespan extension by dietary restriction. One central focus has been on nutrient-responsive signal transduction pathways including Insulin/IGF-1, AMP kinase, protein kinase A and the TOR pathway. Here we describe the increasingly prominent links between TOR signaling and aging in invertebrates. Longevity studies in mammals are not discussed here. Instead, we highlight studies in mouse models which indicate that dampening the TOR pathway leads to widespread protection from an array of age-related diseases.
Introduction
To say that caloric intake and aging are tightly coupled is not news. Calorie excess and an increasingly sedentary lifestyle have resulted in obesity on a grand scale, not only in first world countries but world wide [1-4]. Obesity is a major risk factor for a range of age-associated diseases including, but not limited to, type II diabetes, cardiovascular disease and many forms of cancer [5, 6]. Conversely, dietary restriction, defined as a reduction in caloric intake without malnutrition, results in lifespan extension and protection from many of the same diseases.
Why are excess calories bad? This question has been debated for decades and is still not satisfactorily answered. One thing to remember, at least in regions where the obesity epidemic is occurring, is that humans (as mimicked by laboratory animals on ad libitum diets) now have access to cheap high-calorie foods [7, 8]. This, coupled with a transition to a sedentary lifestyle, has likely placed caloric intake out of register with much of our evolutionary past, when long periods of consistent access to high calorie diets are thought to have been rare. The human body is optimized for one range of caloric intake and exercise, but it is encountering another.
Although they are under intense scrutiny, the mechanisms that underlie obesity-induced pathology or lifespan extension by dietary restriction have remained stubbornly refractory. Mechanistically, it is not clear that excess calories and dietary restriction are two ends of the same register. Obesity research will not be discussed in detail here. Instead, readers are referred to recent reviews on the topic [9-12]. Here, we will focus on studies to define the pathways that sense nutrients and modulate lifespan extension by dietary restriction.
Cells and tissues have a myriad ways of detecting nutrient levels in the environment and, when engaged, mediate an array of overlapping downstream responses. For instance, the insulin pathway is responsive to glucose levels in the bloodstream, with islet cells producing and secreting insulin in response to glucose, and with peripheral tissues like skeletal muscle and fat responding directly to insulin. The TOR pathway is activated by several signals (see below), but amino acid levels may be the primary efferent. Other sensors, which may also activate the TOR pathway, detect cellular energy stores. For instance, AMP kinase activity is determined by cellular AMP/ATP ratios [13]. In both invertebrate and mammalian aging model organisms, dietary restriction leads to reduced insulin/IGF-1 and TOR signaling, but increased AMP kinase activity. Making molecular dissection more challenging, all of these pathways (and others) communicate on several levels presumably to bring about an integrated cellular response. In this review, we focus on recent evidence that the TOR pathway modulates aging, and that reduced TOR signaling may be a primary mechanism by which dietary restriction extends longevity and offsets age-related disease in aging model organisms.
The target of rapamycin kinase is a conserved regulator of growth
The target of rapamycin (TOR) kinase acts as a central regulator of eukaryotic growth and cell division in response to nutrient and growth factor cues. TOR proteins are highly conserved from yeast to humans, and were first identified (and named) from studies of the growth inhibiting properties of the anti-fungal compound rapamycin [14]. Yeast have two TOR paralogs, TOR1 and TOR2, while other eukaryotes have but one (mTOR in mammals) [15-17].
TOR kinases exist in two complexes termed TORC1 and TORC2. TORC1 and TORC2 have distinct cellular functions, but both are essential for viability [18, 19]. In yeast, Tor1 is exclusively found in TORC1, while Tor2 functions in both complexes. In mammals, mTOR functions in both complexes. TOR binds to specific accessory proteins in each complex; among these are Raptor (yeast Kog1) in TORC1 and Rictor (yeast Avo3) in TORC2 (Table 1). TOR kinases act as signal integrators that modulate permissive signals for cell growth by responding to the status of nutrients, energy signals, growth factors, and cellular stresses. TORC1 is activated by nutrient availability, particularly amino acids, and coordinates protein synthesis and degradation to promote growth when nutrients are plentiful. TORC2 is responsive to growth factors, such as insulin and insulin-like growth factors, and promotes stress responses necessary for cell survival. In addition, TORC2 plays an important role in organizing the actin cytoskeleton [20, 21]. TORC2 also mediates cross-talk between the insulin signaling pathways and TOR signaling by phosphorylating Akt, a phosphatidyl 3-OH kinase that acts downstream of the insulin receptor [22]. Insulin signaling and Akt, in turn, also modulate TORC1 function (see below) [23], providing a potential link between TORC1 and TORC2 activity. Many features of the upstream regulation and downstream processes controlled by both TOR complexes remain poorly understood. The elucidation of these events is an ongoing subject of intense study.
Table 1. Identified orthologous components of TOR complex 1 and TOR complex 2.
Components of the TORC1 and TORC2 complexes in C. elegans, D. melanogaster, and mammals were identified based on orthology mapping from the yeast protein sequences available on the Saccharomyces cerevisiae Genome Database http://www.yeastgenome.org.
| TORC1 | |||
|---|---|---|---|
|
| |||
| S. cervesisiae | C. elegans | D.melanogaster | Mammals |
| TOR1/TOR2 | let-363 | TOR-PA | mTOR |
| KOG1 | daf-15 | Raptor-PA | Raptor |
| LST8 | C10H11.8 | CG3004-PA | mLST8 |
| TCO89 | - | - | - |
| TORC2 | |||
|---|---|---|---|
|
| |||
| S. cervesisiae | C. elegans | D.melanogaster | Mammals |
| TOR2 | let-363 | TOR-PA | mTOR |
| AVO1 | - | - | - |
| AVO2 | - | - | - |
| BIT61 | - | - | - |
| LST8 | C10H11.8 | CG3004-PA | mLST8 |
| SLM1 | - | - | - |
| SLM2 | - | - | - |
| AVO3/TSC11 | F29C12.2 | Rictor-PA | Rictor |
Rapamycin is produced by the bacterium Streptomyces hygroscopicus [24] and specifically inhibits the function of the two TOR proteins in yeast through a mechanism which is still not completely understood. Rather than competing for substrate binding directly, as is the case with many kinase inhibitors, rapamycin serves as the lynchpin in a trimolecular interaction that also includes TOR and FKBP12 [25, 26]. This complex inhibits TOR-mediated phosphorylation of downstream targets in vivo, perhaps by disrupting protein-protein interactions within TORC1. The non-competitive nature of rapamycin action may underlie its unusual specificity among kinase inhibitors. TORC2, unlike TORC1, is thought to be rapamycin-insensitive [19, 21]. However, in certain cell types, long-term exposure of cells in culture to rapamycin leads to inhibition of TORC2 assembly and reduced TORC2 activity [27].
