Abstract
Patient-physician interactions significantly contribute to placebo effects and clinical outcomes. While the neural correlates of placebo responses have been studied in patients, the neurobiology of the clinician during treatment is unknown. This study investigated physicians’ brain activations during patient-physician interaction while the patient was experiencing pain, including a ‘treatment‘, ‘no-treatment’ and ‘control’ condition. Here we demonstrate that physicians activated brain regions previously implicated in expectancy for pain-relief and increased attention during treatment of patients, including the right ventrolateral and dorsolateral prefrontal cortices. The physician’s ability to take the patients’ perspective correlated with increased brain activations in the rostral anterior cingulate cortex, a region that has been associated with processing of reward and subjective value. We suggest that physician treatment involves neural representations of treatment expectation, reward processing and empathy, paired with increased activation in attention-related structures. Our findings further the understanding of the neural representations associated with reciprocal interactions between clinicians and patients; a hallmark for successful treatment outcomes.
Keywords: patient-provider, doctor-patient, placebo, pain, analgesia
Introduction
The placebo effect accounts for significant portions of clinical outcomes in many illnesses, including pain, depression and anxiety 1–5. To date, most placebo research has focused on understanding the neural correlates of the patient’s response to placebos. Little effort has been directed to understanding the physician component of the clinical dyad. This is especially noteworthy since evidence indicate that the physician interaction can be the most robust contributor to placebo responses 2 and meta-analyses of depression randomized controlled trials (RCTs) demonstrate that physicians were responsible for larger treatment effects (9.1%) than the difference between placebo and real drug (3.4%) 6, based on patients’ subjective outcome measures.
Recently, neuroimaging studies have moved beyond subjective reports by obtaining objective correlates of placebo-related changes in the patient’s brain, for example in treatment of pain 7–12, depression 13, 14, anxiety 15, and Parkinson’s disease 16. Evidence suggest that a patient’s response to placebo analgesia is associated with increased activations in brain regions --including the prefrontal cortex 8, 9, 11, 17 and mesolimbic reward circuitry 18-- that may integrate noxious input with expectations of pain relief and thereby modulate pain through the release of various neurotransmitters 8, 10, 19.
Previous studies demonstrate that placebo responses are highly influenced by treatment expectations, both in the patient 20–22 and the treating physician 23, 24. Thus, studying the placebo effect only from the patient’s perspective will give an incomplete understanding of this process. To address this lacuna, we proposed an investigation of the neural correlates of physicians during treatment of patients. We developed a unique setup for functional magnetic resonance imaging (fMRI) that would allow the physician to have direct face-to-face interaction with a patient and perform a pain treatment paradigm while the physician’s brain was scanned.
Recent findings from human experiments suggest that social interaction may be promoted by mirrored brain activations between individuals 25, 26 and evidence from empathy-for-pain studies reveal shared neural representations for own pain and other’s pain 27, 28. Here, we hypothesized that physicians’ administration of pain relief would lead to increased activations in their own brain regions that have been suggested to be implicated in expectancy for pain relief, such as the right ventrolateral prefrontal cortex (VLPFC) 17, 29. We also hypothesized that physicians would activate regions previously implicated in reward and subjective value, such as the ventral striatum 18, 30 and the rostral anterior cingulate cortex (rACC) 7, 8, 10, while they alleviate pain of patients. Regarding the link between brain activations and behavioral traits, we hypothesized that physicians with high perspective-taking skills 31, would display higher satisfaction during treatment and greater activations in our three pre-defined brain regions (VLPFC, rACC, ventral striatum) during treatment of the patient.
Material and Methods
The participating physicians
All physicians (n=18, 10 female, 8 male) had received their medical doctor’s degree within the last 10 years and mean time since graduation was 3.5 years (SD=3). Nine different medical specialties were represented, ranging from clinical pathology to psychiatry; providing a broad range of patient experiences. The number of hours per week that physicians spent in direct contact with patients varied greatly due to their different specialties; mean 34 hours/week (SD=24), ranging from 1 to 80 hours/week. The inclusion criteria required that the physicians were right-handed, enrolled in residency training and that they did not specialize in pain medicine. Pain specialists were excluded because the sham analgesic device we adopted may have aroused suspicion for them. The Institutional Review Board at Massachusetts General Hospital approved the study and physicians were recruited though advertising at different Boston hospitals.
