Abstract
The organization of the eukaryotic genome into chromatin enables DNA to fit inside the nucleus while also regulating the access of proteins to the DNA to facilitate genomic functions such as transcription, replication and repair. The basic repeating unit of chromatin is the nucleosome, which includes 147 bp of DNA wrapped 1.65 times around an octamer of core histone proteins comprising two molecules each of H2A, H2B, H3 and H4 [1]. Each nucleosome is a highly stable unit, being maintained by over 120 direct protein–DNA interactions and several hundred water mediated ones [1]. Accordingly, there is considerable interest in understanding how processive enzymes such as RNA polymerases manage to pass along the coding regions of our genes that are tightly packaged into arrays of nucleosomes. Here we present the current mechanistic understanding of this process and the evidence for profound changes in chromatin dynamics during aging. This article is part of a Special Issue entitled: Histone chaperones and Chromatin assembly.
Keywords: Histone chaperone, Histone modification, Aging, Lifespan extension, Chromatin, Transcription
1. Introduction
The vast majority of eukaryotic genes are transcribed by RNA polymerase II (Pol II). During transcription, the DNA template is extensively distorted as it passes through the narrow active center cleft of Pol II [2,3]. Given the intimate interaction between the DNA and Pol II during RNA synthesis, it is inconceivable that transcription can occur in the absence of drastic disruptions to the nucleosome. Indeed, in vitro transcription experiments with nucleosomal templates revealed that Pol II cannot traverse through nucleosomes [4]. As a consequence, nature has evolved additional cellular activities in order to disrupt the chromatin structure to facilitate Pol II passage. These cellular activities include ATP-dependent nucleosome remodelers, which use the energy of ATP hydrolysis to break histone–DNA interactions. ATP-dependent nucleosome remodelers allow the DNA to “inch-worm” around the histone octamer. However, this is extremely energy demanding. For example, movement of the histone octamer along the DNA by ATP-dependent nucleosome remodelers requires the hydrolysis of 2–4 ATP molecules per base pair moved [5]. It is now well appreciated that the action of ATP-dependent nucleosome remodelers is facilitated by a family of proteins collectively termed histone chaperones, which appear to “collect” the histones after the histone–DNA interactions have been broken by ATP-dependent nucleosome remodelers. By acting together, the ATP-dependent nucleosome remodelers and histone chaperones facilitate the removal of histones from the DNA and their assembly onto the DNA. Here we review the large body of evidence for the dynamic eviction of histones from DNA in our cells and the subsequent return of histones to the DNA, collectively termed “histone exchange”, that occurs independent of DNA replication. We will present the current mechanistic understanding of how ATP-dependent chromatin remodelers, histone chaperones and specific histone post-translational modifications function together to promote histone exchange during transcriptional elongation by eukaryotic RNA polymerase II. We will cover the distinct mechanisms that are used to either promote histone exchange or polymerase passage through the nucleosome, depending on the gene's rate of transcription. Highlighting the importance of histone exchange, we will discuss the functional outcome of disrupting this process experimentally and the growing evidence for a decline in these chromatin dynamics with increased organismal age.
2. The logistics of histone exchange
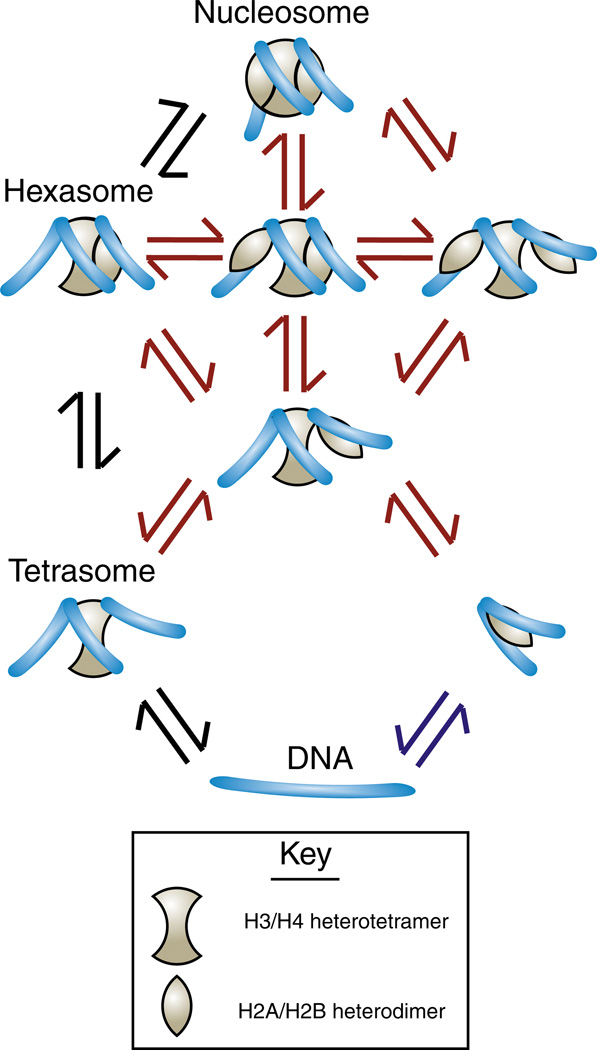
A full understanding of the mechanism of histone exchange requires an appreciation for how the histones are organized within the nucleosome structure [1]. The central core of the nucleosome is a heterotetramer of H3/H4 that is maintained by dimerization of two H3/H4 heterodimers via H3–H3 interactions. The H3/H4 heterotetramer is flanked on either side by a dimer of H2A/H2B (Fig. 1). Although the intact histone octamer can be transferred from one DNA molecule to another in vitro using anionic molecules that transiently neutralize the highly basic histone proteins, this is not how nucleosomes are generally disassembled from the DNA or assembled onto the DNA in the cell. Consistent with the central location of the H3/H4 heterotetramer within the nucleosome, histones H3/H4 are deposited onto the DNA before H2A/H2B during nucleosome formation in our cells (Fig. 1). Subsequently, the H2A/H2B dimers are incorporated on either side of the H3/H4 heterotetramer via protein–protein interactions between the C-terminal portion of each H4 molecule and each H2B molecule. This nucleosome assembly process also involves the wrapping of the DNA around the histone octamer, where the H3/H4 tetramer directs the wrapping of the central turn of the DNA and each H2A/H2B dimer directs the wrapping of the peripheral half-turns of the DNA as it enters and exits the nucleosome (Fig. 1).
Fig. 1.
Stepwise chromatin assembly and disassembly. The black arrows indicate established reversible steps involved in chromatin assembly and disassembly, where H3/H4 is deposited onto the DNA first and removed from the DNA last. The presence of cellular factors that are not shown, such as histone chaperones, histone modifications, ATP-dependent nucleosome remodelers and polymerases, tip the equilibria in one direction or the other. H2A/H2B can also be deposited onto the DNA before H3/H4, although histone chaperones such as NAP1 function to remove non-functional H2A/H2B–DNA complexes in our cells as indicated by the blue arrows [103]. The red arrows indicate more speculative steps in chromatin assembly/disassembly. For example, it is formally possible that an H3/H4 tetramer may join the H2A/H2B dimer–DNA complex to form a hexasome, although this has not been shown. Other putative chromatin assembly and disassembly intermediates are shown where the interaction(s) between the H2A/H2B dimer and DNA exist, while the weaker interactions between the H2A/H2B dimer and H3/H4 tetramer have been released or not yet established. Such intermediates have been observed recently in vitro [6].
Chromatin disassembly in our cells occurs via a stepwise process that is the opposite of chromatin assembly (Fig. 1). The peripherally located histone H2A/H2B dimers are first removed from the nucleosome, followed by removal of H3/H4 from the DNA. Chromatin disassembly of H2A/H2B appears to first involve release of all th e interactions between each H2A/H2B dimer and H3/H4 tetramer, followed by release of the H2A/H2B–DNA interactions, at least in vitro (Fig. 1) [6]. Histone exchange of H3/H4 presumably necessitates eviction of all four core histones from the DNA followed by complete reassembly of the nucleosome, while histone exchange of H2A/H2B would only require the eviction of the peripheral H2A/H2B dimer(s) followed by the reassembly of H2A/H2B. This structural insight will hopefully provide the reader with a framework for a better mechanistic understanding of the process of histone exchange.
3. Evidence for transcription-dependent incorporation of new histones
Much of the experimental evidence for histone exchange has relied on the fortuitous fact that at least some of the histones that return to the DNA during histone exchange are not the same histones that left the DNA in the first place. This has enabled the development of approaches to follow transiently labeled new histones or epitope-tagged newly induced histones, in order to provide evidence for histone exchange. In all the studies of histone exchange to date, it has been critical to either use DNA replication inhibitors or cells in the appropriate cell cycle stage, to avoid the abundant chromatin disassembly and assembly that accompanies DNA replication (reviewed by Zhang et al. in this volume).
It is important for both veterans and newcomers to the field to be reminded of the ingenious studies of Vaughn Jackson and colleagues in the 1980s. Several of the “high impact” mechanistic questions that have been re-raised in the chromatin field in recent years were answered by the Jackson lab decades ago, using density labeling experiments to follow the assembly of newly synthesized histones into octamers in human cells. These ground breaking studies included the unequivocal demonstration that seemingly all canonical parental H3/H4 tetramers remain intact during DNA replication rather than being split into H3/H4 dimers [7]. More relevant to the subject of this review, Jackson provided clear evidence for histone exchange of not only H2A/H2B but also H3/H4 during transcription [8]. Specifically, upon inhibition of DNA replication the extent of incorporation of new H3/H4 into the genome was reduced approximately 10 fold, while inhibition of both DNA polymerase and RNA polymerase passage resulted in a further 2 fold reduction in incorporation of new H3/H4 onto the human genome (Fig. 2) [8]. These results demonstrated that the entire nucleosome is at least sometimes disassembled and reassembled during the process of RNA polymerase passage. Furthermore, the ~5% of H3/H4 histone exchange that occurred independent of both transcription and DNA replication demonstrated that polymerase passage is not the sole driving force for histone exchange (Fig. 2). Rather, nucleosomes within the cell should be viewed as dynamic structures that are in a constant equilibrium between a state of disassembly and reassembly. Polymerase passage presumably directly or indirectly increases the probability for tipping the histone exchange equilibrium transiently towards histone eviction. The question of whether H3/H4 tetramers remain intact during transcription has also been raised in the last few years, confirming Jackson's careful pulse chase experiments of density labeled histones which showed transcription-specific splitting of H3/H4 tetramers into H3/H4 dimers and even into occasional monomers (Fig. 2) [8]. We speculate that the mixing of old and new core histone monomers during transcription may promote the re-establishment of the original pattern of post-translational histone modifications onto the newly incorporated histones via trans-modification pathways following Pol II passage.