The upstream regulatory features of TORC1 activity are better understood than those of TORC2. TORC1 is activated by Akt in response to insulin and other growth factors [23]. Activation of TORC1 by Akt is mediated by inhibition of the tuberous sclerosis complex 2 (TSC2), which inhibits the TORC1-activator Rheb [28-30]. TORC1 is also repressed by the energy sensing AMP-activated protein kinase (AMPK) [31-33]. In addition to its ability to respond directly to nutrient abundance, many other input pathways place TORC1 at a key regulatory nexus in responding to nutrients, growth cues, and cellular energy status.
Modulation of longevity by TOR signaling in invertebrate organisms
The role of TOR as an important longevity pathway has been firmly established from studies performed in three invertebrate model systems: the budding yeast Saccharomyces cerevisiae, nematode Caenorhabditis elegans, and fruit fly Drosophila melanogaster. In each of these organisms, a reduction in TORC1 activity has been shown to be sufficient to increase lifespan significantly. The genetic and pharmacological models of reduced TOR signaling associated with enhanced longevity in each of these organisms are shown in Table 2.
Table 2. Cross species comparison of interventions that reduce TOR signaling, increase life span, and affect aging.
Multiple interventions that reduce TORC1 signaling have been reported to increase life span and affect aging in different model organisms.
| Intervention | S. cerevisiae CLS | S. cerevisiae RLS | C. elegans | D. melanogaster | M. musculus |
|---|---|---|---|---|---|
| Dietary restriction | ✓ | ✓ | ✓ | ✓ | ✓ |
| TOR mutation/knockdown | ✓ | ✓ | ✓ | ✓ | - |
| Raptor mutation/knockdown | - | - | ✓ | - | - |
| Activation of AMP kinase | - | - | ✓ | - | - |
| Activation of Tsc1/2 | - | - | - | ✓ | - |
| Pharmacological inhibition of TORC1 (e.g. rapamycin) | ✓ | ✓ | - | - | - |
| S6 Kinase mutation/knockdown | ✓ | ✓ | ✓ | ✓ | - |
| Ribosomal protein mutation/knockdown | - | ✓ | ✓ | - | - |
| Translation initiation factor mutation/knockdown | - | ✓ | ✓ | - | - |
| Mutations reducing amino acid uptake | ✓ | ✓ | ✓ | - | - |
In the budding yeast, aging can be studied by two assays: replicative and chronological [34, 35]. Replicative lifespan is defined by the proliferative capacity of a mother cell and is measured by counting the number of daughter cells a mother cell produces prior to senescence. Chronological lifespan is defined by the length of time that a cell can survive in a quiescent state, and is typically measured by maintaining cells in stationary phase while periodically monitoring their ability to reenter the cell cycle in response to appropriate cues. Both aging paradigms have been studied extensively and a variety of genetic modifiers of replicative or chronological lifespan have been identified [34, 35]. TOR signaling has been shown to be a particularly potent regulator of both replicative and chronological aging of yeast cells. Replicative and chronological lifespan can both be extended by deletion of TOR1 [36, 37], by pharmacologic inhibition of TORC1 [36, 38], or by deletion of the gene coding for the yeast S6 kinase homolog, SCH9 [37, 39]. Additionally, replicative lifespan can also be increased by mutations of downstream targets of TOR signaling involved in protein synthesis, including multiple ribosomal proteins, rRNA processing factors, and translation initiation factors [37, 40].
As with yeast, worm adult lifespan can be extended by RNAi knock-down or mutation of the gene coding for TOR (let-363), the S6 kinase homolog (rsks-1), several ribosomal protein genes, and a number of genes coding for mRNA translation initiation factors [41-45]. Additional evidence for the importance of TORC1 specifically is provided by the observation that mutation of the gene coding for raptor (daf-15) also increases lifespan [46], as does knock-down of an intestinal peptide transporter (pep-2) thought to act upstream of TORC1 in C. elegans [47].
In flies, dominant negative alleles of TOR or S6 kinase have been reported to increase lifespan, as has overexpression the fly homologs of TSC1 and TSC2, which act as negative regulators of TORC1. A hypomorphic allele of TOR has also been shown to increase lifespan in flies [48]. Interestingly, reducing TOR activity specifically in fat bodies is sufficient to provide lifespan extension in this organism [49].
Dietary restriction (DR) is the best-characterized paradigm for slowing aging across multiple eukaryotic species, including both yeast aging models, worms, flies, fish, spiders, mice, and rats [50-52]. The link between TORC1 activity and nutrient response suggests the hypothesis that DR promotes longevity by reducing TOR activity. This hypothesis is supported by the observations that TOR activity is inhibited by DR and, like DR, reduced TOR signaling is sufficient to slow aging in each of the invertebrate models where DR has been extensively studied. Epistasis studies in all three organisms have further strengthened the model that DR is mediated, at least in part, by reduced TOR signaling [37, 42, 48, 53].
