Abstract
Herpes Simplex Virus-2 (HSV-2) is episodically shed throughout the human genital tract. While high viral load correlates with development of genital ulcers, shedding also commonly occurs even when ulcers are not present, allowing for silent transmission during coitus and contributing to high seroprevalence of HSV-2 worldwide. Frequent viral reactivation occurs despite diverse and complementary host and viral mechanisms within ganglionic tissue that predispose towards latency, suggesting that viral replication may be constantly occurring in a small minority of neurons within the ganglia. Within genital mucosa, the in vivo expansion and clearance rates of HSV-2 are extremely rapid. Resident dendritic cells and memory HSV-specific T cells persist at prior sites of genital tract reactivation, and in conjunction with prompt innate recognition of infected cells, lead to rapid containment of infected cells. Shedding episodes vary greatly in duration and severity within a single person over time: this heterogeneity appears best explained by variation in the densities of host immunity across the genital tract. The fact that immune responses usually control viral replication in genital skin prior to development of lesions provides optimism that enhancing such responses could lead to effective vaccines and immunotherapies.
Herpes simplex virus (HSV-2) is a lifelong infection that is the leading cause of recurrent genital ulcers worldwide. In the immunocompetent host, HSV-2 mucosal ulcerations are normally self-limited. However, systemic complications such as recurrent meningitis, hepatitis, and pneumonitis can occur during acquisition or reactivation of infection, particularly among patients with poor T-cell immunity due to AIDS, organ transplantation or chemotherapy.1-3 Neonatal HSV arises from contact with the virus during childbirth and when untreated results in high mortality (>80%) and neurological morbidity.4-6
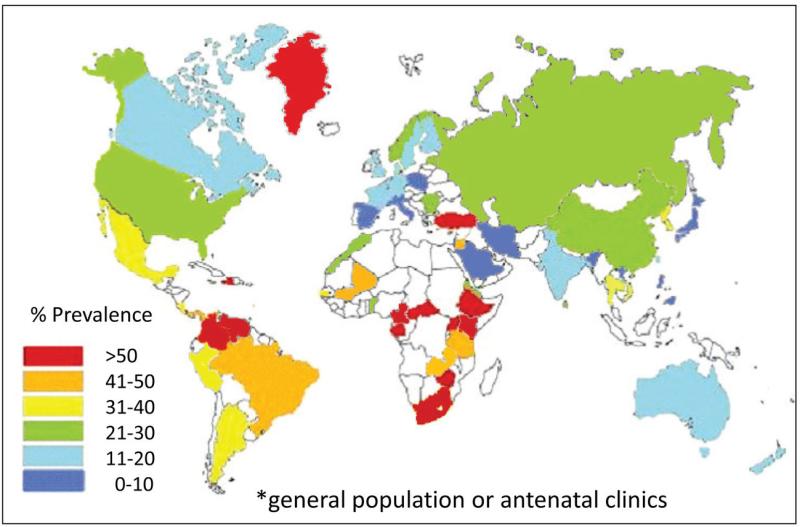
HSV-2 infection is widespread and continues to spread efficiently across the globe. In addition to high worldwide prevalence (Figure 1), recent incidence in several African cohorts approximates 20 infections per 100 person-years.7-10 Global incidence is also estimated to be approximately 23.6 million infections per year.11 The rate of HSV-2 coital acquisition as well as the serologic prevalence of HSV-2 is higher in women than men,12,13 though men who have sex with men remain at high risk of incident infection.7 Prevalent HSV-2 infection has been shown to increase the per coital transmission rate of HIV 5-10 fold.14 Men and women who have acquired HSV-2 have a 2-3 fold increased risk of HIV-1 infection.15,16 In countries in which sexually active adults have a high prevalence of HSV-2 infection, or in subpopulations such as men who have sex with men where HSV-2 infection is common, HSV-2 is a significant epidemiological driver of HIV-1 epidemics.16-18
Figure 1. Prevalence of HSV-2 infection in women.
Map is based upon published studies within the last 15 years using serological assays that can accurately differentiate HSV-2 from HSV-1 infection. White indicates no available data.
In this review, we describe how frequent release of HSV-2 from latency within neurons, as well as highly dynamic interactions between replicating HSV-2 and the host cell-mediated immune response in genital tissues contribute to observed disease manifestations, high global prevalence, and enhanced HIV acquisition risk. We also highlight the unique challenges that the kinetics of these viral-host interactions pose for antiviral and vaccine development. A critical discovery over the last 2 decades is that HSV-2 is frequently shed throughout the human genital tract even when symptomatic ulcerations are not present. While HSV latency is the predominant state of the virus on a per neuron basis within the entire biomass of the ganglia, HSV-2 is rarely quiescent. Within the genital tract, there is a frequent if not constant interplay between the virus, infected keratinocytes, and the host immune cells.
Many of the insights about mucosal HSV-2 infection have come from studies using quantitative detection of HSV-2 by PCR-based techniques. Genital shedding is assessed quantitatively with swabs processed for HSV DNA: this is widely accepted as a more sensitive, but no less specific, measure of shedding as compared to viral culture.19,20 Not only is HSV DNA replication the target of current antiviral therapies,19 but more severe disease manifestations correlate with higher shedding rates.21,22 While 80% of HSV-2 seroprevalent persons report no genital lesions,23 the majority of these people will have HSV DNA commonly detected on genital swabs with repeated sampling. In a cohort of 531 asymptomatic and symptomatic HSV-2 seropositive patients from the general population who were sampled daily over a 30-60 day time period, HSV-2 DNA was detected on 18% of days and occurred in the absence of recognizable genital lesions ⅔ of the time.24 Both clinical and subclinical shedding episodes decrease only modestly over a 20-year time frame in infected persons, highlighting the lifelong transmission potential of an infected person.25 Efficient sexual transmission is therefore explained both by the large reservoir of persistently infected persons with undiagnosed infection and the high frequency of silent reactivation throughout the lifetime of the human host.
Viral shedding manifests as frequent, highly heterogeneous bursts, or episodes. Recent studies indicate that subclinical shedding episodes can occur weekly, and often in multiple areas on the same day, indicating that reactivation occurs despite diverse and complementary host and viral mechanisms within ganglionic tissue that predispose towards latency. Shedding episodes also may vary enormously in duration and viral production over short time periods, due to varying degrees of localized immune control within a person across space and time. While replication and cell-to-cell transfer of HSV-2 are extremely rapid within genital lesions, resident memory HSV-specific T cells persist at the sites of reactivation: these highly localized infiltrates of dendritic cells, and CD4+ and CD8+ lymphocytes, in conjunction with prompt innate recognition of infected cells, often lead to rapid containment of infected cells and virus. Persistent CD4+ T-cells may be deleterious in that they enhance the probability of HIV-1 acquisition. On the other hand, the fact that cell-mediated immune responses control virus replication in genital skin prior to the development of genital lesions provides optimism that enhancing such responses will lead to design and development of immunotherapeutic approaches that control subclinical reactivations, and effective vaccines for the prevention of HSV-2 acquisition.
Antiviral Therapy and Vaccines
Current antiviral therapies limit the severity and frequency of genital lesions, but do not entirely eliminate episodes of subclinical shedding and as such only partially reduce transmission of HSV-2 to new partners. Treatment for mucosal HSV-2 infection has been available for over 30 years. HSV-2 was the prototype for development of small molecule inhibitors of virally encoded replication enzymes. Three decades ago, Gertrude Elion and colleagues demonstrated that the nucleoside analog aciclovir (ACV) was a substrate for HSV-1 and HSV-2 thymidine kinase (TK).26 Viral TK selectively phosphorylates ACV and cellular enzymes add two additional phosphates. ACV-triphosphate is a chain terminator of HSV DNA synthesis. The elucidation of the mechanisms of action of ACV and its subsequent derivatives as clinically effective and safe medications provided the conceptual framework for the development of antiviral agents for HIV, CMV, and Hepatitis B and C.
ACV, its derivative valaciclovir (VCV), and the related nucleoside analog famciclovir (FCV) effectively decrease the severity of genital lesions in immunocompetent and immunosuppressed patients if given early during recurrences. In addition, twice-daily administration has low toxicity and decreases lesion rates by 75% and shedding rates by 80%.27 VCV, an ACV prodrug, achieves higher peak serum concentrations than ACV,28 and FCV has a considerably longer intracellular half-life than ACV and VCV.29 However, recent studies indicate that subclinical breakthrough reactivations, especially of short duration, are common even among fully compliant study participants receiving maximal daily doses of VCV or FCV therapy,30 a phenomenon that may be explained by the rapid kinetics of viral expansion during drug trough levels. VCV was also the first antiviral to reduce transmission of a sexually transmitted viral infection (by ~50% among serodiscordant couples).31 Despite increasing use of these antivirals over the last 25 years, most infected persons remain untreated. Resistance to current anti-HSV-2 antivirals is infrequent and is usually encountered only among severely immunocompromised hosts.32 This characteristic is relatively unique among antiviral agents.