Fig. 2.
Summary of the results of Jackson's analysis of transcription dependent histone exchange [8]. Schematic representation of the form of new histones (H2A/H2B dimers, H3/H4 heterotetramers, H3/H4 dimers or histone monomers — all shown in red by different shapes) that are assembled into human chromatin in the absence or presence of inhibitors of transcription and/or DNA replication. The % numbers indicate the proportion of new histone incorporation, where 100% represents the extent of new histone incorporation when neither replication nor transcription was inhibited. Histone dimer shapes with lines down the middle and of mixed colors indicate monomer exchange that was detected upon the chase, as discussed in the text.
Exchange of H2A/H2B dimers during transcription occurs more globally and more rapidly than H3/H4 exchange [8], which is not too surprising given their peripheral location and weaker interaction with the DNA (Fig. 2). This observation has also been made in other systems. For example, this was observed during chromatin immunoprecipitation (ChIP) analyses following the addition of exogenous epitope-tagged histone proteins into plasmodial slime molds where all nuclei were naturally synchronized in G2 phase, away from the interference of replication-dependent chromatin assembly [9]. Noteworthy, H3/H4 exchange was more apparent in highly transcribed genes as compared to less transcribed genes. The addition of the Pol II inhibitor α-amanitin greatly diminished the histone exchange, indicating that it was a consequence of transcriptional elongation. Related approaches have been used in budding yeast cells synchronized outside of S phase. These studies used galactose-inducible promoters to produce new epitope-tagged histones in G1 phase of the cell cycle and directly compared their incorporation onto the genome to that of endogenous histones tagged with a distinct epitope, referred to here as dual epitope histone labeling. While incorporation of new H2B occurred irrespective of whether genes were active or inactive, H3 was mainly incorporated into transcriptionally active genes [10]. The extension of these studies using tiling arrays covering the yeast genome revealed that replication-independent exchange of histone H3 is a common feature of the coding regions of highly transcribed genes as well as promoter regions [11].
Consistent with the idea that RNA polymerase passage stimulates histone eviction, histone H3 replacement rates within coding regions correlate nicely with Pol II density [10,11]. High levels of new histone incorporation outside of S phase occur at regions of high Pol II occupancy, while low levels of new histone incorporation occur at regions of low Pol II density. Dual epitope histone labeling approaches have also been used in sequential ChIP analyses to reveal the existence of dually labeled nucleosomes [12]. Dually labeled nucleosomes presumably represent the product of splitting the H3/H4 heterotetramer followed by mixing a new H3/H4 dimer with an old H3/H4 dimer. Consistent with the earlier studies by Jackson [8], these split tetramers were only detectable during transcription and only at highly transcribed genes [12]. This suggests that the mechanism of chromatin assembly following Pol II passage is distinct from that used following DNA replication, where the canonical H3/H4 tetramers are never split [13].
One potential caveat of all the yeast studies using newly induced epitope-tagged histones is that these histones are strongly induced from a promoter that is active throughout the cell cycle, while endogenous histone H3 is expressed only during S-phase. As such, it is possible that rates of histone exchange and the extent of H3/H4 tetramer splitting are altered by having an excess of the epitope-tagged free histones present at times in the cell cycle during which free histones are not normally abundant. Notwithstanding, these studies demonstrate the relative potential for histone exchange at different regions of the genome and they nicely complement other approaches using labeled endogenous histones or fluorescence analyses to examine the dynamics of relatively low levels of histones that are usually expressed from the endogenous promoters. One such newly developed system uses CRE recombinase-induced tag exchange to switch epitope tags on the endogenous histone proteins, achieving differential labeling of new and old histones [14]. This approach was used to show replication-independent histone exchange of endogenous histones in yeast and should be valuable for accurate quantitation of histone exchange in vivo.
Fluorescence recovery after photobleaching (FRAP) studies in mammalian cells have provided key insights into the proportion of the cell's chromatin that is available for histone exchange as well as the kinetics of histone exchange. Stable HeLa cell lines expressing H2B-GFP, H3-GFP or H4-GFP from a constitutive promoter were used to follow histone exchange [15]. Because the GFP-tagged histones were expressed at a low level compared to the flood of endogenous canonical histone proteins that are expressed and incorporated onto the DNA exclusively during S phase, the genomic distribution of the GFP-tagged histone that is expressed throughout the cell cycle most likely reflects replication-independent histone exchange. H2B-GFP yielded a distribution similar to that of DNA suggesting that replication-independent histone H2A/H2B dimer exchange occurs throughout the genome [15]. By contrast, H3-GFP and H4-GFP resided more in euchromatic regions consistent with transcription-dependent histone exchange. Upon photobleaching, the recovery of fluorescence requires the exchange of the bleached GFP-tagged histone with a new GFP-tagged histone. By this approach, it was apparent that the exchange of H2B-GFP is faster than exchange of H3-GFP or H4-GFP. About 3% of the genomic H2B exchanged within minutes and this population disappeared upon inhibition of transcription, consistent with transcription-dependent H2A/H2B dimer exchange. Another 40% of the H2B and 15% of the H3 exchanged slowly, with a half-life of DNA occupancy of around 130 min. Noteworthy, this length of time is significantly shorter than the cell cycle and indicates that extensive histone exchange occurs in mammalian cells independent of DNA replication.
Studies of histone variants in Drosophila revealed that a histone H3 variant, termed H3.3, is often used to replace the evicted histones during transcription [16]. The use of a heat-shock inducible promoter to drive expression of the GFP-tagged histones enabled the new histones to be followed specifically. Transcription-dependent incorporation of H3.3 into the genome is not merely due to the fact that, unlike canonical H3.1, it is expressed throughout the cell cycle. Drosophila H3.1 and H3.3 only differ by 4 amino acids (5 amino acids differ between the human proteins) and three of these amino acids differing between H3.1 and H3.3 specify the use of either the replication-dependent histone chaperone CAF-1 or the replication-independent histone chaperone HIRA [16,17]. Phosphorylation on histone H4 serine 47 (H4 S47p) also helps specify which histone chaperone binds to which histone variant, where H4 S47p promotes the interaction of HIRA with H3.3/H4 but reduces the interaction of CAF-1 with H3.1/H4 [18]. The existence of a histone variant in metazoans that is preferentially incorporated during transcription-dependent histone exchange provides a powerful tool for the study of global histone exchange independent of DNA replication [17,19].
The relative rates of histone exchange between open reading frames (ORFs) and promoters differ between yeast and metazoans. In yeast, promoters have very high rates of histone exchange while most transcribed genes have low rates of histone exchange, with the exception of highly transcribed genes. However, in Drosophila, ORFs have the highest histone exchange rates and these are proportional to transcription rates, while promoters have little histone exchange. The ingenious approach used for the Drosophila histone exchange studies, termed CATCH-IT, relies on a brief metabolic labeling of native histones with a methionine analog which is then covalently attached to biotin [20]. The chromatin is digested to mononucleosomes and captured on strepdavidin affinity beads, followed by DNA sequence analysis. This CATCH-IT approach should be very powerful for mapping nucleosome exchange rates, based on the extent of newly synthesized H3/H4 incorporation, over mammalian genomes, in different cell types and during stem cell differentiation.
All the studies described so far provided evidence for histone exchange based on the observation of newly synthesized histones being incorporated into the genome outside of DNA replication. Although these studies clearly showed the occurrence of histone exchange and the proportion of the genome from which histone exchange occurs, they did not reveal whether the DNA ever exists in a histone-depleted form in between histone eviction and histone replacement. Instead, the profound extent of histone eviction during RNA polymerase passage was made apparent by ChIP analyses of steady state bulk histone occupancy where more than 50% histone depletion occurs in highly transcribed yeast ORFs. Within the ORFs of the galactose-inducible yeast genes for example, the density of histones inversely correlates with Pol II occupancy, and this histone eviction is dependent on transcriptional elongation [21–23]. Using careful time course analyses of the first wave of Pol II passage upon gene induction, it is clear that histone eviction temporally follows RNA polymerase entry into the ORF. Conversely, during the last wave of transcription upon glucose induced repression, the histones return to the DNA within 1 min of Pol II passage. This depletion of histones from actively transcribed genes is not unique to galactose-inducible genes, as a global microarray study of histone occupancy in yeast identified a partial loss of histones H3 and H4 from the ORFs of the most highly transcribed yeast genes [24]. The depletion of histones from highly transcribed genes presumably reflects a state where the constant dynamic equilibrium of histone eviction and replacement is tipped towards the disassembled state. Indeed histone occupancy per se over a particular region of the genome can be considered to reflect the state of the equilibrium between histone eviction and histone replacement, and therefore indicates the rate of histone exchange at that particular genomic region.
The ultimate evidence for the extent of the transient physical removal of histones from the DNA and their return to the DNA following RNA polymerase passage was provided by the analysis of yeast mutants lacking particular histones chaperones. As discussed below, the absence of the histone chaperones FACT or Spt6 cripples the return of histones to the DNA after RNA polymerase passage. The requirement for reassembly of histones after RNA polymerase passage appears to be quite global because free histones, which are usually barely detectable, accumulate upon inactivation of FACT in a transcription-dependent manner [25]. Taken together, there is unquestionable evidence for the disassembly of nucleosomes during Pol II passage and their subsequent reassembly behind Pol II. Below, we will discuss whether this occurs during the transcription of all genes and the mechanisms that determine whether the outcome of Pol II passage is the transient removal of all four core histones, just H2A/H2B, or no histones at all.
4. What is the mechanism of histone exchange during transcription?
It is clear from the evidence discussed above that H2A/H2B dimer exchange (removal from DNA and replacement with new H2A/H2B) is more prevalent and faster than H3/H4 exchange during transcription. It is not clear whether this is due to preferential return of the original H3/H4 proteins to the DNA rather than their being replaced by new H3/H4, or whether simultaneous breaking of all the DNA–H3/H4 interactions (which is required for histone eviction) occurs less frequently than it does for the H2A/H2B–DNA interactions. In agreement with the observation of more prevalent H2A/H2B exchange during transcription in vivo, in vitro transcription systems have been established in which only one H2A/H2B dimer is lost from a mononucleosome during a single round of Pol II passage [26]. Although these studies used a minimal transcription system lacking the accessory factors present in the cell that modulate the chromatin structure, it is noteworthy that the nucleosome was not translocated along the DNA by Pol II passage, as has been observed for RNA polymerase III passage [27]. Instead, the nucleosome remained in its original position on the DNA following Pol II passage suggesting that the mechanisms used for traversing the nucleosome by these two polymerases are distinct.