Possible mechanisms by which TOR modulates aging in invertebrate organisms
There has been much interest in understanding potential molecular mechanisms by which inhibition of TORC1 can slow aging across such disparate species as yeast, worms, and flies. Dissecting out specific downstream targets of TOR signaling that are causally involved in modulating longevity has been difficult, however, because of the particularly complex (and often poorly understood) network through which TOR signaling influences multiple aspects of cellular physiology. Current experimental data suggest that at least four distinct outputs of TOR signaling influence aging in invertebrates: regulation of mRNA translation, autophagy, stress response pathways, and metabolic changes associated with mitochondrial function.
TORC1 activity promotes mRNA translation through at least two different downstream targets: S6 kinase (S6K1) and eukaryotic initiation factor 4E (eIF4E) binding proteins (4E-BP) [54]. Activation of S6K1, in turn, promotes the activity of other translation initiation factors, such as eukaryotic initiation factor 4B (eIF4B), and stimulates production of ribosomal proteins and ribosome biogenesis [55]. As mentioned above, decreased S6K1, translation initiation factor activity, or ribosomal protein expression has been found to promote longevity in yeast, worms, and flies. Thus, perturbing mRNA translation in a manner similar to the effect of reducing TOR activity is sufficient to phenocopy the lifespan extension associated with reduced TOR signaling in yeast, worms, and flies.
In the yeast replicative aging model, further evidence supports the idea that much of the lifespan extension from TOR inhibition can be directly attributed to altered mRNA translation. Epistasis studies place DR, Tor1, Sch9, and multiple ribosomal proteins in a single epistasis group with respect to replicative lifespan [37, 40]. A global reduction in translation in response to DR or in long-lived mutants in this epistasis group is associated with a relative increase in translation of GCN4 mRNA [56, 57]. GCN4 codes for a starvation-responsive transcription factor [58] and its deletion prevents full lifespan extension in these mutants [40]. Thus, one model for how TOR signaling and regulation of mRNA translation could modulate aging in multicellular eukaryotes is through differential translation of specific target messages, such as GCN4. Functional homologs of Gcn4 are present and are translationally regulated; however it remains to be determined whether they also modulate longevity in these organisms.
Regulation of carbon metabolism and stress resistance seem to be the primary mechanisms by which TOR signaling modulates yeast chronological lifespan. In addition to significantly increasing lifespan, deletion of either TOR1 or SCH9 causes a change in carbon metabolism away from alcoholic fermentation toward mitochondrial respiration [59, 60] and an induction of stress response genes [39, 61]. These changes are necessary for increased lifespan, suggesting that they play a causal role in the chronological aging process. To date, there is little evidence that reduced expression of translation initiation factors or ribosomal proteins significantly extend chronological lifespan, which differentiates this aging model from other invertebrate systems.
In worms, evidence suggests that both mRNA translation and regulation of autophagy are important TOR-regulated modifiers of longevity. Enhanced autophagy, a process involved in recycling damaged macromolecules, is a potential avenue to lifespan extension. As discussed above, knock-down of S6 kinase, translation initiation factors, and ribosomal proteins is known to increase adult lifespan in worms [41-45]. In response to DR or TOR-inhibition, autophagy is also induced and this increase in autophagy is required for lifespan extension [53, 62, 63]. It remains unclear whether induction of autophagy promotes longer lifespan or is simply necessary for the extension of lifespan associated with these interventions.
Does mTOR activity promote aging in mammals
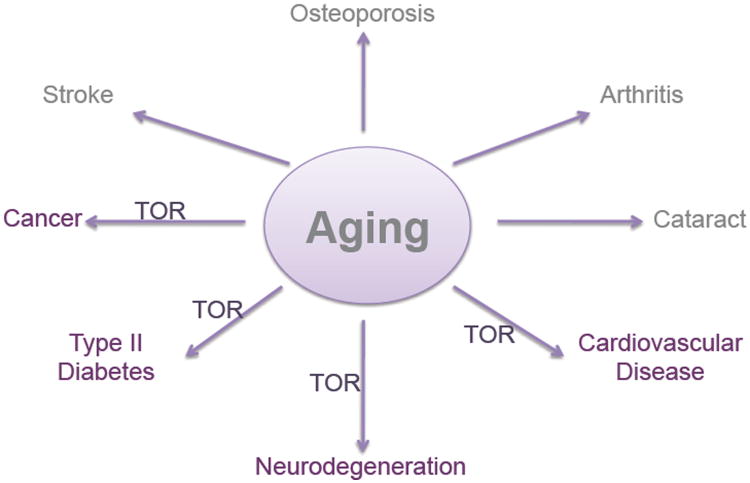
The data from invertebrate organisms unquestionably demonstrates that TOR signaling plays an important role in aging across multiple evolutionarily divergent species. Does TOR activity modulate mammalian aging as well? Although no definitive answer to this question is yet available, there is a growing body of evidence that TOR signaling does influence a variety of aging-related processes in rodents and possibly hints of similar effects in people (Figure 1).
Figure 1. TOR signaling and Age-Associated Disease.
Schematic depicts age-related pathologies for which there is a clear link between TOR signaling and disease progression.
DR is known to both dramatically increase lifespan and retard the onset of a variety of age-associated pathologies in rodents [50-52]. The invertebrate studies indicate that reduced TOR signaling is likely to account for the lifespan extension associated with DR. Studies are currently underway as part of the National Institute on Aging (NIA) Interventions Testing Program to determine whether inhibition of mTOR by feeding mice rapamycin is sufficient to mimic DR and increase lifespan in a mammal [64, 65]. In the absence of such data, however, it is informative to examine whether reduced TOR signaling can delay a spectrum of diseases that are influenced by DR.
A complicating issue in mammals is that TOR has different functions in different tissues, and pharmacological inhibition of the TORC1 complex may be beneficial in some tissues and detrimental in others. It is beyond the scope of this review to discuss in detail specific functions of TOR in different tissues. Here, we focus on studies examining the efficacy of TOR inhibition for diseases in which aging is considered a major risk factor. To summarize, many mouse models of age-associated disease, including cancer, Huntington's and cardiovascular diseases, display improvement upon pharmacological inhibition of TOR [54].