To date, no effective human vaccine exists. Attempts at using recombinant glycoprotein D (gD2), an envelope protein that generates a substantial neutralizing antibody response, have recently been shown to be unsuccessful.33 A better understanding of the immunology of acquisition of HSV-2 is needed to successfully develop a vaccine that will reduce coital acquisition.34
Acquisition of HSV-2
Infection is acquired almost invariably via sexual contact with infected genital secretions and as such is decreased by condom use.35 The relationship between viral reactivation rate and titer, and transmission is unknown. A retrospective study estimated a median of 40 sexual acts,36 or ~3.5% per coital transmission risk, prior to transmission. While genital shedding episodes associated with visible lesions are of longer duration and account for the highest genital viral loads, epidemiologic studies indicate that the majority of transmissions occur during asymptomatic shedding of virus (Box 1).37,38 This may be explained by the fact that couples are probably more likely to avoid coitus during severe lesions. Moreover, many asymptomatic episodes, while associated with lower peak viral loads and duration compared to recurrences, are still associated with viral loads exceeding 106 HSV DNA copies and duration exceeding three days.24
Pathogenesis of primary infection and establishment of latency
During successful acquisition, viruses access permissive nucleated cells in the mid to basal epidermis via microscopic breeches in the epidermis that occur with coitus. The HSV replication cycle is governed by processes that promote transcription of viral DNA, translation of mRNA, DNA replication, viral assembly and viral egress prior to cell lysis,39,40 in concert with numerous immune evasion strategies at each stage of replication.41 Viral replication within keratinocytes leads inevitably to cell death. After entry into the cytosol, viral protein production occurs in a cascade-like fashion. Immediate early gene products are produced within hours and promote immune evasion, neurovirulence and synthesis of early proteins required for viral DNA replication. Late proteins provide structural components of the capsid and are necessary for viral egress. The timeline between viral entry and cell lysis is approximately 24 hours.40 In the absence of antiviral therapy, HSV spreads rapidly between epithelial cells and subsequent cell lysis leads to vesicle or ulcer formation. The virus enters sensory neuronal axons via a plexus of free nerve endings in the epidermis and is transported into neuronal cell bodies in the dorsal root ganglia, where it replicates and spreads to other neurons.
Latency and Reactivation
The viral genome is maintained within ganglia for the life of a host in a quiescent condition termed latency (Box 1). Reactivation within ganglia leads to transport of HSV towards genital skin or mucosa. Subsequent replication within epidermal cells follows passage of viruses across the axonal epithelial gap and leads to discrete shedding episodes, which are commonly sub-clinical.42 Several lines of evidence suggest that asymptomatic detection of HSV DNA with a genital swab denotes viral replication within epithelial cells rather than simply viral release from neurons or micro-trauma from swabbing: the plexus of free nerve endings arborizes and terminates in the stratum granulosum of the mid-epidermis (Figure 2); a minimum of 10 epithelial cells separates neuron endings from the genital skin surface meaning that the architecture of this layer of epithelial cells must be substantially disrupted for swabs to contact the mid-epidermis; moreover, per cell amplification of HSV DNA is several logs higher in cultured epithelial cells than in neurons;43,44 importantly aciclovir therapy, which interacts with viral genes only made during the replication cycle of the virus, reduces the frequency and titer of such shedding, though this could be due to activity in neurons as well as keratinocytes.
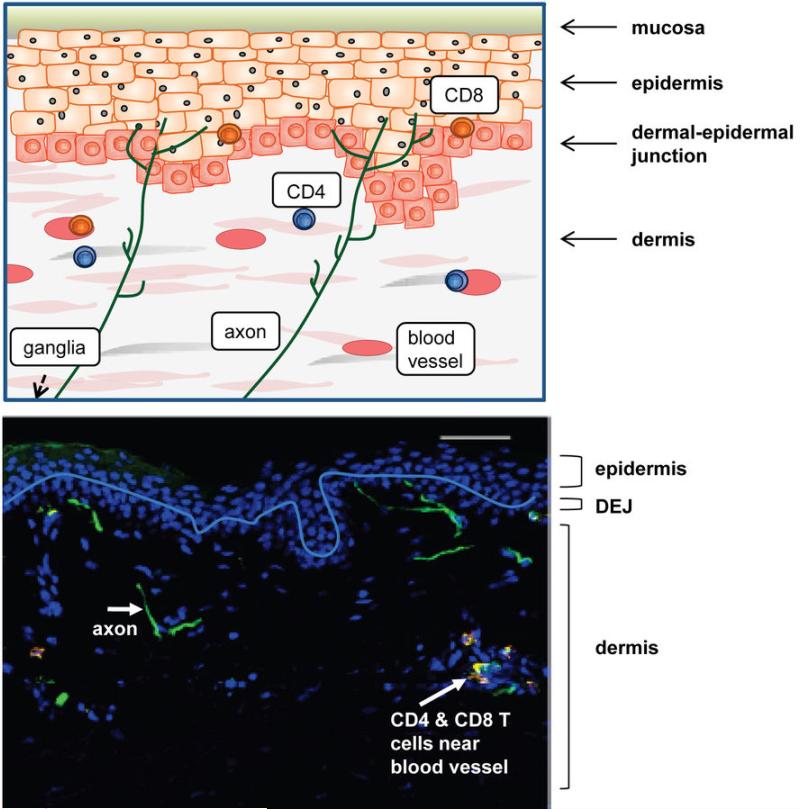
Figure 2. Schematic and histologic representation of healthy genital mucosa.
Axons arrive from the dorsal root ganglia and terminate at the dermal-epidermal junction or within the mid-layer of the epidermis. HSV-2 is released from axonal termini across the neuron-epithelial gap, and can enter epithelial cells where virus is amplified and rapidly spreads to contiguous skin cells. Low numbers of CD4+ and CD8+ T cells are present in the dermis and dermal-epidermal junction after entering the genital skin through blood vessels. The white line denotes the dermal-epidermal junction.
Symptomatic episodes, characterized by closely arrayed visible ulcers and vesicles on a bed of erythema, are termed recurrences (Box 1). Mathematical estimates derived from examining cell size and histologic structure of genital mucosa suggest that a single visible ulcer of at least 1mm diameter implies the death of >14,000 epithelial cells.45 Recurrences occur infrequently in most persons (0-10 times yearly). This observation led to the view that latency was comprehensively controlled within all neurons in sacral ganglia requiring infrequent “trauma,” environmental stimuli such as ultraviolet light exposure or immune suppression (transient or sustained) to elicit reactivation.
In the last decade, several experimental and clinical studies have altered the concept that viral reactivation is infrequent and usually results in genital lesions. Recent data support the concept that a minority of viral genomes in ganglia may be reactivating at any given point in time. The frequent detection of subclinical episodes of mucosal shedding in over 90% of persons who possess HSV-2 antibodies undermines the idea that viral release from ganglia is uncommon and occurs with the same frequency as recurrences, 19,45-49 and may suggest that neuronal stimulation leading to viral release is a common event. Detailed studies in which HSV-2-infected persons were sampled at 6 hour intervals for at least one month revealed that shedding episodes occur roughly every 10 days and that most subclinical reactivations are rapidly cleared in 2 to 12 hours.46 HSV-2-specific lymphocytes persist in genital skin contiguous to the neuronal termini of sensory nerve roots,50 suggesting an active role in immune surveillance perhaps due to frequent contact with released HSV.
Because direct observation of viral release from neural root ganglia towards the skin is not possible in humans due to lack of access to such tissue, one of the unknowns in HSV pathogenesis is the frequency and pattern of viral release/leak from neurons. While available cell culture systems,43 and guinea pig models,51-53 can capture the passage of HSV from neuronal cell bodies to epithelial monolayers, they lack sufficient semblance to recapitulate the full complexity of viral dynamics and immunologic response of human infection. Statistical analysis of HSV shedding patterns derived from sampling a wide anatomic area with genital swabs suggests that many episodes involve multiple concurrent ganglionic reactivations, and that not all virus released from neuronal endings in the genital tract necessarily leads to detectable shedding episodes.47 A mathematical model of HSV reactivation that assumed the total body of dorsal root ganglia releases small amounts of virus in a nearly constant fashion (10 – 100 HSV DNA copies/day) most closely fit available shedding datasets.45 This concept of “slow viral drip” is in concert with the observation that 6.3% of neuronal cell bodies harbor HSV DNA while only 0.6% contain >100 HSV DNA copies.54 Many infected neuronal cell bodies are surrounded by host T-cells. These calculations help harmonize the idea of a nearly constant “leak” of HSV at the whole organism level, with the well known concept that the overwhelming majority of viral DNA with neurons is in a latent form. It is the wide anatomic distribution of these reactivating virions that appears to result in frequent antigenic exposure to the host across many micro-regions in the genital tract (Figure 3).