How is a single H2A/H2B dimer removed from a nucleosome? Recent studies employing single molecule FRET analysis have identified a new intermediate of the nucleosome disassembly pathway [6]. This altered nucleosome has lost the protein–protein contacts between the H2A/H2B dimer and the H3/H4 tetramer while still maintaining the H2A/H2B–DNA interactions (Fig. 1). Although this analysis was not performed in the context of ongoing transcription or in the presence of chromatin modulating activities such as histone chaperones and ATP-dependent nucleosome remodelers, it indicates that the first step during spontaneous nucleosome disassembly is the disruption of the H3/H4 tetramer–H2A/H2B dimer interaction. This was followed by H2A/H2B dimer release from the DNA [6]. The removal of the H2A/H2B dimer from the nucleosome fits well with the location of the major kinetic barrier to Pol II passage, which is immediately upstream of the high affinity H3/H4 tetramer–DNA interaction within the nucleosome [28].
4.1. The role of histone chaperones in histone exchange
In vitro, the release of the H2A/H2B dimer from a nucleosome during Pol II passage is facilitated by a histone chaperone termed FACT [29]. FACT (discussed in detail in the review by Tim Formosa in this volume) was originally identified biochemically by its ability to promote Pol II transcriptional elongation through a chromosomal template in vitro. Mechanistically, it is intriguing to ask how can a histone chaperone, which is not an enzyme, remove histones from the stable nucleosome structure? For FACT, part of this answer probably lies in its ability to bind to both H3/H4 and H2A/H2B at the same time using different interfaces [29]. FACT may utilize its interaction with H3/H4 to ratchet an H2A/H2B dimer off the nucleosome. In other studies, FACT has been observed to alter the overall structure of the nucleosome without the removal of an H2A/H2B dimer [30]. It is tempting to speculate that this may reflect the intermediate state of the nucleosome prior to H2A/H2B removal where the H2A/H2B dimer–H3/H4 tetramer interactions have been disrupted but all the DNA–histone contacts remain intact (Fig. 1).
In vivo, FACT appears to be more involved in reassembling chromatin following Pol II passage. This is apparent from the observation that inactivation of the FACT component Spt16 in yeast results in loss of histones from the bodies of highly transcribed genes [22,31,32]. Yeast lacking FACT fail to efficiently restore histone occupancy on the GAL10 ORF upon transcriptional repression [22]. Tellingly, the absence of FACT not only caused depletion of H2A/H2B within ORFs, but also caused depletion of H3/H4. As such, FACT is implicated in the return of not just H2A/H2B dimers to the DNA, but also in the return of H3/H4 to the DNA (Fig. 3). Furthermore, it appears that FACT is more involved in returning the displaced H3/H4 histones to the DNA rather than new H3/H4, because inactivation of Spt16 favors the incorporation of new histones onto the genome [33]. This could be explained if FACT were also involved, directly or indirectly, in removal of the H3/H4 from in front of Pol II.
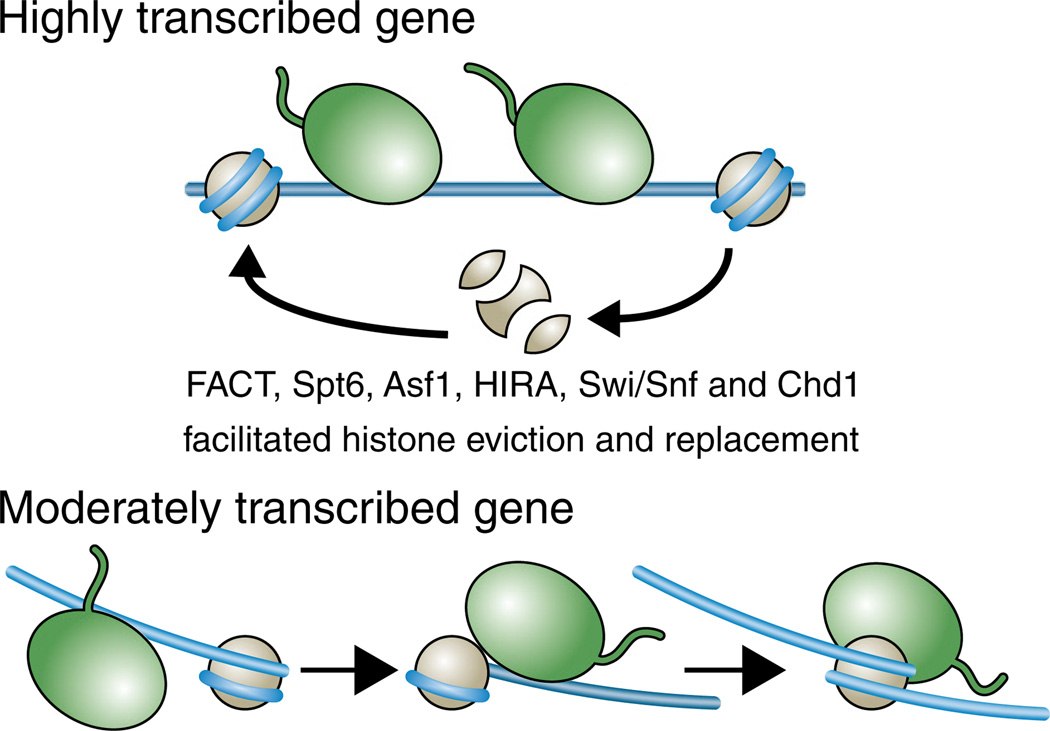
Fig. 3.
Different mechanisms for RNA polymerase II transcription through chromatin templates, depending on the rate of transcription. The green shape represents Pol II. Nucleosomes are completely disassembled and reassembled during high rates of transcription and this is facilitated by histone chaperones and ATP-dependent nucleosome remodelers. Other genes lose only H2A/H2B during transcription. Moderate and low levels of transcription are not necessarily accompanied by eviction of histones from the DNA, because the slow movement of Pol II along the DNA may allow time for rebinding of the DNA to the histone octamer behind the polymerase before all the histone interactions ahead of the polymerase have been released.
Spt6 is an H3/H4 histone chaperone that has convincingly been shown to promote chromatin reassembly following Pol II passage in yeast [31,32]. Global mapping of the genes that utilize Spt6 to return H3/H4 to the DNA during transcriptional elongation identified mainly highly transcribed genes [31]. As discussed below, one of the functional outcomes of failure to reassemble chromatin following transcription is transcriptional initiation from accessible cryptic sites within ORFs in yeast. Cryptic internal initiation was first discovered in yeast lacking Spt6 or Spt16 [22,31,32]. However, cryptic internal initiation has been used as an assay for factors that might be involved in reassembly of chromatin after transcription, including three other H3/H4 histone chaperones Asf1, HIR, and Rtt106 and the ATP-dependent nucleosome remodeling protein Chd1 [34]. In agreement, other studies have shown that lack of Asf1 or Chd1 leads to initiation from cryptic promoters within coding regions at many genes [35,36] and lack of fission yeast HIRA also results in cryptic initiation [37]. As such, many histone chaperones/chromatin remodelers appear to be involved, directly or indirectly, in the reassembly of chromatin behind RNA polymerase II (Fig. 3).
Asf1 is widely considered to be an upstream histone H3/H4 chaperone that hands histones to downstream replication-independent chaperones such as HIR or replication-dependent histone chaperones such as CAF-1. Consistent with a role in replication-independent histone exchange, Asf1 associates with promoters and coding regions of active genes that are sites of histone exchange and Asf1 has been shown to mediate the eviction of histone H3 (but not H2B) and its deposition during transcription elongation in yeast [35]. As would be expected for a scenario where Asf1 hands histones to HIR for chromatin assembly during transcription, yeast lacking either Asf1 or HIR have similarly reduced bulk histone occupancy within transcribed genes [23]. Unexpectedly, the source of the histones that are preferentially reassembled onto transcribed genes by these two histones chaperones is quite distinct, where the asf1 mutant preferentially incorporated new H3/H4 and the hir1 mutant preferentially incorporated old H3/H4 [23]. These results suggest that the normal function of Asf1 is to promote the reassembly of the preexisting H3/H4 onto DNA while the normal function of HIR is to promote the assembly of new H3/H4 following transcription. As such, the situation in the cell is more complicated than Asf1 merely handing histones to the HIR complex to deposit them following RNA polymerase passage.
4.1.1. Splitting of the H3/H4 tetramer during transcription
There is convincing evidence for splitting of the H3/H4 tetramer during transcription, although it is not clear whether this occurs on the DNA or off the DNA. Nearly 25 years ago, Vincent Allfrey noted that mammalian nucleosomes enriched in actively transcribed regions have increased accessibility of the sulfhydryl groups of cysteine 110 of H3 [38]. Within the nucleosome, cysteine 110 normally interacts with H113 of the other H3 molecule at the H3/H4 dimer–H3/H4 dimer interface (Fig. 4B). Although there is no evidence to support this idea, it is possible that the increased accessibility of the sulfhydryl of H3 C110 during transcription may be due to splitting of the H3/H4 tetramer during transcriptional elongation to reveal the H3–H3 interface. Noteworthy, this transcription-dependent accessibility of the H3–H3 interface was reversed upon transcriptional repression. Electron microscopy analysis of the nucleosomes that had accessible H3 C110 sulfhydryl groups revealed them to adopt an extended U-shape without any loss of histone proteins, consistent with their representing an unfolded nucleosomal intermediate during transcription [39]. The potential for splitting of the H3/H4 tetramer whilst still within the nucleosomal context is supported by the observation of DNase I sensitivity at the dyad in yeast [40], although it should be noted that yeast lack a cysteine at H3 amino acid 110 that helps to hold the H3/H4 tetramer together in larger eukaryotes. H3/H4 tetramer splitting was also observed in Jackson's density labeling experiments specifically during transcription (Fig. 1) [8], and in sequential ChIP analyses of distinctly epitope-tagged new and old histones at highly transcribed genes [12]. It is noteworthy here that the Asf1 histone chaperone interacts with H3/H4 via the H3/H4 dimer–H3/H4 dimer interface [41], suggesting that if Asf1 is directly mediating chromatin disassembly then it must be accompanied by the H3/H4 tetramer splitting into two H3/H4 dimers. It will be interesting to discern whether the splitting of H3/H4 tetramers during transcription is unique to nucleosomes carrying the histone variant H3.3, given that splitting of H3.3/H4 tetramers but not H3.1/H4 tetramers has been observed during DNA replication in human cells [13]. The canonical yeast H3 more closely resembles H3.3 than H3.1, suggesting that there may be more mixing of old and new H3/H4 dimers occurring in yeast.