TOR and Cancer
A primary hallmark of DR in rodents is a dramatic reduction in age-associated cancer incidence and growth rate (*pull some refs from Sabatini's PI3K paper: PMID: 15395906, PMID: 13067072,) [52]. Indeed, it has been speculated that reduced cancer occurrence may account for a large fraction of the longevity benefit associated with DR in rodents [66]. Mutations of mTOR pathway components (although not mTOR itself) and/or elevated mTOR activity have been discovered in many cancers including lymphomas, melanomas, gliomas, and central nervous system malignancies as well as carcinomas of lung, bladder, kidney, ovary, breast, prostate, stomach, pancreas, and head and neck [67]. Upregulation of mTOR signaling due to mutation in its upstream negative regulators, TSC1 or TSC2, results in an increased risk of renal cell carcinoma [68, 69]. Additionally, upregulation of mTOR signaling is correlated with poor tumor prognosis for melanoma [70], prostate [71], pancreas [72], thyroid [73], breast [74] and cervical cancers [75]. These findings have prompted extensive development and clinical testing of rapamycin and its derivatives (termed rapalogs) in a wide variety of tumors.
The anti-proliferative, anti-angiogenetic, apoptosis-sensitizing effects of rapamycin make it an attractive anti-cancer therapy. Treating cells with rapamycin leads to cell cycle arrest at G1 due insufficient cell mass and size [14, 76-78]. Administration of rapamycin was also shown to enhance autophagy, inhibiting malignant glioma cell growth [79]. The anti-proliferative effect of rapamycin in cancers is being further evaluated in clinical trials, which are showing that this and other mTOR inhibitors have good efficacy with mild side effects [80-82]. Temsirolimus, a rapamycin analog, has received FDA approval for treatment of metastatic renal cell carcinoma, while clinical trials of another rapalog, everolimus, are showing promise [83].
The major value of rapamycin may be its application in cancer therapies in combination with other anti-tumor agents. Sensitivity to rapamycin appears to be cancer-type specific. Similarly, different tumors vary in their response to DR (ref: 4,7,10-14 from Sabatini's paper). Tumor responsiveness to DR has been correlated with P13K pathway activity, which influences signaling through the AKT pathway (Sabatini's PI3K paper: PMID: 19279572). Typically only <10% of patients respond to rapamycin therapy; however, clinical trials are underway to evaluate the effects of using mTOR inhibitors in combination with irradiation, chemotherapy, or other anti-angiogenic agents [81]. For example, when in combination with the caspase-3 inhibitor Z-DEVE, rapamycin increases radiation sensitivity in lung cancer cells [84]. Various other combinatorial therapy trials are also currently underway.
TOR and Vascular Disease
Dietary restriction lowers the risk factors associated with age-associated cardiovascular disease, such as blood pressure, cholesterol, and hypertension. Similarly, inhibition of the mTOR signaling pathway by rapamycin administration has cardioprotective benefits in various mouse models of cardiac hypertrophy and heart failure [85]. Cardiac hypertrophy is characterized by enlargement of the heart as a consequence of increased cell size and enhanced protein synthesis [86]. Elevated PI3K/Akt/mTOR pathway activity has been implicated in this inappropriate enlargement of the heart [87]. mTOR inhibition by rapamycin treatment regresses cardiac hypertrophy induced by pressure overload [87-89] and inhibits angiotensin II-induced increases in protein synthesis in cardiac myocytes [90]. Rapamycin improved parameters of cardiac hypertrophy, such as increased left ventricular end-systolic dimensions, depressed fractional shortening, ejection fraction, as well as regressing left ventricular fibrosis in mice with hypertrophy and heart failure [89, 91], thus suggesting that rapamycin may be a tool to improve cardiac function and repair cardiac hypertrophy. These effects are accompanied by suppression of ribosomal S6 protein and eIF4E phosphorylation which occurs due to pressure overload [89].
Rapamycin also attenuates hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy in mice [92], and rapamycin-coated stents are approved for use to inhibit growth of vascular smooth muscle cell growth in patients with vascular disease after coronary angioplasty [93-95]. These data indicate that mTOR plays a key role in cardiac and vascular growth and homeostasis. With age there is an increase in the prevalence of cardiovascular diseases, such as congestive heart failure and coronary artery disease. Studies are demonstrating that modulation of mTOR activity might be a cardioprotective strategy that could be used to decrease the mortality associated with age-related cardiovascular disease.
TOR signaling has also been implicated in a variety of ophthalmological conditions. Rodent models of corneal neovascularization as well as retinal and choroidal angiogensis showed improvement upon treatment with rapamycin (Shi et al 2006, Kwon et al 2006, ref:34 Kleinmman et al 2007, 35 Dejneka et al 2004) and clinical trials are currently underway to evaluate the efficacy of rapamycin in humans with choroidal neovascularization and neovascular age-related macular degeneration (refs: 36 MacuSight press release, 37 clinical trials.gov Chappelow and Kaiser 2008, reviewed in).
TOR and Metabolic Disease
Not surprisingly, age-related obesity and metabolic disease are essentially completely prevented by DR. Although it remains unclear how much of the lifespan extension from DR in rodents is due to the absence of obesity-associated metabolic effects, in people diabetes and obesity are major health risks among the elderly. A number of long-lived mouse models have phenotypes consistent with improved metabolic function. Examples include Ames and Snell dwarf mice which have reduced metabolic rate [97]; GHR/BP-/-mice which are small, have reduced fasting insulin and glucose levels and delayed sexual maturity [98]; and FIRKO (insulin receptor knockout in adipose) mice which are small and have reduced fat mass and lower fasting insulin [99]. Investigators have taken advantage of these observations by challenging many potentially long-lived models with the stress of nutrient overload to accelerate metabolic disease in young mice. Many long-lived genetic models exhibit resistance to this high calorie challenge. For instance, p66shc-/- mice show reduced atherogenesis [100], S6K1-/- (S6 kinase 1) mice are protected against obesity [101], and mice overexpressing Sirt1 show better glucose tolerance among other phenotypes [102]. These findings make short-term nutrition overload experiments a strategy to increase the likelihood that a mouse genetic model in question might have enhanced longevity.