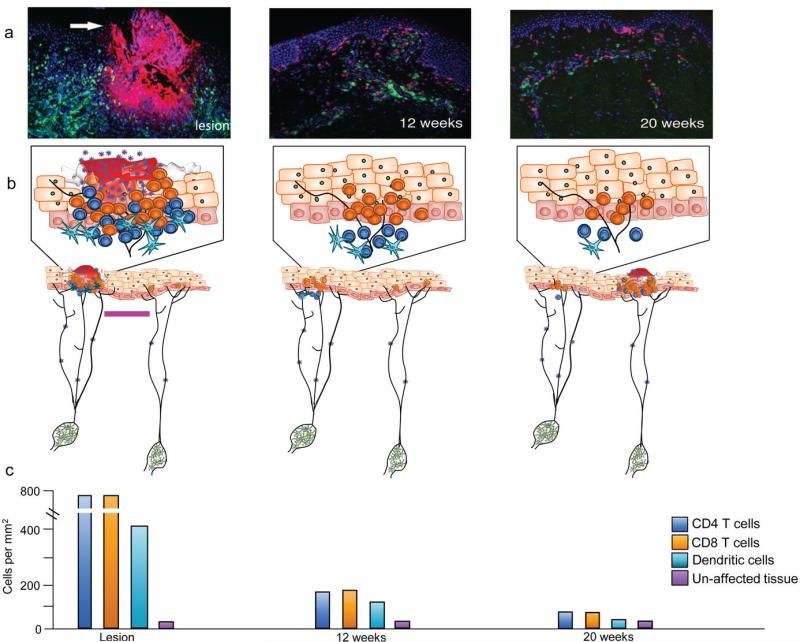
Figure 3. Schematic and histologic depiction of HSV-2 reactivation dynamics.
HSV is continually and slowly released due to concurrent reactivation of HSV within multiple ganglia. This leads to HSV release into multiple spatially discrete mucosal sites. Periodically, epithelial cells become infected, leading to rapid viral spread, multiple infected cells, micro-ulcer or ulcer formation (where high numbers of keratinocytes are lysed and virions are shed), and dense CD4+ and CD8+ T-cell infiltration. Following containment of HSV at a single site, T cells persist at that location for several months in an immunosurveillance capacity. HSV-2 reactivates at other sites where CD4+ and CD8+ lymphocyte density is lower allowing for a genital tract shedding episode, which initiates in a different region. (a) Fluorescent micrographs of genital skin biopsies from three time-points: active lesion (left; arrow) and at 12 (middle) and 20 (right) weeks post-healing. Lesion stained for HSV-2 (red) and CD8+ T cells (green); 12 and 20 weeks post healing stained for CD4+ (green) and CD8+ (red) cells. (b) HSV-2 drips from neurons (green) and periodically will infect epithelial cells resulting in lesion formation, where high densities of CD4+ (dark blue) and CD8+ (orange) lymphocytes, and to a lesser extent dendritic cells (light blue), traffic to clear viral infection (left panel, left neuronal cell body). Not all virus released from neuronal endings in the genital tract necessarily leads to detectable shedding episodes; epithelial cells only millimeters away from a lesion site can remain unaffected (left panel, right neuronal cell body). Bar = 3 mm. (c) Lymphocytes and dendritic cells remain localized at the lesion site for months after healing and decrease in numbers over time.
Latency is defined at the cellular level as the presence of fully competent viral genomes in cell nuclei without production of infectious virus (Box 1). While recent evidence suggests the presence of latent genomes in autonomic tissues,55 as well as within sensory neuron cell bodies, there is no evidence to date that non-replicating viral DNA can persist within keratinocytes or other non-neural tissues. For this reason, we believe that detection of a newly initiated HSV DNA shedding episode in the genital tract implies recent HSV replication in, and transport from, neuronal ganglia. Neuronal latency is interrupted when translation of a full complement of viral lytic proteins occurs (Figure 4).56 This is followed by HSV glycoprotein E- and I-dependent anterograde transport of viral capsids and glycoproteins down axons towards the genital tract.44,57 The architecture and function of ganglia are preserved without apparent cell loss. Accordingly, patients with genital herpes do not experience genital dysesthesia between recurrences even after decades of frequent reactivation.42
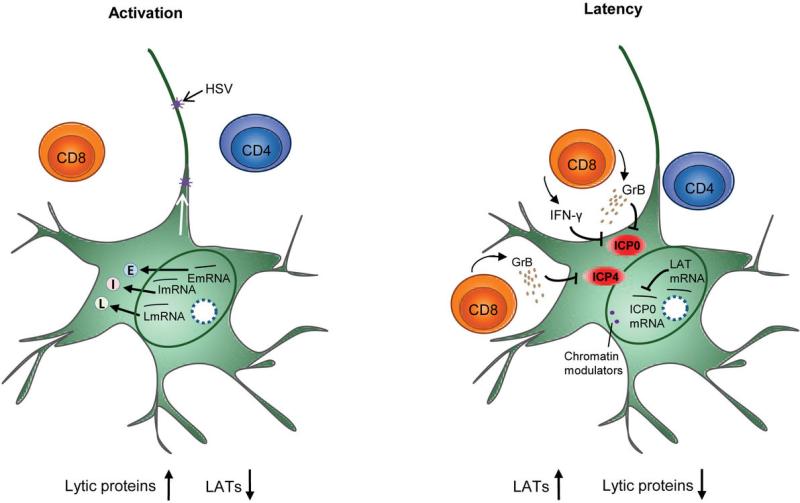
Figure 4. HSV-2 and host interactions in nerve root ganglia.
Depiction of the molecular interactions in the ganglia showing the inverse relationship between LAT transcripts and lytic proteins. The top inset (activation) depicts active transcription of viral RNA and high lytic protein expression accompanied by low LAT production. The bottom inset (latency) depicts control of HSV protein expression in neuronal cell bodies in the ganglion by local resident CD8+ T-cells: IFN-γ and granzyme B released by host T cells may inhibit lytic protein expression. LAT production is high and lytic protein production is low.
Molecular features of latency
Factors that determine the balance between latency and reactivation within a single neuron are not completely understood. In the 1980's Stevens et al. defined a series of latency-associated transcripts (LATs) in neuronal cell bodies within trigeminal and sacral ganglia.58 While the exact role of LATs in maintaining latency is still debated, viral genomes are found in cells expressing and not expressing LATs, suggesting that viruses within different neurons may be in distinct stages of latency and reactivation.54,59 Even in mouse ganglia, where spontaneous reactivation does not occur, abundant expression of proteins from all stages of HSV replication is evident in a small percentage of trigeminal neuronal cell bodies,60 which are targets for an HSV-directed inflammatory infiltrate.61,62
LAT transcripts block HSV-1-induced neuronal apoptosis in animal models,63 and may also protect from CD8+ T-cell and granzyme-induced apoptosis.64 HSV-2 expresses two 22-nucleotide microRNAs (part of the LAT) in human dorsal root ganglia that are antisense to ICP0, an immediate early protein critical for lytic replication (Figure 4).65 These microRNAs perform post-transcriptional regulation that could potentially be involved in maintenance of latency.66 Undefined cell-type specific factors also cause HSV to preferentially express gene products that modulate host chromatin structures, which favor down-regulation of immediate-early gene products. This contrasts with epidermal cells where an alternative chromatin structure favors lytic gene expression.67
Host immunity in ganglia
Whereas the ganglia were once considered immunoprivileged sites where HSV could exist with low antigenic presence and transcription of few available viral genes,58 there is now evidence that host immune mechanisms may favor latency. HSV-1-specific effector memory CD8+ and CD4+ T cells are permanently retained within trigeminal ganglia in infected humans,68 though it is unknown if similar cell subsets exist within the dorsal root ganglia during HSV-2 infection. Histologic evidence is consistent with a noncytocidal mechanism controlling levels of virus in neurons. As has been demonstrated for hepatitis B infection, CD8+ T-cells are known to maintain control over virus-infected cells using noncytocidal as well as directly lytic mechanisms.69 Mice have a similar localization of lymphocytes in trigeminal ganglia, and CD8+ T cells prevent spontaneous reactivations even though viral proteins are present in miniscule amounts and neurons do not express MHC molecules necessary for T-cell binding.61 This mechanism of memory effector function results from CD4+ T-cell help early during infection. Memory CD8+ T cells produce IFN-γ, which has a partial dampening effect on early viral replication within neurons, and disruption of these cells promotes reactivation.70,71 CD8+ T cells may polarize their T-cell receptors towards infected neurons and release granzyme B lytic granules (Figure 4). Granzyme B induces apoptotic pathways in fibroblasts, but not in neurons where it cleaves ICP4, a viral protein needed for transcription of subsequent critical early and late HSV genes. Early viral replication may therefore be limited, ultimately favoring viral latency without lethal effects to the neuron.72
Our opinion is that HSV latency is the overwhelmingly predominate state on a per neuron basis, but that HSV is rarely quiescent within the entire biomass of ganglia and that there may be an evolving interplay between virus, neurons and acquired immune cells.73 The idea that reactivation is a common, if not constant, phenomenon perhaps induced by repeated neuronal stimulation, does not obscure the role that ganglionic immune responses might have on disease phenotype. In mice, the number of infected neurons and average genomic copies per neuron correlate with total ganglionic latent viral load.74 Ganglionic viral load is determined in part by inoculums during primary infection,75 remains stable during chronic infection,76 and in guinea pigs and mice predicts reactivation rate,75,77 which is in turn inversely correlated with ganglionic CD8+ T-cell density.75In silico models suggest that even small adjustments in the average amount of HSV released from neurons per day may have important effects on shedding frequency.45 Some heterogeneity of reactivation frequency in humans might be due to variability of host immune control in neural ganglia.