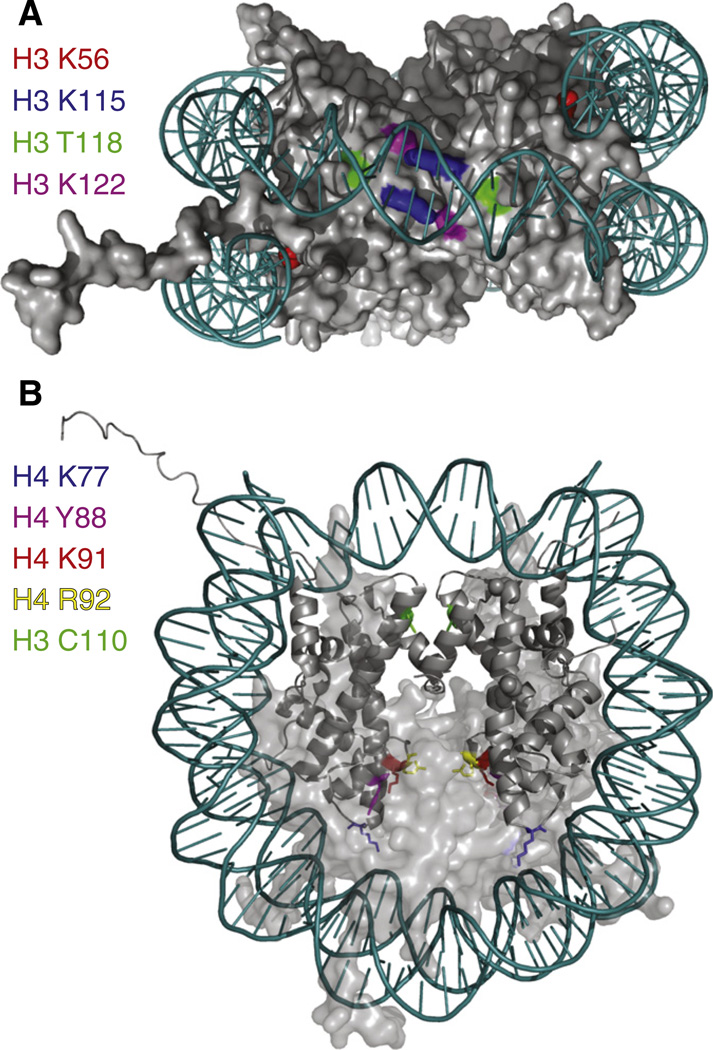
Fig. 4.
Locations of histone post-translational modifications that may alter histone–DNA interactions (A) and histone dimer–dimer interactions (B). In A, all histones are shown in a space-filling form. In B, the histone H3/H4 tetramer is shown in ribbon form and histones H2A/H2B are shown in a space-filling form and H2A/H2B have been made semi-transparent to reveal the amino acids on H4 that are at the interface between the H2A/H2B dimers and H3/H4 tetramer.
Why do tetramers of H3.3/H4 split into heterodimers and intermix with new H3.3/H4 dimers following DNA replication, while the old H3.1/H4 tetramers remain intact [13]? One possible explanation for this is that different mechanisms may exist for replication-dependent chromatin disassembly and/or assembly at euchromatin versus heterochromatin, where H3.3 is enriched in euchromatin. Perhaps this is also related to the fact that different histone chaperones assemble H3.3 and H3.1 onto chromatin. If CAF-1 were to deposit only tetramers of new H3.1/H4, while HirA were to deposit only dimers of H3.3/H4, this could account for the observed mixing of old and new heterodimers for H3.3/H4 and not for H3.1/H4. By this scenario, it is likely that tetramers of H3.1/H4 and H3.3/H4 may both be capable of transiently splitting during chromatin disassembly ahead of the DNA replication fork, but if the new H3.1/H4 are always deposited as tetramers by CAF-1 onto the DNA, there will be no opportunity for the old H3.1/H4 dimers to mix with new H3.1/H4 dimers. Clearly more research is required to test these ideas.
Why should we even care whether or not old H3/H4 dimers mix with new H3/H4 dimers during DNA replication? The reason is because the mixing of an old H3/H4 dimer with a new H3/H4 dimer following DNA replication may enable the old H3/H4 dimer to template its pattern of histone modification onto the new H3/H4 dimer, in effect promoting epigenetic inheritance of histone modification patterns. To test this idea, it will be interesting to determine whether patterns of histone modifications that are carried by H3.3 (which does undergo old/new dimer mixing during replication) are more effectively inherited than patterns of histone modifications that are carried by H3.1 (which do not undergo old/new dimer mixing during replication).
4.1.2. ATP-dependent nucleosome remodelers, histone variants and template topology contribute to histone exchange
ATP-dependent nucleosome remodelers promote the breaking of histone–DNA interactions during histone exchange. For example, Swi/Snf is required for transcriptional elongation of the mouse Hsp70 gene in vivo [42]. The Swi/Snf remodeling complex can also alter chromatin structure facilitating transcriptional elongation on the GAL genes in yeast [43]. Chd1 is an ATP-dependent nucleosome remodeling complex that is specifically involved in incorporation of the replication-independent H3.3 variant onto DNA in Drosophila [44], a function that it does in concert with the HIRA histone chaperone [44,45]. Another replication-independent histone variant, H2A.Z, is specifically deposited onto DNA by the Swr1 ATP-dependent nucleosome remodeling complex via partial unwrapping of the nucleosomal DNA and replacement of an H2A/H2B dimer with an H2A.Z/H2B dimer [46]. Noteworthy, nucleosomes including both H2A.Z and H3.3 are particularly prone to disassembly in vivo [47]. As such, the cell appears to incorporate replication-independent histone variants as a mechanism to tip the equilibrium of histone eviction and replacement toward eviction to promote factor access to the DNA.
Changes in DNA superhelical tension that accompany transcription also contribute to the eviction of histones from the DNA. RNA polymerase passage induces positive superhelical stress ahead of the polymerase and negative superhelical stress behind it. Within the nucleosome, the DNA is wrapped around the nucleosome in a left-handed (negative superhelical) manner and therefore transcription-induced positive stress promotes right-handed coils in the DNA and disruption of the nucleosome. Indeed, transcription-induced positive stress has been shown to promote displacement of H2A/H2B dimers from the nucleosome [48]. Interestingly, positively supercoiled DNA can induce a chiral transition within the H3/H4 tetramer that is thought to occur via a rotation of the two H3/H4 dimers around their H3–H3 interface [49]. This chiral transition state of the H3/H4 tetramer is not compatible with binding of the H2A/H2B dimer [50]. As such, it is tempting to speculate that the positive supercoiling induced by RNA polymerase passage may induce a chiral transition at the H3–H3 interface that leads to displacement of H2A/H2B. It would also be interesting to determine whether the increased accessibility of the H3 C110 sulfhydryl groups at the H3–H3 interface during transcription, discussed above, reflects the chiral transition of the H3/H4 tetramer in vivo. If this were the case, it would provide an elegant mechanism for coupling H2A/H2B removal to Pol II passage.
4.1.3. Low level transcription is not associated with histone displacement from the DNA
Eviction of histones from the DNA may not be obligatorily coupled to the passage of RNA polymerases. The analysis of histone H3 depletion from ORFs in the absence of the Spt6 chromatin reassembly factor demonstrates that transcription at low levels does not displace nucleosomes, while transcription at high levels does displace histones [31]. However, it is still possible that histone chaperones other than Spt6 are responsible for reassembling chromatin after Pol II passage at genes that are transcribed at a low rate. Regardless, transcription studies on mononucleosome templates in vitro have revealed a mechanism for nucleosome survival during Pol II passage [51]. Once Pol II arrives at a strong DNA–histone interaction site, the DNA ahead of the polymerase unwinds from the histone octamer and a small intranucleosomal DNA loop containing transcribing Pol II is formed (Fig. 3). This DNA loop formation leads to restoration of the DNA–histone interactions behind Pol II. This mechanism could explain why the original H3/H4 histones are retained rather than being exchanged for new H3/H4 at moderately transcribed genes, because in this model all the H3/H4-DNA interactions are not simultaneously broken at any point in time. However, rate of transcription per se is not the only determinant of the extent of histone H3/H4 exchange, as some genes show rates of histone exchange that are higher or lower than would be predicted from the Pol II transcription rate alone [52].
5. Histone modifications that regulate histone exchange
Histone proteins undergo a wide variety of post-translational modifications on specific amino acid residues during genomic processes. The vast majority of these modifications occur on the exposed N-terminal tails of the histones that extend out beyond the nucleosomal DNA. However, where examined the histone N-terminal tails are not essential for histone exchange. For example, the sites of chromosomal incorporation of newly induced histones H2B and H3 lacking their N-terminal tails in G1 phase arrested cells is unchanged from that of full length histones [10]. It is possible that the N-terminal tails of the histones act in a redundant manner during histone exchange. Indeed, the N-terminal tails of histones slow down Pol II passage along chromatin templates in vitro [53]. In Drosophila, it has been noted that N-terminally acetylated histones turn over more rapidly than unacetylated histones [54]. Whether the N-terminal histone acetylation is causative or correlative for histone exchange is unknown.
Structurally, it makes more sense to consider the potential influence of histone modifications that affect the histone–DNA interactions on histone exchange, rather than those on the histone N-terminal tails. The founding member of this class of histone modification is acetylation of H3 lysine 56 (H3 K56Ac). Within the nucleosome structure, H3 K56 contacts the DNA via a water molecule (Fig. 4A). Addition of an acetyl group is predicted to break the interactions between H3 K56 and the DNA as it enters and exits the nucleosome. Indeed, there is extensive evidence to indicate that H3 K56Ac disrupts the nucleosome structure both in vivo and in vitro. Single molecule FRET experiments have revealed that H3 K56Ac increases the unwrapping of the ends of the nucleosomal DNA from the histone octamer by a factor of 7-fold [55]. By promoting this “breathing” of the nucleosomal DNA, H3 K56Ac can enhance the binding of LexA to its nucleosome-buried DNA binding site by at least 3-fold [56]. In yeast, failure to acetylate H3 K56 leads to increased superhelical density and decreased nuclease accessibility, suggesting that H3 K56Ac loosens the chromatin structure in vivo [57,58]. During histone eviction from the yeast PHO5 promoter, the proportion of histone H3 carrying K56Ac drastically increases in a local manner, while a mutation to mimic K56 acetylation increases the rate of histone eviction from this promoter [59]. A similar situation is seen within ORFs where Asf1, presumably through its role in promoting H3 K56 acetylation, drives histone exchange during transcriptional elongation [35]. Indeed, transcription coupled incorporation of H3 K56Ac promotes transcription of heterochromatic regions [60]. Genome-wide, histones carrying K56Ac mark regions of new histone incorporation and high levels of replication-independent histone exchange in yeast [61]. Furthermore, the rate of histone exchange globally is reduced in yeast unable to acetylate H3 K56Ac [62]. Taken together all the evidence indicates that acetylation of H3 K56 promotes histone eviction and subsequent histone exchange, at least in yeast. It is noteworthy however, that yeast lacking the H3 K56 histone acetyl transferase Rtt109, unlike asf1 mutants, do not display cryptic internal initiation which is a consequence of failure to properly reassemble chromatin after transcription [34]. As such, Asf1 would appear to have roles in chromatin assembly after RNA polymerase passage that are distinct from its function in H3 K56 acetylation and histone eviction.