The mTOR signaling pathway has been implicated in both Type 1 and Type 2 diabetes. The hallmark of Type 1 diabetes is reduced insulin production due to destruction of pancreatic β-cells, while Type 2 diabetes arises initially from an insulin resistance. Insulin-induced protein translation and proliferation [103] as well as growth induced by glucose and branched amino acids [104] is dependent on mTOR signaling in pancreatic β-cells. Meanwhile, desensitization of cells to insulin has been linked in part to S6 kinase-dependent phosphorylation and inhibition of the insulin-receptor substrate proteins (IRS1 and 2) [105, 106]. Thus chronic TOR activation results in insulin resistance (i.e. hyperglycemia and Type 2 diabetes). Treatment of mouse models with rapamycin reduces specific features of diabetic nephropathy, including inhibition of renal or glomerular hypertrophy [107, 108].
In addition mTOR plays a role in lipid metabolism. mTOR expression is increased during adipogenesis [109, 110]. Administration of rapamycin results in inhibition of adipocyte differentiation as well as reduced adipogenesis and lipogenesis. Evidence of involvement of the TOR signaling pathway in metabolism is further supported by studies of mice defective downstream components of mTOR signaling. An adipose-specific knockout of Raptor results in lean mice that are resistant to diet-induced obesity and insulin resistance [111]. These mice also exhibit increased energy expenditure due to enhanced mitochondrial uncoupling in adipose. Changes in S6 kinase-dependent IRS phosphorylation were not assessed. Interestingly, the consequences of knocking out Raptor expression vary dependent on the chosen tissue; a complete knockout results in developmental lethality [112]. Whereas the adipose-specific deletion of Raptor may have beneficial consequences, skeletal muscle-specific Raptor knockout mice have a muscular dystrophy phenotype [113].
S6K1-deficient mice have smaller islets and exhibit hypersensitivity to insulin and glucose intolerance [114]. These mice also display reduced weight gain and resistance to obesity due to age or challenge with a high calorie diet compared to wild-type mice [101]. Similarly disruption of 4E-BP1, another downstream component of mTOR signaling, results in mice with reduced adipose tissue [115]. Furthermore, in Drosophila, the melted mutant, defective in dTOR signaling, displays reduction in body size due to decreased fat accumulation [116]. Reduction of TOR activity in Drosophila also results in reduced glucose and lipid levels, maintenance of insulin sensitivity, decreased heart failure rate and increased life-span [49].
TOR and Neurodegeneration
DR has been suggested to have a neuroprotective effect and to decrease age-related neuronal loss [117, 118]. The underlying mechanisms for this neuroprotective effect of DR are poorly understood. DR is implicated in regulating adult neuronal stem cells, increasing neurogenesis in young rats, [119] and reducing decline of neurogenesis in older rats through a cell survival promoting effect [120].
Neurological disorders such as Huntington's, and Alzheimer's diseases as well as normal brain function might benefit from pharmacological modulation of mTOR signaling. mTOR signaling has been shown to play a role in protection against polyglutamine toxicity, which is associated with several human diseases, including Huntington's disease. Rapamycin treatment reduces polyglutamine toxicity in fly and mouse models via a mechanism that involves reduction of mTOR activity [121] and induction of an autophagy response that might be involved in clearance of protein aggregates [122]. It is speculated that this autophagy response induced by rapamycin treatment may help remove protein aggregates in other neurodegenerative disorders, such as β-amyloid accumulation in Alzheimer's patients. mTOR also plays a role in regulation of translation of tau mRNA and degradation of tau [123], a protein thought to contribute to the neurofibrillary tangles found in the brains of Alzheimer's patients. In Drosophila models, rapamycin treatment decreases tau levels, reducing toxicity caused by tau [124].
In response to oxidative stress, mTOR signaling is inhibited, inducing expression of stress response genes, such as YDR533C in yeast [125]. Autosomal recessive mutation of the human homolog of YDR533C, DJ-1, is associated with early onset Parkinson's disease, which has led to the postulation that inhibition of mTOR signaling in Parkinson's patients might provide a neuroprotective effect by induction of DJ-1 expression [126]. In addition, mTOR is a major downstream effector of the tuberous sclerosis complex (TSC), suggesting pharmacologic modulation of mTOR signaling might benefit neuropsychiatric disorders, such as epilepsy, mental retardation and autism, all of which have been linked to TSC mutations [127].
Evidence demonstrating that mTOR is involved in synaptic protein synthesis links the mTOR signaling pathway with normal brain function as well as learning and memory. Synthesis and induction of long-term potentiation and long-term facilitation proteins, important for the long-term synaptic plasticity involved in learning and memory processes, are inhibited by rapamycin treatment [128]. mTOR and components critical for translation are enriched at postsynaptic junctions [129], allowing for rapid synthesis of proteins from pre-transcribed mRNAs upon stimulus of a synapse to induce long-term changes in synaptic function and structure.
Conclusions
While rapamycin may protect from and in some cases be part of a treatment regimen for age-related diseases, there are potential adverse consequences in otherwise healthy patients. Foremost among these may be its activity as an immunosuppressant. As such, it has been approved for clinical use following kidney transplant as a means to inhibit organ rejection [130]. As with many cancer trials, rapamycin is primarily used in combination therapy with other immunosuppressants such as cyclosporine. How long-term rapamycin administration at a lower dose would affect the immune system in healthy individuals is not well understood. However, concerns in this regard are sufficient to make rapamycin a questionable candidate for administration to healthy individuals to promote healthspan. It is worth noting that reduced immune function may be associated with dietary restriction (DR) in humans [131]. Other side effects of rapamycin treatment regimens have also been reported, although the drug is considered to be generally well-tolerated.