Transition from Neuronal to Mucosal Infection
A key mystery in HSV biology is the fate of virions released from neurons into genital skin. One unique feature of infection is extreme variability between genital reactivations. The same individual over a span of 1-2 weeks may exhibit several subclinical episodes lasting only 2-12 hours, followed by a lesional episode lasting 4-10 days.46 Viral production can differ by a multiple of 10 million between successive episodes.19,46,48 There is a direct correlation between quantity of genomic HSV detected, episode duration and the presence of visible genital lesions. Yet, all mucosal reactivations in the immunocompetent host are notable for extraordinarily rapid expansion (minimum 7.6 log10 HSV DNA copies/day) and decay (minimum 6.2 log10 HSV DNA copies/day) phases.24 Any comprehensive theory of HSV pathogenesis must explain profound heterogeneity in episode severity in the same individual over time, as well as the rapid production and elimination of HSV in the genital tract.
A reliable animal model system for HSV-2 reactivation would be of enormous utility for the field. Spontaneous HSV-2 reactivation is rare in the mouse unless the ganglion is “stressed” or the animal is immunosuppressed, at which time relatively efficient release of virions and expansion of infection into skin/mucosa occurs. Sporadic reactivation occurs in guinea pigs following resolution of primary infection,78 but this model has not been evaluated in enough detail using molecular detection of HSV to understand whether kinetics of reactivation and clearance approximate those of human infection. Guinea pigs can be effectively vaccinated against HSV-2 acquisition with glycoprotein D subunits,79 and an immunotherapeutic benefit is achieved when lower doses of vaccine are used.80 The lack of an effective glycoprotein based vaccine in humans highlights the fact that certain critical features of human and rodent viral immunity probably differ in important ways.
Antiviral Innate Immunity in the Genital Mucosa
Mucosal shedding episodes are likely to be initiated after successful attachment of neuron-derived HSV into at least one epithelial cell. The immune response is initiated soon thereafter. HSV recognition occurs even prior to entry and replication via several pathways including recognition of essential viral fusion proteins (gH and gL),81 and genetic deficits in innate immunity can be associated with severe infections. HSV immediate early protein ICP27 is required for optimal NFκβ expression within 3-5 hours of infection.82 Mutations in NEMO/IKK-γ, a NFκβ regulator, are associated with fatal HSV.83 Autosomal dominant TLR3 deficiency permits high-level viral replication in fibroblasts due to abnormal IFN-β and γ production, resulting in an increased risk of fatal HSV encephalitis (HSE) despite normal resistance to severe forms of other viral infections.84 TRIF deficiency impairs TLR3 and represents another genetic marker for increased risk of sporadic childhood HSV-1 encephalitis.85 TLR2 on the surface of dendritic cells (DCs) and TLR9 within their endosomes detect HSV pathogen associated molecular patterns in quick succession, also activating NFκβ pathways.86 Plasmacytoid dendritic cells (pDCs) subsequently exert a strong antiviral effect by secreting type I interferons.87 Consanguineous family members who develop fatal HSE appear to have a poor IFN-α response to TLR7 and TLR9 agonists due to deficits in TLR signaling within endoplasmic reticula.88 The genetic link with HSV disease severity, particularly risk for early encephalitis, has been reviewed elsewhere.89
Herpetic vesicles are packed with high levels of cytokines, beta chemokines,90 and interferons,91 and early cytokine production appears to be of epithelial origin and is geared towards recruitment and activation of T cells.90 IFN-β is produced by keratinocytes and has a potent antiviral effect during HSV infection of these skin cells,92 whereas IFN-α and IFN-γ appear to have inhibitory effects on viral spread both from neurons to and between epithelial cells.93 Several immediate early viral proteins interrupt activity of type I IFNs,41 and HSV-2 appears to block IFN-α and IFN-β production early during recurrences in humans.94 Type II IFNs are secreted by antigen-specific CD8+ and CD4+ T cells and innate lymphocytes such as nature killer (NK) cells and γδ T cells, which are all present in genital lesions. IFN-γ binds different receptors than type I IFNs but triggers a similar transcriptional response involving STAT-1 and -2; STAT-1 mutations are associated with fatal HSV-1 infections.95
The high frequency of shedding episodes in the genital tract suggests that viral evasion mechanisms frequently, at least partially, overcome innate responses. This may be due to frequent physiologic stresses that temporarily suppress local immunity. Ultraviolet light is one such stress that appears to hamper viral antigen presentation,96 and cause photoisomerization of trans-Urocanic acid (trans-UCA) to cis-Urocanic acid (cis-UCA), which in turn induces upregulation of immunomodulatory factors in keratinocytes.97 This may be one explanation for the observation that UV exposure after HSV inoculation in rats leads to far more severe lesions despite an apparent lack of effect on cell mediated immune response.98 While the relevance of UV exposure to human orolabial HSV infection is clear, the particular stresses that might predispose to genital HSV-2 reactivation are unknown. Nevertheless, because innate mechanisms are operative early during viral-host interaction, interventions that stimulate innate responses to viral antigen hold promise. For example, a TLR7 and TLR8 agonist that induced type I IFNs when applied topically to human genital skin was associated with decreased HSV-2 shedding frequency.99
Induction of acquired mucosal immunity
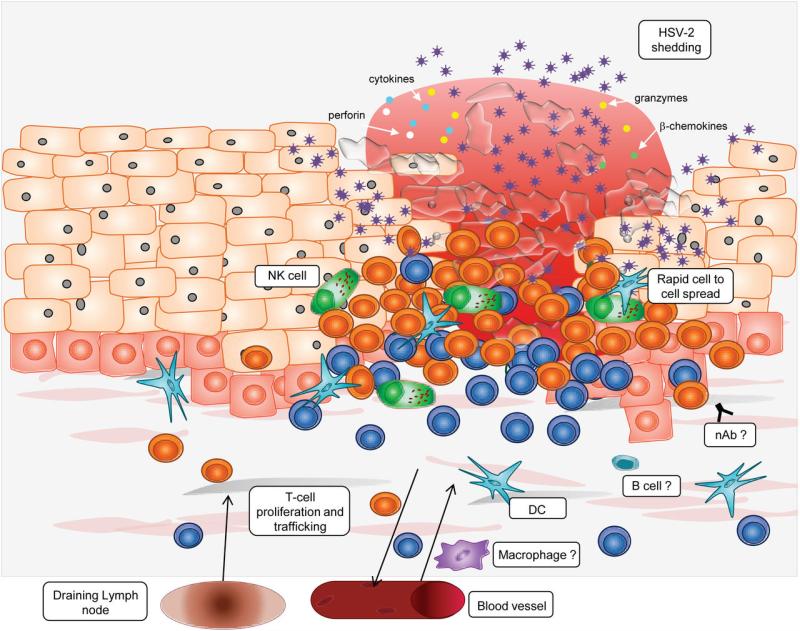
In 1985, Cunningham and colleagues discovered that intense lesion-site inflammation develops during genital lesions containing a wide variety of cells (Figure 5), including T-cell predominance and lesser concentrations of B cells and NK cells in the first 24 hours, followed by monocyte/macrophage infiltrates in the later stage of healing.100 Innate signaling and acquired immune responses are linked via DCs, NK cells and cytokine signaling pathways. Plasmacytoid DCs are typically restricted to blood and lymphatic compartments but infiltrate the dermal-epidermal junction and interface with infected cells, as well as activated T cells and NK cells during lesions. In culture, HSV-exposed pDCs are not permissive for viral replication, but stimulate autologous T lymphocyte proliferation.101 Activated pDCs potently induce cutaneous lymphocyte antigen (CLA) on HSV-2-reactive CD4+ T cells likely through the release of cytokines,102 which subsequently promotes T-cell trafficking to lesion sites.103 pDCs also boost adaptive immune responses in draining lymph nodes.104 pDC depleted mice poorly control HSV after intra-vaginal challenge,87 and severe human infections may result from defective pDCs.105,106 Thus, trafficking of pDCs to the dermal-epidermal junction may play an important role in containment through the recruitment of effector lymphocytes to the site of infection.
Figure 5. Host immunity in the genital mucosa.
There is an intense infiltrate of CD4+ (blue) and CD8+ (orange) T-cells and mixed populations of dendritic cells (blue stellate cells), macrophages (purple cells) and NK cells (green cells). This cellular infiltrate produces IFN- γ, TNF-α, RANTES, granzyme B and perforin. B cells and neutralizing antibodies may also be present. The location and function of macrophages, B cells and neutralizing antibodies have not been fully characterized.