We predict that H3 K56ac is the founding member of a large class of histone modifications that are used by the cell to regulate histone eviction and histone replacement. To date the evidence for other histone modifications with this function is limited. However, the physical locations within the nucleosome of several histone post-translational modifications are too coincidental to ignore. For example, there is a cluster of histone modifications that lie at the interface between the nucleosomal DNA dyad and histone H3 including acetylation and methylation of K115 and K122 and phosphorylation of T118 (Fig. 4A). Interestingly, these modifications often occur together, at least on peptides from bovine histones [63], suggesting that they may act in a concerted manner to disrupt the H3–DNA interactions. Peptide ligation has been used to generate nuclesomes carrying H3 K122Ac and H3 K115Ac [64]. These acetylation marks reduce the free energy for DNA binding to the histone octamer and augment chromatin disassembly in vitro [64,65]. Although the effects of H3 T118 phosphorylation on nucleosome stability has not yet been directly examined in vitro, it is noteworthy that T118I was uncovered as a SIN mutant (Swi/Snf independent) [66]. In vitro, the T118I mutation destabilizes nucleosomes [67,68] and H3 T118I promotes the ability of Pol II to pass the nucleosome barrier [69]. Given the predicted drastic distortion of the nucleosomal DNA that phosphorylation of H3 T118 would cause, it will be fascinating to determine when and where this modification and its neighboring modifications are utilized by the cell.
Other histone modifications may be used by the cell to regulate the interaction between the H2A/H2B dimers and H3/H4 tetramer during histone exchange. One potential example of this type of modification is acetylation of H4 on lysine 91. H4 K91Ac has been detected on newly synthesized histones in yeast and its location at the interface between H2A/H2B and the H3/H4 tetramer is suggestive of a role in regulating the interaction between the histone proteins [70]. Within the nucleosome, H4 K91 forms a salt bridge with E74 (E71 in metazoans) of H2B and structurally it is likely that H4 K91 acetylation would break this interaction (Fig. 4B). Indeed, H4 K91Ac destabilizes the histone octamer [70].Whether H4 K91Ac is used to help maintain the H3/H4 tetramers free of H2A/H2B dimers during the process of histone exchange is not known. H4 K91 is also monoubiquitylated in response to DNA damage in mammals [71], which could potentially distort the nucleosome structure to facilitate repair.
Mapping other post-translational modifications that have been uncovered by mass spectrometry analyses onto the nucleosome structure reveals additional histone modifications that could influence the interaction between the H2A/H2B dimers and H3/H4 tetramer (Fig. 4B). For example, acetylation of H4 K77 was observed by mass spectroscopy of bovine histones [63]. Within the nucleosome structure, H4 K77 mediates Van der Waal's interactions with R92 of H2B (in metazoans, R95 in yeast) and acetylation of K77 may disrupt this H4–H2B interaction. H4 R92 methylation has also been observed by mass spectroscopy of bovine histones [63]. Within the nucleosome H4 R92 forms strong salt bridge interactions with E71 of H2B (in metazoans, E74 in yeast). Like many of the other sites of modification noted above, mutation of H4 K77 and H4 R92 cause interesting phenotypic effects on transcriptional silencing in yeast [72]. Phosphorylation of H4 Y88 [73] is likely to disrupt the aromatic ring stacking interactions between this residue and Y86 of H2B (in yeast, Y83 in metazoans) within the nucleosome. The stacking of Y88 of H4 on Y86 of H2B has been proposed previously to form a spring like structure that maintains tensile strength in the lower half of the nucleosome [74]. As such, one could imagine how phosphorylation of H4 Y88 could potentially trigger release of the H2A/H2B dimer–H3/H4 tetramer interaction. Yeast carrying substitution of Y88 to G are SIN mutants [75], consistent with their having a loosened nucleosome structure. In addition to overcoming the requirement for the ATP-dependent nucleosome remodeler Swi/Snf, Y88G also leads to defects in transcription and proliferation [75]. It is important to appreciate that the nucleosomal locations of these histone residues that mediate interactions with DNA and with other histones (Fig. 4) makes them extremely inaccessible for enzymatic modification. As such, their modification must either occur on free histones or during extensive disruption of the nucleosome structure.
Other histone modifications have been implicated indirectly in histone eviction or histone return to the DNA. FACT-mediated chromatin reassembly following Pol II passage is regulated by H2B K123 monoubiquitylation. In yeast, the absence of H2B ubiquitylation prevents the chromatin structure being properly restored in the wake of elongating Pol II [76]. The function of FACT and H2B ubiquitylation are intertwined, where H2B ubiquitylation is required for the stable accumulation of Spt16 at the GAL1 coding region, and Spt16 regulates the formation of ubiquitylated H2B both globally and at the GAL1 gene. Related observations had been made earlier with an elegant highly reconstituted transcription system in vitro [77]. Consistent with its role in recruitment of FACT to promote chromatin reassembly, monoubiquitylated H2B stabilizes the nucleosome structure in yeast [78].
In mice sperm, acetylation of H4 K16 promotes global histone eviction from the genome during their replacement with protamines [79]. However, it is not known whether this role of H4 K16Ac in histone removal is direct or indirect. Given that H4 K16Ac breaks nucleosome–nucleosome interactions that are involved in chromatin condensation [80], it is possible that H4 K16Ac mediated decondensation of chromosomes is required before histone removal during spermatogenesis.
Although not a histone modification per se, proteolytic cleavage of the N-terminal tail of histone H3, which indirectly removes the N-terminal histone modifications, plays a role in histone eviction. N-terminal truncation of histone H3 after amino acid 21 precedes, and is required for, histone eviction from promoters during transcriptional induction [81]. The same study did not observe histone N-terminal H3 cleavage within the IME1 ORF. However, this particular ORF does not display significant H3 depletion during transcription and therefore may not be the optimal locus to investigate a role for N-terminal tail clipping in promoting histone eviction during RNA polymerase passage. For investigators employing N-terminal epitopes on H3 to examine histone occupancy or histone dynamics, it is important for them to consider the influence of N-terminal H3 clipping when interpreting their results.
6. Functional importance of histone exchange
Clearly the cell has placed a lot of emphasis on developing intricate mechanisms to mediate the eviction and replacement of histones during Pol II passage. This implies that improper histone exchange is likely to be detrimental to the cell. But what are those detrimental consequences? Failure to evict histones ahead of Pol II would presumably hinder RNA polymerase passage through ORFs in cells, as it does in vitro. Consistent with this idea, two of the histone chaperones that promote histone exchange within ORFs, FACT and Spt6 (Fig. 3), are both essential in yeast. Failure to restore the histones onto the DNA in the wake of Pol II passage results in cryptic transcription internal initiation and the synthesis of their encoded proteins [34]. The consequence of making truncated proteins via cryptic internal initiation is presumably deleterious. Surprisingly, Drosophila lacking the transcription specific histone variant H3.3 are viable (although the males are infertile) [82], but this is largely due to compensatory upregulation of the canonical histone H3.1 genes for replication-independent chromatin assembly.
Histone exchange that occurs independent of transcription and DNA replication, for example at promoters and enhancers, provides an opportunity for transient access of factors to the genome. This is likely to be essential for enabling effective transcriptional activation and repression to occur. Without histone exchange, the pattern of histone modifications and any information carried within this pattern would remain static long after the enzymes that established the histone modifications had left that genomic region. One can see how this would be particularly problematic in post mitotic cells that lack the opportunity to refresh the histone proteins during DNA replication. By constantly refreshing the histones with new histones lacking the “old” histone modification pattern, histone exchange allows the chromatin structure to be highly responsive to changes in the environment.
Histone exchange is likely to be especially important in pluripotent cells. Histone FRAP studies on pluripotent embryonic stem cells revealed that histone exchange is significantly faster and more extensive than in differentiated cells [83]. This rapid fluorescence recovery of the histones in stem cells was due to a population (18% for H2B and 25% for H3) of loosely bound (yet not free) histones that were exchanging within seconds. The size and speed of recovery of this dynamic histone pool in stem cells is much greater than in differentiated cells. The mechanistic reason for the “hyperdynamic” chromatin state of stem cells is not presently known. Also, whether the hyperdynamic chromatin is the cause or consequence of the unique gene expression pattern and histone post-translational modification signature in pluripotent cells remains to be determined.
7. Changes in chromatin dynamics during aging
There is growing evidence that the chromatin structure and chromatin dynamics change during the aging process, and the functional consequences of these changes are likely to be profound. Although rates of histone exchange have not yet been directly examined in aged cells, there are hints that they will be significantly altered. For example, the replication-independent histone variant H3.3 accumulates on the genome of cells that are no longer replicating, such as neurons [84,85]. The reason for this is probably due to the rather trivial fact that the canonical H3.1 and H3.2 that are expressed during S-phase are no long expressed in post mitotic cells. There is an interesting correlation between the fact that neurons have genomes that are globally transcribed (approximately 80% of genes are expressed in adult mouse brain) [86] and have a genome that is mostly packaged with H3.3. As such, it is tempting to speculate that global transcription of the neuronal genome is a consequence of their genome being packaged by the “looser” histone H3.3 variant that may promote more frequent histone exchange and increased access of the transcription machinery to the DNA. In fact, the H3.3 variant appears to become more abundant during aging in general, not just in neurons. For example, H3.3 levels increase during postnatal development of chicken and mice [87].