What is apparent is that reduced TOR signaling is sufficient to increase lifespan in invertebrate organisms and, if brought about in the right manner, can protect against at least a subset of age-related diseases in mammals (Figure 1). These findings make further mechanistic studies relating TOR signaling to aging an imperative. It will be particularly important to determine which downstream effects of mTOR activation accelerate aging and in what tissues these actions occur. Likewise, a better understanding of the relationship between TOR signaling, insulin/IGF-1 signaling and other signaling pathways involved in responding to DR will be required. Importantly, the field awaits the findings from the NIA Intervention Testing Program rapamycin study on mouse longevity [64, 65]. Will lifespan be extended by inhibition of TOR? If so, this would mark the first pathway shown to modulate aging in four commonly used model aging systems: yeast, worms, flies, and mice. As such, examination of the already clinically relevant TOR inhibitors for effects across a spectrum of age-associated diseases in people would seem to be warranted.
References
- 1.Berghofer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. BMC Public Health. 2008;8:200. doi: 10.1186/1471-2458-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James WP. J Intern Med. 2008;263:336–52. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 3.Levitt NS. Heart. 2008;94:1376–82. doi: 10.1136/hrt.2008.147306. [DOI] [PubMed] [Google Scholar]
- 4.Witkos M, Uttaburanont M, Lang CD, Arora R. J Cardiometab Syndr. 2008;3:173–6. doi: 10.1111/j.1559-4572.2008.00012.x. [DOI] [PubMed] [Google Scholar]
- 5.Artham SM, Lavie CJ, Milani RV, Ventura HO. Postgrad Med. 2008;120:34–41. doi: 10.3810/pgm.2008.07.1788. [DOI] [PubMed] [Google Scholar]
- 6.Hjartaker A, Langseth H, Weiderpass E. Adv Exp Med Biol. 2008;630:72–93. doi: 10.1007/978-0-387-78818-0_6. [DOI] [PubMed] [Google Scholar]
- 7.Bellisari A. Obes Rev. 2008;9:165–80. doi: 10.1111/j.1467-789X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 8.Peters JC, Wyatt HR, Donahoo WT, Hill JO. Obes Rev. 2002;3:69–74. doi: 10.1046/j.1467-789x.2002.00059.x. [DOI] [PubMed] [Google Scholar]
- 9.Pradhan A. Nutr Rev. 2007;65:S152–6. doi: 10.1111/j.1753-4887.2007.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 10.Crowley VE. Ann Clin Biochem. 2008;45:245–55. doi: 10.1258/acb.2007.007193. [DOI] [PubMed] [Google Scholar]
- 11.Bier DM. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:97–106. doi: 10.1159/000146254. discussion 107-10. [DOI] [PubMed] [Google Scholar]
- 12.Speakman J, Hambly C, Mitchell S, Krol E. Lab Anim. 2008;42:413–32. doi: 10.1258/la.2007.006067. [DOI] [PubMed] [Google Scholar]
- 13.Hardie DG. Nat Rev Mol Cell Biol. 2007;8:774–85. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 14.Heitman J, Movva NR, Hall MN. Science. 1991;253:905–9. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 15.Powers T, Dilova I, Chen CY, Wedaman K. Curr Top Microbiol Immunol. 2004;279:39–51. doi: 10.1007/978-3-642-18930-2_3. [DOI] [PubMed] [Google Scholar]
- 16.De Virgilio C, Loewith R. Int J Biochem Cell Biol. 2006;38:1476–81. doi: 10.1016/j.biocel.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Martin DE, Hall MN. Curr Opin Cell Biol. 2005;17:158–66. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Helliwell SB, Howald I, Barbet N, Hall MN. Genetics. 1998;148:99–112. doi: 10.1093/genetics/148.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Mol Cell. 2002;10:457–68. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 20.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 21.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 22.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Oncogene. 2006;25:6361–72. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 24.Vezina C, Kudelski A, Sehgal SN. J Antibiot (Tokyo) 1975;28:721–6. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 25.Stan R, McLaughlin MM, Cafferkey R, Johnson RK, Rosenberg M, Livi GP. J Biol Chem. 1994;269:32027–30. [PubMed] [Google Scholar]
- 26.Choi J, Chen J, Schreiber SL, Clardy J. Science. 1996;273:239–42. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- 27.Zeng Z, Sarbassov dos D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, Giles FJ, Sabatini DM, Andreeff M, Konopleva M. Blood. 2007;109:3509–12. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoki K, Li Y, Xu T, Guan KL. Genes Dev. 2003;17:1829–34. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoki K, Li Y, Zhu T, Wu J, Guan KL. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 30.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Mol Cell. 2002;10:151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 31.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. J Biol Chem. 2002;277:23977–80. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 32.Krause U, Bertrand L, Hue L. Eur J Biochem. 2002;269:3751–9. doi: 10.1046/j.1432-1033.2002.03074.x. [DOI] [PubMed] [Google Scholar]
- 33.Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, Kemp BE, Witters LA, Mimura O, Yonezawa K. Genes Cells. 2003;8:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 34.Steinkraus KA, Kaeberlein M, Kennedy BK. Annu Rev Cell Dev Biol. 2008;24:29–54. doi: 10.1146/annurev.cellbio.23.090506.123509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabrizio P, Longo VD. Methods Mol Biol. 2007;371:89–95. doi: 10.1007/978-1-59745-361-5_8. [DOI] [PubMed] [Google Scholar]