Monocytes are recruited to inflamed skin sites and form monocyte derived dendritic cells (MDDCs), which locally cross-present viral antigen to memory and effector T cells.107 In mice, HSV infected MDDCs undergo apoptosis and are phagocytosed by uninfected MDDCs.108 This occurs in draining lymph nodes, where viral DNA is not present by PCR, but antigen presentation results in HSV-specific T-cell activation within 6 hours and proliferation within 30 hours.109 Langerhans cells, the most prominent antigen presenting cells in skin, surprisingly do not prime Th1 cells,110 whereas other migratory DC subsets, which first recognize viral presence in skin, are critical in this role.111 Of migratory DCs, the CD103+ subset appears specifically adapted for cross-presentation to naïve CD8+ T cells, while CD11b+ dermal DCs successfully present to CD4+ lymphocytes. CD103+ DCs play a dominant role in presenting antigen to CD8+ T cells in murine skin following secondary infection as well.112 Whereas Langerhans cells appear to be the first DCs to emigrate from skin following infection, CD103+ migration from infected skin appears to predominate more than 24 hours after viral inoculation.113 The redundancy within the antigen presentation system underscores the importance of rapid pathogen recognition and lymphocyte activation.114,115
Antibody response
Several pieces of evidence suggest that humoral immunity may be important in controlling HSV infection. Neutralizing antibodies can bind viral envelope glycoproteins necessary for viral entry develop during infection.116 The presence of maternal antibodies specific to HSV-2 but not HSV-1 reduces neonatal transmission of HSV-2.117 Antiserum limits extent of mucosal and dorsal root ganglia infection, as well as recurrence rate in the guinea pig model, provided that antibody is delivered in high quantities during the first 24 hours of infection.118 Neutralizing antibodies are capable of binding virus in the gap between neuron endings and epithelial cells and may limit bi-directional viral transfer between these tissues.119 IgG antibody subsets predominate in the female genital tract and Fc receptors on epithelial cells are required for transcytosis of IgG across these cells and for protection against HSV-2 challenge.120 IgG appears to be a necessary component of the protective immune response in a humanized mouse model,121 and vaccine studies in guinea pigs using glycoprotein subunit products continue to show promising results, though induction of a robust CD4+ T-cell response may explain this phenomenon.122
On the other hand, human vaccine studies using glycoprotein product have shown no protection against HSV-2 and only limited protection against HSV-1, despite generation of adequate neutralizing antibody titers.123,124 While T-cell immunodeficiency is a clear risk factor for severe infection, patients with agammaglobulinemia appear to be at no higher risk of severe infection. HSV-1 encodes complement-interacting and IgG Fc-binding glycoproteins (gC and gE) that may limit the in vivo effects of antibody-dependent cell-mediated cytotoxicity.125,126 In addition, the effect of mucosal antibodies may be limited as spread of infection within an ulcer occurs via direct cell-to-cell spread of virus across tight junctions that are inaccessible to antibodies.127 For these reasons, the overall role of neutralizing antibodies in protection is unknown.
T-Cell Immune Response
Substantial evidence in both humans and mouse models of HSV supports a critical role for highly localized T-cell responses in viral containment.128,129 In mouse models, Th1-mediated protection occurs via MHC class II (DC and B cell) initiated CD4+ memory T-cell re-stimulation within vaginal mucosa. Activated CD4+ cells produce IFN-γ, which limits viral production and spread.130 Yet, infection of mouse flank results in a rapid influx of a highly localized, non-migratory subset of HSV-specific CD8+ memory cells that remain at the inoculation site for at least 100 days. These cells display lower homeostatic replication and greater expression of E-cadherin and extracellular matrix-binding proteins than circulating HSV-specific CD8+ lymphocytes. Their memory effector phenotype provides local protection against viral re-inoculation several months after initial exposure, while infection at a separate skin locus is poorly controlled.129 Transplantation of CD8+ memory cells prior to HSV-1 inoculation in a mouse does not prevent establishment of latency but substantially limits accumulation of virus and infected cells in the skin and the innervating dorsal root ganglia.131 Chronic re-exposure to HSV antigen does not appear to induce T-cell exhaustion.132
Similarly, functionally competent HSV-2-specific cytotoxic CD8+ T cells and CD4+ T-cells densely populate the site of genital recurrences in humans (Figure 5).133-135 CD8+ T cells selectively infiltrate adjacent to HSV-2 infected cells during lesions with no excess CD8+ T-cell density even two-centimeters away from the active lesion,50 representing a dramatic illustration of the important spatial dynamics between the virus and host. HSV-2 specific CD8+ lymphocytes also persist at the dermal-epidermal junction close to peripheral nerve endings (Figures 3). Recent studies in which these CD8+ T cells are captured by laser dissection microscopy and then evaluated for transcriptional changes have shown that perforins, granzymes A and B, and a variety of antiviral cytokines are expressed during clinical quiescence, signifying an active immunosurveillance role after episode clearance.136
CD4+ T-cell infiltration occurs slightly earlier during lesion formation than CD8+ T-cell infiltration.135 DCs and CD4+ lymphocytes occupy a deeper location than CD8+ T cells, congregating in the peri-vascular space in post-capillary venule tufts in rete ridges of the upper dermis. CD4+ T cells also persist locally for at least 6 months whether or not patients receive antiviral therapy (Figures 3), and along with NK cells continue to express IFN-γ early following exposure to HSV antigen after lesion healing.128 IFN-γ tissue levels and interferon gene products expression levels remain elevated in tissue for weeks after lesion healing, though it is not known if this cytokine is produced by macrophages or lymphocyte subsets.94 The dense lymphocyte infiltration during lesions and persistence afterwards has relevance to HIV-1 co-infection. CD4+ lymphocytes in genital skin are enriched for chemokine receptor CCR5. CCR5-tropic strains of HIV-1 replicate in higher numbers in cells derived from healed lesion areas than from control skin, providing a biological mechanism for enhanced HIV-1 acquisition in HSV-2 infected persons.128
An unanswered question in human infection is whether lymphocyte replenishment at the site of an HSV-2 reactivation is due to trafficking from secondary immunologic organs or local expansion. For circulating CD8+ cytotoxic lymphocytes to enter infected tissue, CD4+ T cells must first encounter antigen presented by DCs in the submucosa,107 and then produce interferon-γ. IFN-γ-induced chemokine production by epithelial cells allows for cytotoxic lymphocyte homing to locations where viral antigen is present on infected cell surfaces.137 Plasma central memory CD4+ and CD8+ T cells specific for HSV-2 constitutively express CLA while lymphocytes specific for non-skin associated herpes viruses lack this antigen: CLA promotes chemotaxis by adhering to upregulated E-selectin on HSV-2-infected epithelial cells.102,138 CD8+ T-cell HSV-specific precursors are recruited into the CTL response within 24 hours following mouse footpad inoculation.139 The potential importance of lymphocyte trafficking is demonstrated by the fact that lower levels of HSV-specific CD8+ cytotoxic T lymphocytes among peripheral blood mononuclear cells in HIV-infected persons predict more severe recurrences.140
This multi-step process of viral antigen transport from site of infection to secondary lymphoid compartments, antigen presentation to cognate CD8+ T cells, lymphocyte activation and expansion within secondary lymphatic tissues, and migration back to the site of reactivation occur while the number of HSV-2-infected cells rapidly expands. Greater control might be attained if mucosal effector cells persisted for a more extensive time period. In mouse models, the immunodominant structural protein gB is presented on the surface of infected cells with kinetics of an immediate early protein, allowing for CD8+ T-cell activation within 2 hours;141 local CD8+ re-expansion from local DC and CD4+ T-cell priming may therefore be a key component in controlling localized infection.107
More general investigations of viral immunity, such as that of murine cytomegalovirus, suggest that proliferating, terminally differentiated effector memory T cells persist at the point of viral infection.142 Following certain viral infections of the skin and gut, non re-circulating memory CD8+ T cells that display replicative and cytolytic function remain at the infection site, allowing for immediate recognition and clearance of pathogens at the portal of entry.143 The ability of fully competent effector memory T cells to traffic to the periphery and then persist in previously infected tissues, while maintaining an ability to rapidly recognize and eliminate infected cells, is therefore not likely to be unique to HSV infection,144 though HSV-2 has provided a unique opportunity to observe these phenomena in the human host.
Temporospatial fluctuations in densities of localized CD8+ T cells, CD4+ T cells, DCs, macrophages, NK cells and IFN-γ50,94,128 may explain the enormous diversity of shedding episode severity witnessed in the same infected person over time. Replication and spread of virus is explosive during some reactivations leading to thousands of infected cells within 24 hours. In support of this finding, surface glycoprotein gE can mediate cell-to-cell spread at keratinocyte tight junctions, facilitating efficient and rapid infection of tightly packed epithelial cells.145 Yet, the host controls most reactivations within several hours before formation of genital lesions occurs. Differences of only 20-30 minutes in the average lifespan of a virally infected cell appear to determine whether virus is contained as a sub-clinical shedding episode lasting <24 hours rather than a 4-8 day clinical recurrence.146 Thus, the spatial interactions between viral spread and containment can explain the varying heterogeneity of episodes within a single immunocompetent individual.