Cellular senescence is a state of irreversible growth arrest in culture. Whether or not a link exists between cellular senescence and in vivo aging is controversial and currently unproven. Notwithstanding, akin to the observations made during in vivo aging, H3.3 levels increase during the culture of human fibroblasts, which is a model for the entry into cell senescence [85]. Accordingly, the levels of the histone H3.3 specific chaperone HIRA also increase during aging in baboons [88]. Cellular senescence can be induced by multiple factors in vitro including the BRAF oncogene. Interestingly, knockdown of HIRA blocks BRAF-induced senescence [89], indicating that normal levels of HIRA may be important for entry into the senescent state. Perhaps related, HIRA is required for the formation of senescence-associated heterochromatin foci (SAHF) [90]. However, SAHF do not appear to accumulate during aging in vivo, at least not as morphologically distinct structures that can be visualized by microscopy in either mouse or baboon [91].
H2A.Z, the other histone variant that is incorporated into the genome during replication-independent histone exchange is also linked to aging. Although H2A.Z levels do not appear to change during the aging process, H2A.Z knockdown in human fibroblasts causes premature entry into senescence [92]. Similarly, knockdown of p400, the human counterpart of the Swr1 H2A.Z histone exchanger, also induces premature senescence in culture [93]. However, the physiological significance of these observations is unclear, as primary human cells are highly prone to premature senescence when stressed.
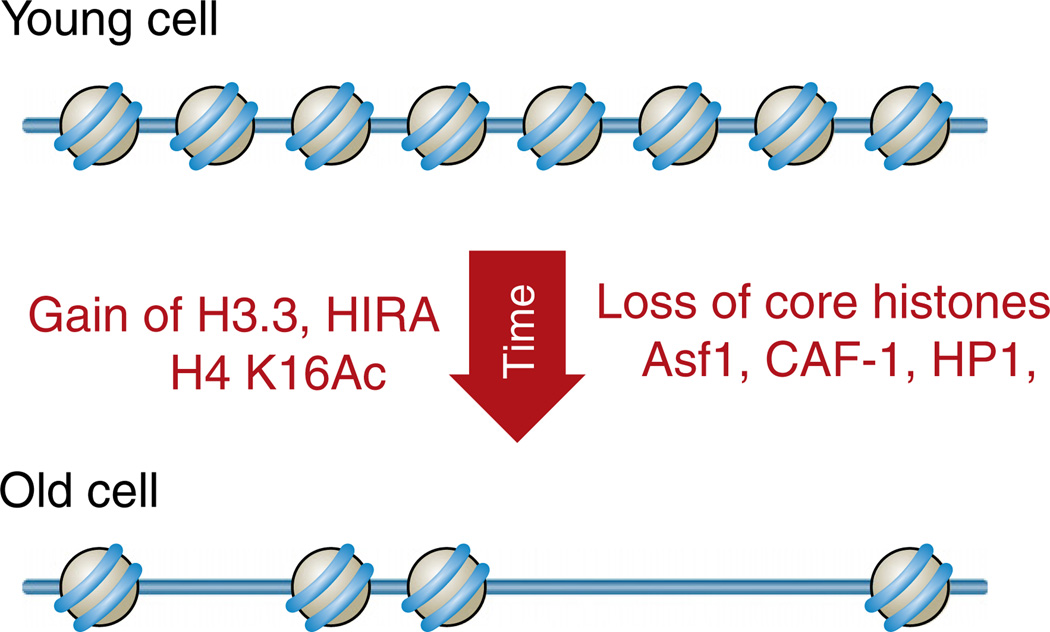
While the levels of the histone variants that are incorporated during replication-independent histone exchange either rise during aging or influence aging, the levels of the canonical histone proteins drastically drop during replicative aging (Fig. 5). Human fibroblasts that have been through a high number of population doublings show a decrease in total histone protein levels compared to those that have been through a low number of population doublings [94]. This is accompanied by a drastic decrease in the levels of the histone chaperones required for the incorporation of canonical histones during DNA replication, CAF-1 and Asf1 [94]. This profound loss of the core histones with increasing numbers of cell divisions has also been observed in yeast, where it leads to expression of otherwise silenced genes [95,96]. Meanwhile, total histone H3 protein levels do not change between 10 day old and 40 day old Drosophila (a situation that largely represents post-mitotic aging) [97].
Fig. 5.
Changes to the chromatin structure during aging. The schematic summarizes observations made from mammalian systems, although the profound loss of nucleosomes from the genome during aging has also been observed in yeast.
Studies have only just begun to examine the changes to histone modifications during the aging process (reviewed by [98]). These include an increase in levels of H4 K16Ac with replicative age in both yeast and with increasing numbers of population doublings of human fibroblasts in culture [94,96]. Increased H4 K16Ac levels would reduce the nucleosome–nucleosome interactions, opening up the chromatin structure in old cells. There is a decrease in the levels of H3 K56Ac during in vitro aging of fibroblasts [94]. From the function of H3 K56Ac shown above, a drop in H3 K56Ac levels in old cells would be predicted to reduce the rate of histone exchange. This drop in H3 K56Ac levels in old cells is likely to be a consequence of the reduced levels of Asf1 in old cells [94], which is required for H3 K56 acetylation [99]. However, given that at least half of the genome is nucleosome-free in old cells, this reduction in levels of H3 K56Ac may not impact accessibility of the genome too greatly. In worms, excess H3 K4 methylation, which correlates with active transcription, is detrimental for longevity, as deficiency in the ASH-2 methyltransferase complex extend lifespan [100]. Noteworthy, longevity in worms reflects postmitotic aging, not mitotic/replicative aging that was the focus of the other studies in yeast and in cultured mammalian cells.
Heterochromatin is the repressed and less dynamic form of chromatin, which is maintained by the binding of heterochromatin protein 1 (HP1) [101]. Although mainly speculative, HP1 may act to repress transcription by stabilizing the nucleosome structure and in doing so HP1 may reduce the rate of histone exchange. As such, it is interesting to note that cells from aged humans have less HP1 and loss of the specific modification that recruits HP1 to chromatin, H3 K9me3 [102] (Fig. 5). However, other studies report that HP1 levels increase during replicative senescence of human fibroblasts [91] and that levels of HP1 and H3 K9me3 increase in 40 day old Drosophila as compared to 10 day old flies [97]. Clearly the situation is confusing and much more research is required to fully understand how the chromatin structure changes during replicative aging and in vivo aging, and whether it is different in different organisms.
In general, the changes to the chromatin structure during aging infer that cells that have divided more times may have a more open and dynamic chromatin state. Whether this is programmed or an unfortunate consequence of aging is not clear. However, supplying additional histones can extend replicative lifespan, at least in yeast, indicating that the decay of chromatin during aging is a cause of aging [95]. Functional analyses of the molecular consequences of the altered chromatin structure during aging hold the potential to reveal key mediators of the aging process. In summary, while our understanding of the mechanisms of histone exchange has grown rapidly in recent years, we still have a lot to learn about the full implications of histone exchange on the activities of the genome.
Acknowledgments
We are very grateful to Leisa McCord of the Department of Biochemistry and Molecular Genetics UT MD Anderson Cancer Center for assistance with the figures. We would also like to acknowledge Siddhartha Roy, for his assistance with analysis of the nucleosome structure. JKT is supported by grants from NIH GM and NCI, and a CPRIT Rising Star award, CD is supported by a Susan G. Komen for the Cure Fellowship.
Footnotes
This article is part of a Special Issue entitled: Histone chaperones and Chromatin assembly.
Note added in proof
A recent careful in vitro analysis of the effect of H3 T118 phosphorylation revealed that it increases nucleosome mobility by 26 fold and increases DNA accessibility near the normally inaccessible dyad by 6 fold [104].