- 36.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Genes Dev. 2006;20:174–84. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaeberlein M, Powers RW, III, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 38.Medvedik O, Lamming DW, Kim KD, Sinclair DA. PLoS Biol. 2007;5:230–241. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 40.Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, Dang N, Johnston ED, Oakes JA, Tchao BN, Pak DN, Fields S, Kennedy BK, Kaeberlein M. Cell. 2008;133:292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curran SP, Ruvkun G. PLoS Genet. 2007;3:e56. doi: 10.1371/journal.pgen.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 43.Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Aging Cell. 2007;6:111–9. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Syntichaki P, Troulinaki K, Tavernarakis N. Nature. 2007;445:922–6. doi: 10.1038/nature05603. [DOI] [PubMed] [Google Scholar]
- 45.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 46.Jia K, Chen D, Riddle DL. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 47.Meissner B, Boll M, Daniel H, Baumeister R. J Biol Chem. 2004;279:36739–45. doi: 10.1074/jbc.M403415200. [DOI] [PubMed] [Google Scholar]
- 48.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, Bodmer R, Oldham SM. Cell Metab. 2006;4:133–42. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy BK, Steffen KK, Kaeberlein M. Cell Mol Life Sci. 2007;64:1323–8. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masoro EJ. Mech Ageing Dev. 2005;126:913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 52.Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Charles C. Thomas; Springfield, IL: 1988. [Google Scholar]
- 53.Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wullschleger S, Loewith R, Hall MN. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 55.Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Growth Factors. 2007;25:209–26. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 56.Yang R, Wek SA, Wek RC. Mol Cell Biol. 2000;20:2706–17. doi: 10.1128/mcb.20.8.2706-2717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valenzuela L, Aranda C, Gonzalez A. J Bacteriol. 2001;183:2331–4. doi: 10.1128/JB.183.7.2331-2334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinnebusch AG. Annu Rev Microbiol. 2005;59:407–50. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 59.Lavoie H, Whiteway M. Eukaryot Cell. 2008 doi: 10.1128/EC.00330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beck T, Hall MN. Nature. 1999;402:689–92. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- 62.Jia K, Levine B. Autophagy. 2007;3:597–9. doi: 10.4161/auto.4989. [DOI] [PubMed] [Google Scholar]
- 63.Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Science. 2003;301:1387–91. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 64.Nadon NL, Strong R, Miller RA, Nelson J, Javors M, Sharp ZD, Peralba JM, Harrison DE. AGE. 2008;30:187–199. doi: 10.1007/s11357-008-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R. Aging Cell. 2007;6:565–75. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 66.Spindler SR. Mech Ageing Dev. 2005;126:960–6. doi: 10.1016/j.mad.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 67.Guertin DA, Sabatini DM. Trends Mol Med. 2005;11:353–61. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Sampson JR, Patel A, Mee AD. J Med Genet. 1995;32:848–50. doi: 10.1136/jmg.32.11.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Saleem T, Wessner LL, Scheithauer BW, Patterson K, Roach ES, Dreyer SJ, Fujikawa K, Bjornsson J, Bernstein J, Henske EP. Cancer. 1998;83:2208–16. [PubMed] [Google Scholar]
- 70.Daido S, Yamamoto A, Fujiwara K, Sawaya R, Kondo S, Kondo Y. Cancer Res. 2005;65:4368–75. doi: 10.1158/0008-5472.CAN-04-4202. [DOI] [PubMed] [Google Scholar]
- 71.Mita MM, Mita AC, Chu QS, Rowinsky EK, Fetterly GJ, Goldston M, Patnaik A, Mathews L, Ricart AD, Mays T, Knowles H, Rivera VM, Kreisberg J, Bedrosian CL, Tolcher AW. J Clin Oncol. 2008;26:361–7. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H, Nakamori S, Monden M, Aozasa K. Clin Cancer Res. 2004;10:2846–50. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 73.Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V, Miyakawa M, Isozaki O, Murakami H, Tsushima T, Burman KD, De Micco C, Ringel MD. J Med Genet. 2004;41:161–70. doi: 10.1136/jmg.2003.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Nat Cell Biol. 2001;3:245–52. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 75.Faried LS, Faried A, Kanuma T, Sano T, Nakazato T, Tamura T, Kuwano H, Minegishi T. Oncol Rep. 2006;16:57–63. [PubMed] [Google Scholar]
- 76.Terada N, Lucas JJ, Szepesi A, Franklin RA, Domenico J, Gelfand EW. J Cell Physiol. 1993;154:7–15. doi: 10.1002/jcp.1041540103. [DOI] [PubMed] [Google Scholar]
- 77.Morice WG, Brunn GJ, Wiederrecht G, Siekierka JJ, Abraham RT. J Biol Chem. 1993;268:3734–8. [PubMed] [Google Scholar]
- 78.Albers MW, Williams RT, Brown EJ, Tanaka A, Hall FL, Schreiber SL. J Biol Chem. 1993;268:22825–9. [PubMed] [Google Scholar]
- 79.Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S. Cancer Res. 2005;65:3336–46. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 80.Hwang M, Perez CA, Moretti L, Lu B. Curr Med Chem. 2008;15:1192–208. doi: 10.2174/092986708784310459. [DOI] [PubMed] [Google Scholar]
- 81.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Strimpakos AS, Karapanagiotou EM, Saif MW, Syrigos KN. Cancer Treat Rev. 2008 doi: 10.1016/j.ctrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 83.Le Tourneau C, Faivre S, Serova M, Raymond E. Br J Cancer. 2008;99:1197–203. doi: 10.1038/sj.bjc.6604636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim KW, Hwang M, Moretti L, Jaboin JJ, Cha YI, Lu B. Autophagy. 2008;4:659–68. doi: 10.4161/auto.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balasubramanian S, Johnston RK, Moschella PC, Mani SK, Tuxworth WJ, Jr, Kuppuswamy D. Cardiovasc Hematol Agents Med Chem. 2009;7:52–63. doi: 10.2174/187152509787047603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frey N, Katus HA, Olson EN, Hill JA. Circulation. 2004;109:1580–9. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 87.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Circulation. 2003;107:1664–70. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 88.Sanada S, Node K, Asanuma H, Ogita H, Takashima S, Minamino T, Asakura M, Liao Y, Ogai A, Kim J, Hori M, Kitakaze M. J Am Coll Cardiol. 2002;40:991–7. doi: 10.1016/s0735-1097(02)02057-0. [DOI] [PubMed] [Google Scholar]