The margin between episode containment within the first hours of viral replication and progression to a prolonged episode appears to be extremely narrow, and may pertain to the lack of a localized host immune response at small clusters of productively infected epidermal cells. A possible complementary explanation involves immunomodulatory effects that HSV has on activated T cells. During lesion formation, HSV infects 5-20% of skin-resident CD4+ and CD8+ lymphocytes via a virologic synapse from epidermal cells.147 Activated T cells do not support viral replication but HSV alters lymphocyte receptor signaling leading to a shift in function from cytolytic to immunosuppressive.148 Once viral replication bypasses cytolytic activity early during a reactivation, stimulation of CD8+ T-cell expansion by CD4+ T cells and DCs is necessary for viral clearance. This delay may allow for enhanced viral spread prior to clearance.107 Wide spatial dispersion of virus occurs during reactivations, which may serve to bypass areas of high T-cell density.149
Overall, these observations suggest a model of pathogenesis with the following features: 1) due to focal HSV reactivation within sections of ganglia perhaps resulting from incomplete host immunological control, the virus leaks from neurons into the genital tract frequently, leading to recurrent, detectable shedding episodes (Figure 3); 2) the major determinant of episode severity is whether escape from innate and acquired immune surveillance occurs early during viral spread; local immunologic control in the ganglia (Figure 4) as well as genital keratinocytes may be altered by environmental stimuli; 3) rapid containment of viral replication is required to prevent genital lesions; 4) there appears to be a threshold of immune cell density above which control is achieved; and 5) these rapid interactions between virus and host are highly localized in time and space, and mucosal CD8+ and CD4+ T-cell levels slowly decline over time at a site of a prior recurrence (Figure 3), allowing for subsequent herpetic lesion formation at the same site.
Implications for vaccines and immunotherapy
These new insights on viral-host interactions offer both potential and challenges for the development of novel vaccine and immunotherapeutic approaches for HSV-2 infection. A successful vaccine product would limit the frequent leak of HSV from dorsal root ganglia, as well as the extremely rapid expansion of virus early during episodes, both of which appear to occur despite multiple host control mechanisms within neural and mucosal tissue. Enhanced efficacy of antivirals may be obtained by precisely defining the pharmacodynamics and pharmacokinetics of the drugs in tissue. Effective delivery of drugs to the neural/epithelial cell junction may reduce initial seeding or localized bursts of initial rapid viral replication. The extremely quick expansion of virus early during reactivation, even in the presence of antiviral therapy, may imply that intracellular drug levels need to exceed the inhibitory concentration of drug throughout the dosing cycle.
An HSV-2 vaccine would have substantial beneficial effects at both the individual and population levels.150 An ideal vaccine would significantly decrease the probability of acquisition per coital act. However, an immunotherapeutic vaccine that decreased disease severity and shedding frequency (and therefore transmission likelihood) would also be a welcome addition to the field. The recent demonstration that the host often controls HSV at the point of release as well as the frequent, rapid clearance of virus by the host immune system suggests potential for effective immunotherapy. Given the importance of dense aggregates of lymphocytes and dendritic cells in containing HSV within mucosa and possibly ganglia, an immunogen that increases mucosal innate and HSV-specific T-cell density and function appears to offer a reasonable chance of success in enhancing the localized containment of viral infection in tissue. The importance of these localized host responses in the clearance of HSV-2 infection suggests that measurement of HSV-specific CD8+ and CD4+ mucosal density and function at the site of reactivation is likely to be a more fruitful correlate of clinical outcome than assessment of host immunity in PBMCs, which may not reflect the host immune functions at localized tissue sites. Host immune cell density and functionality within dorsal root ganglia may also be of critical importance, though this will be considerably more difficult to assess from clinical samples.
In summary, recent studies indicate a nearly constant kinetic interaction between HSV-2 and the ganglionic and mucosal immune system as HSV virions appear to leak frequently into the mucosa. The immunocompetent host is able to contain HSV-2 in a localized manner for extended time periods (weeks). The rapid expansion kinetics of reactivated virus and the wide anatomic distribution of virion release provide a favorable milieu for frequent shedding. The ability of the host to contain reactivation in a defined anatomic area for extended time periods provides promise for the development of immunotherapeutic approaches for viral containment both in the infected person as well as the development of immunogens to prevent transmission to sexual partners and from mothers to infants.
Box 1. Glossary of Terms.
Asymptomatic shedding
The detection of HSV-2 DNA or the isolation of HSV in tissue culture from the genital/rectal mucosa at times when no characteristic signs of clinical genital herpes are present; sometimes called subclinical infection.
Clinical herpes
The presence of genital ulcerations, fissures and/or vesicles, usually in association with pain, itching, and/or dysuria. The symptoms may vary with location of the lesion and are also called “symptomatic episodes.”
Mucosal shedding
Detection of HSV-2 on a mucosal surface by either culture or DNA PCR, resulting fromHSV-2 release from neurons followed by replication in the genital epithelium.
Recurrences/Symptomatic episodes
Any instance of clinical herpes after the first episode following virus acquisition. An episode of clinically recognized herpes usually lasts from 1 to 7 days, and is often associated with multiple days in which HSV is isolated from mucosal or skin swabs/samples.
Viral latency
Anytime post-acquisition that virus remains in a dormant, reversible non-replicative state in the ganglia.
Viral leak
Release of virus down axons from neuronal cell bodies within the ganglia to the dermalepidermal junction in the genital skin/mucosa; the antecedent process that results in subsequent infection of adjacent epithelial cells.
Viral reactivation
HSV-2 DNA replication and production of virions within ganglia representing a break from viral latency.
Acknowledgements
We are grateful to Dr. Mindy Miner for her outstanding contribution in the editorial preparation of the manuscript.
References
- 1.Whitley RJ, Lakeman F. Herpes simplex virus infections of the central nervous system: therapeutic and diagnostic considerations. Clin Infect Dis. 1995;20:414–420. doi: 10.1093/clinids/20.2.414. [DOI] [PubMed] [Google Scholar]
- 2.Flewett TH, Parker RG, Philip WM. Acute hepatitis due to Herpes simplex virus in an adult. J Clin Pathol. 1969;22:60–66. doi: 10.1136/jcp.22.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hull HF, Blumhagen JD, Benjamin D, Corey L. Herpes simplex viral pneumonitis in childhood. J Pediatr. 1984;104:211–215. doi: 10.1016/s0022-3476(84)80994-4. [DOI] [PubMed] [Google Scholar]
- 4.Brown Z, et al. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med. 1987;317:1246–1251. doi: 10.1056/NEJM198711123172002. [DOI] [PubMed] [Google Scholar]
- 5.Brown EL, et al. Effect of maternal herpes simplex virus (HSV) serostatus and HSV type on risk of neonatal herpes. Acta Obstet Gynecol Scand. 2007;86:523–529. doi: 10.1080/00016340601151949. [DOI] [PubMed] [Google Scholar]
- 6.Whitley R, et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med. 1991;324:450–454. doi: 10.1056/NEJM199102143240704. [DOI] [PubMed] [Google Scholar]
- 7.Okuku HS, et al. Factors associated with herpes simplex virus type 2 incidence in a cohort of human immunodeficiency virus type 1-seronegative Kenyan men and women reporting high-risk sexual behavior. Sex Transm Dis. 2011;38:837–844. doi: 10.1097/OLQ.0b013e31821a6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobian AA, et al. Factors associated with the prevalence and incidence of herpes simplex virus type 2 infection among men in Rakai, Uganda. J Infect Dis. 2009;199:945–949. doi: 10.1086/597074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobngwi-Tambekou J, et al. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis. 2009;199:958–964. doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobian AA, et al. Male circumcision and herpes simplex virus type 2 infection in female partners: a randomized trial in rakai, Uganda. J Infect Dis. 2012;205:486–490. doi: 10.1093/infdis/jir767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86:805–812. A. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corey L, Wald A. Genital Herpes. In: Holmes KK, et al., editors. Sexually Transmitted Diseases. McGraw-Hill Inc.; New York: 1999. pp. 285–312. Chp. 21. [Google Scholar]
- 13.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A–35A. [PubMed] [Google Scholar]
- 14.Serwadda D, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003;188:1492–1497. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 15.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 16.Freeman E, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 17.Freeman E, et al. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect. 2007;83(Suppl 1):i17–24. doi: 10.1136/sti.2006.023549. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Raddad L, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS ONE. 2008;3:e2230. doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wald A, et al. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacks SL, et al. Introduction: Is viral shedding a surrogate marker for transmission of genital herpes? Antiviral Res. 2004;63(Suppl 1):S3–9. doi: 10.1016/j.antiviral.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N Engl J Med. 1999;341:1432–1438. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- 22.Tronstein E, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. Jama. 2011;305:1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu F, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 24.Schiffer JT, Wald A, Selke S, Corey L, Magaret A. The kinetics of mucosal herpes simplex virus-2 infection in humans: evidence for rapid viral-host interactions. J Infect Dis. 2011;204:554–561. doi: 10.1093/infdis/jir314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phipps W, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis. 2011;203:180–187. doi: 10.1093/infdis/jiq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elion GB, et al. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977;74:5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiffer JT, Magaret A, Selke S, Corey L, Wald A. Detailed analysis of mucosal herpes simplex virus-2 replication kinetics with and without antiviral therapy. J Antimicrob Chemother. 2011;66:2593–2600. doi: 10.1093/jac/dkr346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beutner KR. Valacyclovir: a review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antiviral Res. 1995;28:281–290. doi: 10.1016/0166-3542(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 29.Cirelli R, Herne K, McCrary M, Lee P, Tyring SK. Famciclovir: review of clinical efficacy and safety. Antiviral Res. 1996;29:141–151. doi: 10.1016/0166-3542(95)00941-8. [DOI] [PubMed] [Google Scholar]