References
- 1.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Bushnell DA, Wang D, Calero G, Kornberg RD. Structure of an RNA polymerase II–TFIIB complex and the transcription initiation mechanism. Science. 2010;327:206–209. doi: 10.1126/science.1182015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung AC, Cramer P. Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature. 2011;471:249–253. doi: 10.1038/nature09785. [DOI] [PubMed] [Google Scholar]
- 4.Chang CH, Luse DS. The H3/H4 tetramer blocks transcript elongation by RNA polymerase II in vitro. J. Biol. Chem. 1997;272:23427–23434. doi: 10.1074/jbc.272.37.23427. [DOI] [PubMed] [Google Scholar]
- 5.Fyodorov DV, Kadonaga JT. Dynamics of ATP-dependent chromatin assembly by ACF. Nature. 2002;418:897–900. doi: 10.1038/nature00929. [DOI] [PubMed] [Google Scholar]
- 6.Bohm V, Hieb AR, Andrews AJ, Gansen A, Rocker A, Toth K, Luger K, Langowski J. Nucleosome accessibility governed by the dimer/tetramer interface. Nucleic Acids Res. 2011;39:3093–3102. doi: 10.1093/nar/gkq1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson V. Deposition of newly synthesized histones: hybrid nucleosomes are not tandemly arranged on daughter DNA strands. Biochemistry. 1988;27:2109–2120. doi: 10.1021/bi00406a044. [DOI] [PubMed] [Google Scholar]
- 8.Jackson V. In vivo studies on the dynamics of histone–DNA interaction: evidence for nucleosome dissolution during replication and transcription and a low level of dissolution independent of both. Biochemistry. 1990;29:719–731. doi: 10.1021/bi00455a019. [DOI] [PubMed] [Google Scholar]
- 9.Thiriet C, Hayes JJ. Replication-independent core histone dynamics at transcriptionally active loci in vivo. Genes Dev. 2005;19:677–682. doi: 10.1101/gad.1265205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamai A, Imoberdorf RM, Strubin M. Continuous histone H2B and transcription-dependent histone H3 exchange in yeast cells outside of replication. Mol. Cell. 2007;25:345–355. doi: 10.1016/j.molcel.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Dion MF, Kaplan T, Kim M, Buratowski S, Friedman N, Rando OJ. Dynamics of replication-independent histone turnover in budding yeast. Science. 2007;315:1405–1408. doi: 10.1126/science.1134053. [DOI] [PubMed] [Google Scholar]
- 12.Katan-Khaykovich Y, Struhl K. Splitting of H3–H4 tetramers at transcriptionally active genes undergoing dynamic histone exchange. Proc. Natl. Acad. Sci. U. S. A. 2011;108:1296–1301. doi: 10.1073/pnas.1018308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, Long C, Chen X, Huang C, Chen S, Zhu B. Partitioning of histone H3–H4 tetramers during DNA replication-dependent chromatin assembly. Science. 2010;328:94–98. doi: 10.1126/science.1178994. [DOI] [PubMed] [Google Scholar]
- 14.Verzijlbergen KF, Menendez-Benito V, van Welsem T, van Deventer SJ, Lindstrom DL, Ovaa H, Neefjes J, Gottschling DE, van Leeuwen F. Recombination-induced tag exchange to track old and new proteins. Proc. Natl. Acad. Sci. U. S. A. 2010;107:64–68. doi: 10.1073/pnas.0911164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura H, Cook PR. Kinetics of core histones in living human cells: little exchange of H3 and H4 and some rapid exchange of H2B. J. Cell Biol. 2001;153:1341–1353. doi: 10.1083/jcb.153.7.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell. 2002;9:1191–1200. doi: 10.1016/s1097-2765(02)00542-7. [DOI] [PubMed] [Google Scholar]
- 17.Mito Y, Henikoff JG, Henikoff S. Genome-scale profiling of histone H3.3 replacement patterns. Nat. Genet. 2005;37:1090–1097. doi: 10.1038/ng1637. [DOI] [PubMed] [Google Scholar]
- 18.Kang B, Pu M, Hu G, Wen W, Dong Z, Zhao K, Stillman B, Zhang Z. Phosphorylation of H4 Ser 47 promotes HIRA-mediated nucleosome assembly. Genes Dev. 2011;25:1359–1364. doi: 10.1101/gad.2055511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ooi SL, Henikoff JG, Henikoff S. A native chromatin purification system for epigenomic profiling in Caenorhabditis elegans. Nucleic Acids Res. 2010;38:e26. doi: 10.1093/nar/gkp1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deal RB, Henikoff JG, Henikoff S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science. 2010;328:1161–1164. doi: 10.1126/science.1186777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristjuhan A, Svejstrup JQ. Evidence for distinct mechanisms facilitating transcript elongation through chromatin in vivo. EMBO J. 2004;23:4243–4252. doi: 10.1038/sj.emboj.7600433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwabish MA, Struhl K. Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 2004;24:10111–10117. doi: 10.1128/MCB.24.23.10111-10117.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Seol JH, Han JW, Youn HD, Cho EJ. Histone chaperones regulate histone exchange during transcription. EMBO J. 2007;26:4467–4474. doi: 10.1038/sj.emboj.7601870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CK, Shibata Y, Rao B, Strahl BD, Lieb JD. Evidence for nucleosome depletion at active regulatory regions genome-wide. Nat. Genet. 2004;36:900–905. doi: 10.1038/ng1400. [DOI] [PubMed] [Google Scholar]
- 25.Morillo-Huesca M, Maya D, Munoz-Centeno MC, Singh RK, Oreal V, Reddy GU, Liang D, Geli V, Gunjan A, Chavez S. FACT prevents the accumulation of free histones evicted from transcribed chromatin and a subsequent cell cycle delay in G1. PLoS Genet. 2010;6:e1000964. doi: 10.1371/journal.pgen.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kireeva ML, Walter W, Tchernajenko V, Bondarenko V, Kashlev M, Studitsky VM. Nucleosome remodeling induced by RNA polymerase II: loss of the H2A/H2B dimer during transcription. Mol. Cell. 2002;9:541–552. doi: 10.1016/s1097-2765(02)00472-0. [DOI] [PubMed] [Google Scholar]
- 27.Studitsky VM, Kassavetis GA, Geiduschek EP, Felsenfeld G. Mechanism of transcription through the nucleosome by eukaryotic RNA polymerase. Science. 1997;278:1960–1963. doi: 10.1126/science.278.5345.1960. [DOI] [PubMed] [Google Scholar]
- 28.Bondarenko VA, Steele LM, Ujvari A, Gaykalova DA, Kulaeva OI, Polikanov YS, Luse DS, Studitsky VM. Nucleosomes can form a polar barrier to transcript elongation by RNA polymerase II. Mol. Cell. 2006;24:469–479. doi: 10.1016/j.molcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Belotserkovskaya R, Oh S, Bondarenko VA, Orphanides G, Studitsky VM, Reinberg D. FACT facilitates transcription-dependent nucleosome alteration. Science. 2003;301:1090–1093. doi: 10.1126/science.1085703. [DOI] [PubMed] [Google Scholar]
- 30.Xin H, Takahata S, Blanksma M, McCullough L, Stillman DJ, Formosa T. yFACT induces global accessibility of nucleosomal DNA without H2A–H2B displacement. Mol. Cell. 2009;35:365–376. doi: 10.1016/j.molcel.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanovska I, Jacques PE, Rando OJ, Robert F, Winston F. Control of chromatin structure by spt6: different consequences in coding and regulatory regions. Mol. Cell. Biol. 2011;31:531–541. doi: 10.1128/MCB.01068-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan CD, Laprade L, Winston F. Transcription elongation factors repress transcription initiation from cryptic sites. Science. 2003;301:1096–1099. doi: 10.1126/science.1087374. [DOI] [PubMed] [Google Scholar]
- 33.Jamai A, Puglisi A, Strubin M. Histone chaperone spt16 promotes redeposition of the original h3–h4 histones evicted by elongating RNA polymerase. Mol. Cell. 2009;35:377–383. doi: 10.1016/j.molcel.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Cheung V, Chua G, Batada NN, Landry CR, Michnick SW, Hughes TR, Winston F. Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol. 2008;6:e277. doi: 10.1371/journal.pbio.0060277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwabish MA, Struhl K. Asf1 mediates histone eviction and deposition during elongation by RNA polymerase II. Mol. Cell. 2006;22:415–422. doi: 10.1016/j.molcel.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Quan TK, Hartzog GA. Histone H3K4 and K36 methylation, Chd1 and Rpd3S oppose the functions of Saccharomyces cerevisiae Spt4–Spt5 in transcription. Genetics. 2010;184:321–334. doi: 10.1534/genetics.109.111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson HE, Wardle J, Korkut SV, Murton HE, Lopez-Maury L, Bahler J, Whitehall SK. The fission yeast HIRA histone chaperone is required for promoter silencing and the suppression of cryptic antisense transcripts. Mol. Cell. Biol. 2009;29:5158–5167. doi: 10.1128/MCB.00698-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen TA, Allfrey VG. Rapid and reversible changes in nucleosome structure accompany the activation, repression, and superinduction of murine fibroblast protooncogenes c-fos and c-myc. Proc. Natl. Acad. Sci. U. S. A. 1987;84:5252–5256. doi: 10.1073/pnas.84.15.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bazett-Jones DP, Mendez E, Czarnota GJ, Ottensmeyer FP, Allfrey VG. Visualization and analysis of unfolded nucleosomes associated with transcribing chromatin. Nucleic Acids Res. 1996;24:321–329. doi: 10.1093/nar/24.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee MS, Garrard WT. Transcription-induced nucleosome ‘splitting’: anunderlying structure for DNase I sensitive chromatin. EMBO J. 1991;10:607–615. doi: 10.1002/j.1460-2075.1991.tb07988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.English CM, Adkins MW, Carson JJ, Churchill ME, Tyler JK. Structural basis for the histone chaperone activity of Asf1. Cell. 2006;127:495–508. doi: 10.1016/j.cell.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corey LL, Weirich CS, Benjamin IJ, Kingston RE. Localized recruitment of a chromatin-remodeling activity by an activator in vivo drives transcriptional elongation. Genes Dev. 2003;17:1392–1401. doi: 10.1101/gad.1071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwabish MA, Struhl K. The Swi/Snf complex is important for histone eviction during transcriptional activation and RNA polymerase II elongation in vivo. Mol. Cell. Biol. 2007;27:6987–6995. doi: 10.1128/MCB.00717-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konev AY, Tribus M, Park SY, Podhraski V, Lim CY, Emelyanov AV, Vershilova E, Pirrotta V, Kadonaga JT, Lusser A, Fyodorov DV. CHD1 motor protein is required for deposition of histone variant H3.3 into chromatin in vivo. Science. 2007;317:1087–1090. doi: 10.1126/science.1145339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg AD, Banaszynski LA, Noh KM, Lewis PW, Elsaesser SJ, Stadler S, Dewell S, Law M, Guo X, Li X, Wen D, Chapgier A, DeKelver RC, Miller JC, Lee YL, Boydston EA, Holmes MC, Gregory PD, Greally JM, Rafii S, Yang C, Scambler PJ, Garrick D, Gibbons RJ, Higgs DR, Cristea IM, Urnov FD, Zheng D, Allis CD. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140:678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2004;303:343–348. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- 47.Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, Felsenfeld G. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat. Genet. 2009;41:941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levchenko V, Jackson B, Jackson V. Histone release during transcription: displacement of the two H2A–H2B dimers in the nucleosome is dependent on different levels of transcription-induced positive stress. Biochemistry. 2005;44:5357–5372. doi: 10.1021/bi047786o. [DOI] [PubMed] [Google Scholar]
- 49.Hamiche A, Carot V, Alilat M, De Lucia F, O'Donohue MF, Revet B, Prunell A. Interaction of the histone (H3–H4)2 tetramer of the nucleosome with positively supercoiled DNA minicircles: potential flipping of the protein from a left- to a right-handed superhelical form. Proc. Natl. Acad. Sci. U. S. A. 1996;93:7588–7593. doi: 10.1073/pnas.93.15.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson S, Danowit R, Wunsch A, Jackson V. NAP1 catalyzes the formation of either positive or negative supercoils on DNA on basis of the dimer–tetramer equilibrium of histones H3/H4. Biochemistry. 2007;46:8634–8646. doi: 10.1021/bi6025215. [DOI] [PubMed] [Google Scholar]