- 89.Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, Dart A, Du XJ. J Hypertens. 2006;24:1663–70. doi: 10.1097/01.hjh.0000239304.01496.83. [DOI] [PubMed] [Google Scholar]
- 90.Sadoshima J, Izumo S. Circ Res. 1995;77:1040–52. doi: 10.1161/01.res.77.6.1040. [DOI] [PubMed] [Google Scholar]
- 91.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Circulation. 2004;109:3050–5. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 92.Paddenberg R, Stieger P, von Lilien AL, Faulhammer P, Goldenberg A, Tillmanns HH, Kummer W, Braun-Dullaeus RC. Respir Res. 2007;8:15. doi: 10.1186/1465-9921-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Serruys PW, Kutryk MJ, Ong AT. N Engl J Med. 2006;354:483–95. doi: 10.1056/NEJMra051091. [DOI] [PubMed] [Google Scholar]
- 94.Woods TC, Marks AR. Annu Rev Med. 2004;55:169–78. doi: 10.1146/annurev.med.55.091902.105243. [DOI] [PubMed] [Google Scholar]
- 95.Hausleiter J, Kastrati A, Mehilli J, Vogeser M, Zohlnhofer D, Schuhlen H, Goos C, Pache J, Dotzer F, Pogatsa-Murray G, Dirschinger J, Heemann U, Schomig A. Circulation. 2004;110:790–5. doi: 10.1161/01.CIR.0000138935.17503.35. [DOI] [PubMed] [Google Scholar]
- 96.McCay CM, Crowell MF, Maynard LA. J Nutr. 1935;10:63–79. [Google Scholar]
- 97.Hunter WS, Croson WB, Bartke A, Gentry MV, Meliska CJ. Physiol Behav. 1999;67:433–7. doi: 10.1016/s0031-9384(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 98.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Endocrinology. 2003;144:3799–810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 99.Bluher M, Kahn BB, Kahn RC. Science. 2003;299:572–74. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 100.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 101.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Nature. 2004;431:200–5. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 102.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Proc Natl Acad Sci U S A. 2008;105:9793–8. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McDaniel ML, Marshall CA, Pappan KL, Kwon G. Diabetes. 2002;51:2877–85. doi: 10.2337/diabetes.51.10.2877. [DOI] [PubMed] [Google Scholar]
- 104.Briaud I, Dickson LM, Lingohr MK, McCuaig JF, Lawrence JC, Rhodes CJ. J Biol Chem. 2005;280:2282–93. doi: 10.1074/jbc.M412179200. [DOI] [PubMed] [Google Scholar]
- 105.Manning BD. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. J Cell Biol. 2004;166:213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inoki K. Diabetes Res Clin Pract. 2008;82(1):S59–62. doi: 10.1016/j.diabres.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 108.Sakaguchi M, Isono M, Isshiki K, Sugimoto T, Koya D, Kashiwagi A. Biochem Biophys Res Commun. 2006;340:296–301. doi: 10.1016/j.bbrc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 109.Cho HJ, Park J, Lee HW, Lee YS, Kim JB. Biochem Biophys Res Commun. 2004;321:942–8. doi: 10.1016/j.bbrc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 110.Kim JE, Chen J. Diabetes. 2004;53:2748–56. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- 111.Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 112.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 113.Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Ruegg MA. Cell Metab. 2008;8:411–24. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 114.Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Nature. 2000;408:994–7. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 115.Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, Harper ME, Tremblay ML, Sonenberg N. Nat Med. 2001;7:1128–32. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- 116.Teleman AA, Chen YW, Cohen SM. Dev Cell. 2005;9:271–81. doi: 10.1016/j.devcel.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 117.Gillette-Guyonnet S, Vellas B. Curr Opin Clin Nutr Metab Care. 2008;11:686–92. doi: 10.1097/MCO.0b013e328313968f. [DOI] [PubMed] [Google Scholar]
- 118.Levenson CW, Rich NJ. Nutr Rev. 2007;65:412–5. doi: 10.1111/j.1753-4887.2007.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 119.Lee J, Seroogy KB, Mattson MP. J Neurochem. 2002;80:539–47. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 120.Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Neurobiol Aging. 2004;25:333–40. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 121.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Nat Genet. 2004;36:585–95. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 122.Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Cell Death Differ. 2009;16:46–56. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- 123.An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, Iqbal IG, Winblad B, Pei JJ. Am J Pathol. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB. Curr Biol. 2006;16:230–41. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 125.de Nobel H, Lawrie L, Brul S, Klis F, Davis M, Alloush H, Coote P. Yeast. 2001;18:1413–28. doi: 10.1002/yea.793. [DOI] [PubMed] [Google Scholar]
- 126.Vasseur S, Afzal S, Tardivel-Lacombe J, Park DS, Iovanna JL, Mak TW. Proc Natl Acad Sci U S A. 2009;106:1111–6. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Crino PB, Henske EP. Neurology. 1999;53:1384–90. doi: 10.1212/wnl.53.7.1384. [DOI] [PubMed] [Google Scholar]
- 128.Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. Proc Natl Acad Sci U S A. 2002;99:467–72. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Asaki C, Usuda N, Nakazawa A, Kametani K, Suzuki T. Brain Res. 2003;972:168–76. doi: 10.1016/s0006-8993(03)02523-x. [DOI] [PubMed] [Google Scholar]
- 130.Morath C, Arns W, Schwenger V, Mehrabi A, Fonouni H, Schmidt J, Zeier M. Nephrol Dial Transplant. 2007;22(8):viii61–viii65. doi: 10.1093/ndt/gfm652. [DOI] [PubMed] [Google Scholar]
- 131.Jolly CA. Curr Opin Lipidol. 2007;18:53–7. doi: 10.1097/MOL.0b013e3280115416. [DOI] [PubMed] [Google Scholar]