- 30.Johnston C, et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet. 2012 doi: 10.1016/S0140-6736(11)61750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corey L, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 32.Reyes M, et al. Acyclovir-resistant genital herpes among persons attending sexually transmitted disease and human immunodeficiency virus clinics. Arch Intern Med. 2003;163:76–80. doi: 10.1001/archinte.163.1.76. [DOI] [PubMed] [Google Scholar]
- 33.Belshe RB, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2102;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen J. Immunology. Painful failure of promising genital herpes vaccine. Science. 2010;330:304. doi: 10.1126/science.330.6002.304. [DOI] [PubMed] [Google Scholar]
- 35.Wald A, et al. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA. 2001;285:3100–3106. doi: 10.1001/jama.285.24.3100. [DOI] [PubMed] [Google Scholar]
- 36.Wald A, et al. Knowledge of partners' genital herpes protects against herpes simplex virus type 2 acquisition. J Infect Dis. 2006;194:42–52. doi: 10.1086/504717. [DOI] [PubMed] [Google Scholar]
- 37.Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Intern Med. 1992;116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 38.Mertz GJ, et al. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex Transm Dis. 1985;12:33–39. doi: 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541–553. doi: 10.1086/514600. quiz 554-545. [DOI] [PubMed] [Google Scholar]
- 40.Roizman B, K.D. Herpes simplex viruses and their replication. In: Knipe DM, H.P., Griffin DE, et al., editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- 41.Koelle D, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 42.Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 43.McGraw HM, Friedman HM. Herpes simplex virus type 1 glycoprotein E mediates retrograde spread from epithelial cells to neurites. J Virol. 2009;83:4791–4799. doi: 10.1128/JVI.02341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGraw HM, Awasthi S, Wojcechowskyj JA, Friedman HM. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not Us9. J Virol. 2009;83:8315–8326. doi: 10.1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiffer JT, et al. Frequent Release of Low Amounts of Herpes Simplex Virus From Neurons: Results of a Mathematical Model. Sci Transl Med. 2009;1:7ra16. doi: 10.1126/scitranslmed.3000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mark KE, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198:1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crespi C, Cumberland W, Wald A, Corey L, Blower S. Longitudinal study of herpes simplex virus type 2 infection using viral dynamic modelling. Sex Transm Infect. 2007;83:359–364. doi: 10.1136/sti.2006.022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wald A, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 49.Magaret A, Wald A, Huang M, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45:1618–1620. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanberry LR, Kern ER, Richards JT, Overall JC., Jr. Recurrent genital herpes simplex virus infection in guinea pigs. Intervirology. 1985;24:226–231. doi: 10.1159/000149647. [DOI] [PubMed] [Google Scholar]
- 52.Scriba M. Recurrent genital Herpes simplex virus (HSV) infection of guinea pigs. Med Microbiol Immunol. 1976;162:201–208. doi: 10.1007/BF02120998. [DOI] [PubMed] [Google Scholar]
- 53.Bertke AS, Patel A, Krause PR. Herpes simplex virus latency-associated transcript sequence downstream of the promoter influences type-specific reactivation and viral neurotropism. J Virol. 2007;81:6605–6613. doi: 10.1128/JVI.02701-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang K, Lau T, Morales M, Mont E, Straus S. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal Ganglia at the single-cell level. J Virol. 2005;79:14079–14087. doi: 10.1128/JVI.79.22.14079-14087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohashi M, Bertke AS, Patel A, Krause PR. Spread of herpes simplex virus to the spinal cord is independent of spread to dorsal root ganglia. J Virol. 2011;85:3030–3032. doi: 10.1128/JVI.02426-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens JG, C.M. Latent herpes simplex virus in spinal ganglia. Science. 1971;173:843. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- 57.Snyder A, Polcicova K, Johnson DC. Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons. J Virol. 2008;82:10613–10624. doi: 10.1128/JVI.01241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens JG, Haarr L, Porter DD, Cook ML, Wagner EK. Prominence of the herpes simplex virus latency-associated transcript in trigeminal ganglia from seropositive humans. J Infect Dis. 1988;158:117–123. doi: 10.1093/infdis/158.1.117. [DOI] [PubMed] [Google Scholar]
- 59.Chen X, Mata M, Kelley M, Glorioso J, Fink D. The relationship of herpes simplex virus latency associated transcript expression to genome copy number: a quantitative study using laser capture microdissection. J Neurovirol. 2002;8:204–210. doi: 10.1080/13550280290049642. [DOI] [PubMed] [Google Scholar]
- 60.Feldman LT, et al. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A. 2002;99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu T, Khanna K, Chen X, Fink D, Hendricks R. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Decman V, Kinchington PR, Harvey SA, Hendricks RL. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J Virol. 2005;79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perng GC, et al. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 64.Jiang X, et al. The herpes simplex virus type 1 latency-associated transcript can protect neuron-derived C1300 and Neuro2A cells from granzyme B-induced apoptosis and CD8 T-cell killing. J Virol. 2011;85:2325–2332. doi: 10.1128/JVI.01791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Umbach JL, et al. Identification of viral microRNAs expressed in human sacral ganglia latently infected with herpes simplex virus 2. J Virol. 2010;84:1189–1192. doi: 10.1128/JVI.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Umbach JL, et al. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454:780–783. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol. 2008;6:211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 68.Verjans GM, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murray JM, Wieland SF, Purcell RH, Chisari FV. Dynamics of hepatitis B virus clearance in chimpanzees. Proc Natl Acad Sci U S A. 2005;102:17780–17785. doi: 10.1073/pnas.0508913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frank GM, et al. Early CD4(+) T cell help prevents partial CD8(+) T cell exhaustion and promotes maintenance of Herpes Simplex Virus 1 latency. J Immunol. 2010;184:277–286. doi: 10.4049/jimmunol.0902373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu T, Khanna K, Carriere B, Hendricks R. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knickelbein JE, et al. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Divito S, Cherpes TL, Hendricks RL. A triple entente: virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol Res. 2006;36:119–126. doi: 10.1385/IR:36:1:119. [DOI] [PubMed] [Google Scholar]
- 74.Hoshino Y, Qin J, Follmann D, Cohen JI, Straus SE. The number of herpes simplex virus-infected neurons and the number of viral genome copies per neuron correlate with the latent viral load in ganglia. Virology. 2008;372:56–63. doi: 10.1016/j.virol.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoshino Y, Pesnicak L, Cohen J, Straus S. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol. 2007;81:8157–8164. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill JM, et al. Quantitation of herpes simplex virus type 1 DNA and latency-associated transcripts in rabbit trigeminal ganglia demonstrates a stable reservoir of viral nucleic acids during latency. J Virol. 1996;70:3137–3141. doi: 10.1128/jvi.70.5.3137-3141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoshino Y, et al. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J Virol. 2005;79:410–418. doi: 10.1128/JVI.79.1.410-418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stanberry LR, Kern ER, Richards JT, Abbott TM, Overall JC., Jr. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J Infect Dis. 1982;146:397–404. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- 79.Bourne N, et al. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis. 2003;187:542–549. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 80.Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB. Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. J Infect Dis. 2005;192:2117–2123. doi: 10.1086/498247. [DOI] [PubMed] [Google Scholar]
- 81.Leoni V, Gianni T, Salvioli S, Campadelli-Fiume G. Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-kappaB. J Virol. 2012;86:6555–6562. doi: 10.1128/JVI.00295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hargett D, Rice S, Bachenheimer SL. Herpes simplex virus type 1 ICP27-dependent activation of NF-kappaB. J Virol. 2006;80:10565–10578. doi: 10.1128/JVI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niehues T, et al. Nuclear factor kappaB essential modulator-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:1456–1462. doi: 10.1016/j.jaci.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 84.Guo Y, et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med. 2011;208:2083–2098. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sancho-Shimizu V, et al. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J Clin Invest. 2011;121:4889–4902. doi: 10.1172/JCI59259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 88.Casrouge A, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 89.Sancho-Shimizu V, et al. Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr Opin Allergy Clin Immunol. 2007;7:495–505. doi: 10.1097/ACI.0b013e3282f151d2. [DOI] [PubMed] [Google Scholar]