- 51.Kulaeva OI, Gaykalova DA, Pestov NA, Golovastov VV, Vassylyev DG, Artsimovitch I, Studitsky VM. Mechanism of chromatin remodeling and recovery during passage of RNA polymerase II. Nat. Struct. Mol. Biol. 2009;16:1272–1278. doi: 10.1038/nsmb.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gat-Viks I, Vingron M. Evidence for gene-specific rather than transcription rate-dependent histone H3 exchange in yeast coding regions. PLoS Comput. Biol. 2009;5:e1000282. doi: 10.1371/journal.pcbi.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ujvari A, Hsieh FK, Luse SW, Studitsky VM, Luse DS. Histone N-terminal tails interfere with nucleosome traversal by RNA polymerase II. J. Biol. Chem. 2008;283:32236–32243. doi: 10.1074/jbc.M806636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kharchenko PV, Alekseyenko AA, Schwartz YB, Minoda A, Riddle NC, Ernst J, Sabo PJ, Larschan E, Gorchakov AA, Gu T, Linder-Basso D, Plachetka A, Shanower G, Tolstorukov MY, Luquette LJ, Xi R, Jung YL, Park RW, Bishop EP, Canfield TK, Sandstrom R, Thurman RE, MacAlpine DM, Stamatoyannopoulos JA, Kellis M, Elgin SC, Kuroda MI, Pirrotta V, Karpen GH, Park PJ. Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature. 2011;471:480–485. doi: 10.1038/nature09725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumann H, Hancock SM, Buning R, Routh A, Chapman L, Somers J, Owen-Hughes T, van Noort J, Rhodes D, Chin JW. A method for genetically installing site-specific acetylation in recombinant histones defines the effects of H3 K56 acetylation. Mol. Cell. 2009;36:153–163. doi: 10.1016/j.molcel.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimko JC, North JA, Bruns AN, Poirier MG, Ottesen JJ. Preparation of fully synthetic histone h3 reveals that acetyl-lysine 56 facilitates protein binding within nucleosomes. J. Mol. Biol. 2011;408:187–204. doi: 10.1016/j.jmb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adkins MW, Tyler JK. The histone chaperone Asf1p mediates global chromatin disassembly in vivo. J. Biol. Chem. 2004;279:52069–52074. doi: 10.1074/jbc.M406113200. [DOI] [PubMed] [Google Scholar]
- 58.Driscoll R, Hudson A, Jackson SP. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science. 2007;315:649–652. doi: 10.1126/science.1135862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams SK, Truong D, Tyler JK. Acetylation in the globular core of histone H3 on lysine-56 promotes chromatin disassembly during transcriptional activation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9000–9005. doi: 10.1073/pnas.0800057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varv S, Kristjuhan K, Peil K, Looke M, Mahlakoiv T, Paapsi K, Kristjuhan A. Acetylation of H3 K56 is required for RNA polymerase II transcript elongation through heterochromatin in yeast. Mol. Cell. Biol. 2010;30:1467–1477. doi: 10.1128/MCB.01151-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rufiange A, Jacques PE, Bhat W, Robert F, Nourani A. Genome-wide replication-independent histone H3 exchange occurs predominantly at promoters and implicates H3 K56 acetylation and Asf1. Mol. Cell. 2007;27:393–405. doi: 10.1016/j.molcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan T, Liu CL, Erkmann JA, Holik J, Grunstein M, Kaufman PD, Friedman N, Rando OJ. Cell cycle-and chaperone-mediated regulation of H3K56ac incorporation in yeast. PLoS Genet. 2008;4:e1000270. doi: 10.1371/journal.pgen.1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L, Eugeni EE, Parthun MR, Freitas MA. Identification of novel histone post-translational modifications by peptide mass fingerprinting. Chromosoma. 2003;112:77–86. doi: 10.1007/s00412-003-0244-6. [DOI] [PubMed] [Google Scholar]
- 64.Manohar M, Mooney AM, North JA, Nakkula RJ, Picking JW, Edon A, Fishel R, Poirier MG, Ottesen JJ. Acetylation of histone H3 at the nucleosome dyad alters DNA–histone binding. J. Biol. Chem. 2009;284:23312–23321. doi: 10.1074/jbc.M109.003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Javaid S, Manohar M, Punja N, Mooney A, Ottesen JJ, Poirier MG, Fishel R. Nucleosome remodeling by hMSH2–hMSH6. Mol. Cell. 2009;36:1086–1094. doi: 10.1016/j.molcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kruger W, Peterson CL, Sil A, Coburn C, Arents G, Moudrianakis EN, Herskowitz I. Amino acid substitutions in the structured domains of histones H3 and H4 partially relieve the requirement of the yeast SWI/SNF complex for transcription. Genes Dev. 1995;9:2770–2779. doi: 10.1101/gad.9.22.2770. [DOI] [PubMed] [Google Scholar]
- 67.Kurumizaka H, Wolffe AP. Sin mutations of histone H3: influence on nucleosome core structure and function. Mol. Cell. Biol. 1997;17:6953–6969. doi: 10.1128/mcb.17.12.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muthurajan UM, Bao Y, Forsberg LJ, Edayathumangalam RS, Dyer PN, White CL, Luger K. Crystal structures of histone Sin mutant nucleosomes reveal altered protein–DNA interactions. EMBO J. 2004;23:260–271. doi: 10.1038/sj.emboj.7600046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsieh FK, Fisher M, Ujvari A, Studitsky VM, Luse DS. Histone Sin mutations promote nucleosome traversal and histone displacement by RNA polymerase II. EMBO Rep. 2010;11:705–710. doi: 10.1038/embor.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ye J, Ai X, Eugeni EE, Zhang L, Carpenter LR, Jelinek MA, Freitas MA, Parthun MR. Histone H4 lysine 91 acetylation a core domain modification associated with chromatin assembly. Mol. Cell. 2005;18:123–130. doi: 10.1016/j.molcel.2005.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan Q, Dutt S, Xu R, Graves K, Juszczynski P, Manis JP, Shipp MA. BBAP monoubiquitylates histone H4 at lysine 91 and selectively modulates the DNA damage response. Mol. Cell. 2009;36:110–120. doi: 10.1016/j.molcel.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hyland EM, Cosgrove MS, Molina H, Wang D, Pandey A, Cottee RJ, Boeke JD. Insights into the role of histone H3 and histone H4 core modifiable residues in Saccharomyces cerevisiae. Mol. Cell. Biol. 2005;25:10060–10070. doi: 10.1128/MCB.25.22.10060-10070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 74.Dai J, Hyland EM, Yuan DS, Huang H, Bader JS, Boeke JD. Probing nucleosome function: a highly versatile library of synthetic histone H3 and H4 mutants. Cell. 2008;134:1066–1078. doi: 10.1016/j.cell.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santisteban MS, Arents G, Moudrianakis EN, Smith MM. Histone octamer function in vivo: mutations in the dimer–tetramer interfaces disrupt both gene activation and repression. EMBO J. 1997;16:2493–2506. doi: 10.1093/emboj/16.9.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fleming AB, Kao CF, Hillyer C, Pikaart M, Osley MA. H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Mol. Cell. 2008;31:57–66. doi: 10.1016/j.molcel.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 77.Pavri R, Zhu B, Li G, Trojer P, Mandal S, Shilatifard A, Reinberg D. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125:703–717. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 78.Chandrasekharan MB, Huang F, Sun ZW. Ubiquitination of histone H2B regulates chromatin dynamics by enhancing nucleosome stability. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16686–16691. doi: 10.1073/pnas.0907862106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu LY, Wu J, Ye L, Gavrilina GB, Saunders TL, Yu X. RNF8-dependent histone modifications regulate nucleosome removal during spermatogenesis. Dev. Cell. 2010;18:371–384. doi: 10.1016/j.devcel.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 81.Santos-Rosa H, Kirmizis A, Nelson C, Bartke T, Saksouk N, Cote J, Kouzarides T. Histone H3 tail clipping regulates gene expression. Nat. Struct. Mol. Biol. 2009;16:17–22. doi: 10.1038/nsmb.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakai A, Schwartz BE, Goldstein S, Ahmad K. Transcriptional and developmental functions of the H3.3 histone variant in Drosophila. Curr. Biol. 2009;19:1816–1820. doi: 10.1016/j.cub.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pina B, Suau P. Changes in histones H2A and H3 variant composition in differentiating and mature rat brain cortical neurons. Dev. Biol. 1987;123:51–58. doi: 10.1016/0012-1606(87)90426-x. [DOI] [PubMed] [Google Scholar]
- 85.Rogakou EP, Sekeri-Pataryas KE. Histone variants of H2A and H3 families are regulated during in vitro aging in the same manner as during differentiation. Exp. Gerontol. 1999;34:741–754. doi: 10.1016/s0531-5565(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 86.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 87.Urban MK, Zweidler A. Changes in nucleosomal core histone variants during chicken development and maturation. Dev. Biol. 1983;95:421–428. doi: 10.1016/0012-1606(83)90043-x. [DOI] [PubMed] [Google Scholar]
- 88.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL, Pehrson JR, Berger JM, Kaufman PD, Adams PD. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 91.Kreiling JA, Tamamori-Adachi M, Sexton AN, Jeyapalan JC, Munoz-Najar U, Peterson AL, Manivannan J, Rogers ES, Pchelintsev NA, Adams PD, Sedivy JM. Age-associated increase in heterochromatic marks in murine and primate tissues. Aging Cell. 2011;10:292–304. doi: 10.1111/j.1474-9726.2010.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gevry N, Chan HM, Laflamme L, Livingston DM, Gaudreau L. p21 transcription is regulated by differential localization of histone H2A.Z. Genes Dev. 2007;21:1869–1881. doi: 10.1101/gad.1545707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan HM, Narita M, Lowe SW, Livingston DM. The p400 E1A-associated protein is a novel component of the p53→p21 senescence pathway. Genes Dev. 2005;19:196–201. doi: 10.1101/gad.1280205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O'Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat. Struct. Mol. Biol. 2010;17:1218–1225. doi: 10.1038/nsmb.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feser J, Truong D, Das C, Carson JJ, Kieft J, Harkness T, Tyler JK. Elevated histone expression promotes life span extension. Mol. Cell. 2010;39:724–735. doi: 10.1016/j.molcel.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wood JG, Hillenmeyer S, Lawrence C, Chang C, Hosier S, Lightfoot W, Mukherjee E, Jiang N, Schorl C, Brodsky AS, Neretti N, Helfand SL. Chromatin remodeling in the aging genome of Drosophila. Aging Cell. 2010;9:971–978. doi: 10.1111/j.1474-9726.2010.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feser J, Tyler J. Chromatin structure as a mediator of aging. FEBS Lett. 2010 doi: 10.1016/j.febslet.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459:113–117. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Greer EL, Maures TJ, Hauswirth AG, Green EM, Leeman DS, Maro GS, Han S, Banko MR, Gozani O, Brunet A. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466:383–387. doi: 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheutin T, McNairn AJ, Jenuwein T, Gilbert DM, Singh PB, Misteli T. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science. 2003;299:721–725. doi: 10.1126/science.1078572. [DOI] [PubMed] [Google Scholar]
- 102.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andrews AJ, Chen X, Zevin A, Stargell LA, Luger K. The histone chaperone Nap1 promotes nucleosome assembly by eliminating nonnucleosomal histone DNA interactions. Mol. Cell. 2010;37:834–842. doi: 10.1016/j.molcel.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.North JA, Javaid S, Ferdinand MB, Chatterjee N, Picking JW, Shoffner M, Nakkula RJ, Bartholomew B, Ottesen JJ, Fishel R, Poirier MG. Phosphorylation of histone H3(T118) alters nucleosome dynamics and remodeling. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr304. (May 16, Electronic publication ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]