- 90.Mikloska Z, et al. In vivo production of cytokines and beta (C-C) chemokines in human recurrent herpes simplex lesions--do herpes simplex virus-infected keratinocytes contribute to their production? J Infect Dis. 1998;177:827–838. doi: 10.1086/515236. [DOI] [PubMed] [Google Scholar]
- 91.Overall JC, Jr., Spruance SL, Green JA. Viral-induced leukocyte interferon in vesicle fluid from lesions of recurrent herpes labialis. J Infect Dis. 1981;143:543–547. doi: 10.1093/infdis/143.4.543. [DOI] [PubMed] [Google Scholar]
- 92.Torseth JW, Nickoloff BJ, Basham TY, Merigan TC. Beta interferon produced by keratinocytes in human cutaneous infection with herpes simplex virus. J Infect Dis. 1987;155:641–648. doi: 10.1093/infdis/155.4.641. [DOI] [PubMed] [Google Scholar]
- 93.Mikloska Z, Cunningham AL. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J Virol. 2001;75:11821–11826. doi: 10.1128/JVI.75.23.11821-11826.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng T, et al. Evasion of the mucosal innate immune system by herpes simplex virus type 2. J Virol. 2009;83:12559–12568. doi: 10.1128/JVI.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dupuis S, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 96.Otani T, Mori R. The effects of ultraviolet irradiation of the skin on herpes simplex virus infection: alteration in immune function mediated by epidermal cells and in the course of infection. Arch Virol. 1987;96:1–15. doi: 10.1007/BF01310986. [DOI] [PubMed] [Google Scholar]
- 97.Kaneko K, et al. cis-Urocanic acid initiates gene transcription in primary human keratinocytes. J Immunol. 2008;181:217–224. doi: 10.4049/jimmunol.181.1.217. [DOI] [PubMed] [Google Scholar]
- 98.Garssen J, van der Molen R, de Klerk A, Norval M, van Loveren H. Effects of UV irradiation on skin and nonskin-associated herpes simplex virus infections in rats. Photochem Photobiol. 2000;72:645–651. doi: 10.1562/0031-8655(2000)072<0645:eouios>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 99.Mark K, et al. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J Infect Dis. 2007;195:1324–1331. doi: 10.1086/513276. [DOI] [PubMed] [Google Scholar]
- 100.Cunningham AL, Turner RR, Miller AC, Para MF, Merigan TC. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J Clin Invest. 1985;75:226–233. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Donaghy H, et al. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol. 2009;83:1952–1961. doi: 10.1128/JVI.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koelle DM, Huang J, Hensel MT, McClurkan CL. Innate immune responses to herpes simplex virus type 2 influence skin homing molecule expression by memory CD4+ lymphocytes. J Virol. 2006;80:2863–2872. doi: 10.1128/JVI.80.6.2863-2872.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gonzalez JC, et al. Expression of cutaneous lymphocyte-associated antigen and E-selectin ligand by circulating human memory CD4+ T lymphocytes specific for herpes simplex virus type 2. J Infect Dis. 2005;191:243–254. doi: 10.1086/426944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoneyama H, et al. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J Exp Med. 2005;202:425–435. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dalloul A, et al. Severe herpes virus (HSV-2) infection in two patients with myelodysplasia and undetectable NK cells and plasmacytoid dendritic cells in the blood. J Clin Virol. 2004;30:329–336. doi: 10.1016/j.jcv.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 106.Abbo L, et al. Selective defect in plasmacyoid dendritic cell function in a patient with AIDS-associated atypical genital herpes simplex vegetans treated with imiquimod. Clin Infect Dis. 2007;44:e25–27. doi: 10.1086/510426. [DOI] [PubMed] [Google Scholar]
- 107.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 108.Bosnjak L, et al. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–2227. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 109.Mueller SN, Jones CM, Smith CM, Heath WR, Carbone FR. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J Exp Med. 2002;195:651–656. doi: 10.1084/jem.20012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Allan RS, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 111.Allan RS, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 112.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 113.Puttur FK, et al. Herpes simplex virus infects skin gamma delta T cells before Langerhans cells and impedes migration of infected Langerhans cells by inducing apoptosis and blocking E-cadherin downregulation. J Immunol. 2010;185:477–487. doi: 10.4049/jimmunol.0904106. [DOI] [PubMed] [Google Scholar]
- 114.Lee HK, et al. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med. 2009;206:359–370. doi: 10.1084/jem.20080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 116.Cohen GH, et al. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984;49:102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown ZA, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247–1252. doi: 10.1056/NEJM199105023241804. [DOI] [PubMed] [Google Scholar]
- 118.Bourne N, Pyles RB, Bernstein DI, Stanberry LR. Modification of primary and recurrent genital herpes in guinea pigs by passive immunization. J Gen Virol. 2002;83:2797–2801. doi: 10.1099/0022-1317-83-11-2797. [DOI] [PubMed] [Google Scholar]
- 119.Mikloska Z, Sanna P, Cunningham A. Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro. J Virol. 1999;73:5934–5944. doi: 10.1128/jvi.73.7.5934-5944.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Z, et al. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kwant-Mitchell A, Ashkar AA, Rosenthal KL. Mucosal innate and adaptive immune responses against herpes simplex virus type 2 in a humanized mouse model. J Virol. 2009;83:10664–10676. doi: 10.1128/JVI.02584-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Awasthi S, et al. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J Virol. 2011;85:10472–10486. doi: 10.1128/JVI.00849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stanberry LR, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 124.Corey L, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. Jama. 1999;282:331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 125.Lubinski JM, et al. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J Virol. 2002;76:9232–9241. doi: 10.1128/JVI.76.18.9232-9241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lubinski J, et al. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J Exp Med. 1999;190:1637–1646. doi: 10.1084/jem.190.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Collins WJ, Johnson DC. Herpes simplex virus gE/gI expressed in epithelial cells interferes with cell-to-cell spread. J Virol. 2003;77:2686–2695. doi: 10.1128/JVI.77.4.2686-2695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu J, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 130.Iijima N, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wakim LM, Jones CM, Gebhardt T, Preston CM, Carbone FR. CD8(+) T-cell attenuation of cutaneous herpes simplex virus infection reduces the average viral copy number of the ensuing latent infection. Immunol Cell Biol. 2008;86:666–675. doi: 10.1038/icb.2008.47. [DOI] [PubMed] [Google Scholar]
- 132.Mackay LK, et al. Maintenance of T cell function in the face of chronic antigen stimulation and repeated reactivation for a latent virus infection. J Immunol. 2012;188:2173–2178. doi: 10.4049/jimmunol.1102719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koelle DM, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J Infect Dis. 1994;169:956–961. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 134.Koelle DM, et al. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol. 2001;166:4049–4058. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 135.Koelle DM, et al. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peng T, et al. An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of HSV-2 infection. J Virol. 2012 doi: 10.1128/JVI.01237-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009 doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koelle DM, et al. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J Clin Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stock AT, Jones CM, Heath WR, Carbone FR. Rapid recruitment and activation of CD8+ T cells after herpes simplex virus type 1 skin infection. Immunol Cell Biol. 2011;89:143–148. doi: 10.1038/icb.2010.66. [DOI] [PubMed] [Google Scholar]
- 140.Posavad C, Koelle D, Shaughnessy M, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci U S A. 1997;94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mueller SN, et al. The early expression of glycoprotein B from herpes simplex virus can be detected by antigen-specific CD8+ T cells. J Virol. 2003;77:2445–2451. doi: 10.1128/JVI.77.4.2445-2451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Snyder CM, et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 144.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Polcicova K, Goldsmith K, Rainish BL, Wisner TW, Johnson DC. The extracellular domain of herpes simplex virus gE is indispensable for efficient cell-to-cell spread: evidence for gE/gI receptors. J Virol. 2005;79:11990–12001. doi: 10.1128/JVI.79.18.11990-12001.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schiffer JT, et al. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1006614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aubert M, Yoon M, Sloan DD, Spear PG, Jerome KR. The virological synapse facilitates herpes simplex virus entry into T cells. J Virol. 2009;83:6171–6183. doi: 10.1128/JVI.02163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sloan DD, et al. Inhibition of TCR signaling by herpes simplex virus. J Immunol. 2006;176:1825–1833. doi: 10.4049/jimmunol.176.3.1825. [DOI] [PubMed] [Google Scholar]
- 149.Tata S, et al. Overlapping reactivations of herpes simplex virus type 2 in the genital and perianal mucosa. J Infect Dis. 2010;201:499–504. doi: 10.1086/650302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alsallaq RA, et al. Population Level Impact of an Imperfect Prophylactic Vaccine for Herpes Simplex Virus-2. Sexually Transmitted Diseases. 2010;37:290–297. doi: 10.1097/OLQ.0b013e3181d3d023. [DOI] [PMC free article] [PubMed] [Google Scholar]