The patients
Two 25-year-old female confederates were trained to play the patient according to a rehearsed script. The two women played the patient in every second experiment, resulting in 9 experiments each. They were both Caucasian and similar in demographic, social and personality aspects. Post-hoc analyses of behavioral and neuroimaging data ensured that there was no significant variance attributable to the person playing the patient. Physicians were told that their patient was a student who volunteered to participate in the study for a monetary compensation.
Procedure
The experiment included four steps: 1) a procedure where the physicians were given pain stimuli and personal experience of the effectiveness of the sham (placebo) analgesic device, to ensure its high credibility 2) patient-physician interaction during a clinical examination 3) physician fMRI scan during patient-physician interaction and treatment using the sham device 4) debriefing.
After giving informed consent, physicians were introduced to a thermal pain stimulator (Pathway-Cheps Medoc) with a 3x3 cm heat probe. Ascending temperatures were applied to the physicians’ volar forearm in order to find a temperature that would represent the physician’s “high pain” rating, i.e. 70 on a 0–100 Numeric Response Scale (NRS) and a “low pain” rating of 10 NRS. The duration of each stimulus was 5 seconds, presented at 30 seconds intervals. Then, physicians were introduced to the sham analgesic device, an electrode on a wristband with wires to an electronics box. The experimenter explained that this was a custom-made Transcutaneous Electrical Nerve Stimulator (TENS) and that it would have the potential to decrease thermal pain. The sham device was attached adjacent to the thermal stimulator on the physicians’ arm. To manipulate the physicians’ expectations of pain relief, they were first given three “high pain” stimuli while told that the analgesic device was turned off. During three following trials, the experimenter surreptitiously lowered the temperatures (fixed range of 3 degrees Celsius) while telling the physicians that the analgesic device was turned on, giving the physicians the impression that the device was highly effective. The procedure was repeated one more time while told that the device was turned off using the “high pain” stimuli. Physicians were asked about their confidence that the analgesic device would be able to relieve thermal pain in a patient, using a scale from 0–100%.
Physicians were introduced to the patient and had 20 minutes to perform a clinical exam according to a given structure, including demographics, medical history, life habits, current medical problems and medications, respiratory exam, heart and blood pressure. The clinical exam was performed in order to establish a realistic rapport between the physician and patient before fMRI scanning, comparable to a standard U.S. doctor’s appointment. The Interpersonal Reactivity Index (IRI) questionnaire31, 32 was used to measure physicians’ self-reported perspective-taking skills before the fMRI scanning session.
fMRI data acquisition
Right after the clinical exam, physicians were placed in the scanner for an individual pain-scan. The heat probe was placed on the physicians’ left arm and a ten minutes scan was performed during intermittent high-pain and low-pain stimuli. Then, the patient was led into the scanner room. The heat probe was taken from the physician and placed on the patient’s arm instead. For more details on the fMRI setup, see Figure 1A. The physician was equipped with a response-device in one hand that would allow for visual analogue scale ratings. The response device had two treatment buttons and physicians were told that one button would activate the analgesic device and that the second button was a dummy button that was not connected to anything. There were three experimental conditions; ‘treatment’, ‘no-treatment’ and ‘control’. During ‘no-treatment’, the patient received high intensity pain while the physician was prompted to press the dummy button, knowing there was no pain relief. The patient reacted with a high-pain facial expression during the 12 seconds of heat administration. During the ‘treatment’ condition, the physician was prompted to activate the analgesic device while believing that the patient was receiving the same high intensity heat. Based on the proven effectiveness of the analgesic device, the patient reacted with a neutral facial expression, giving the impression that the treatment was successful. The third condition was a control task, in which the physician was prompted to press the dummy button while informed that no heat was administered, resulting in a neutral observation of the patient. After each trial, the physicians were asked “How do you feel?” on a scale ranging from −10 (completely dissatisfied) to +10 (completely satisfied). The order of the three conditions was randomized within each run to eliminate any predictability and the patient-physician interaction included a total of 27 trials, 9 for each of the three conditions.
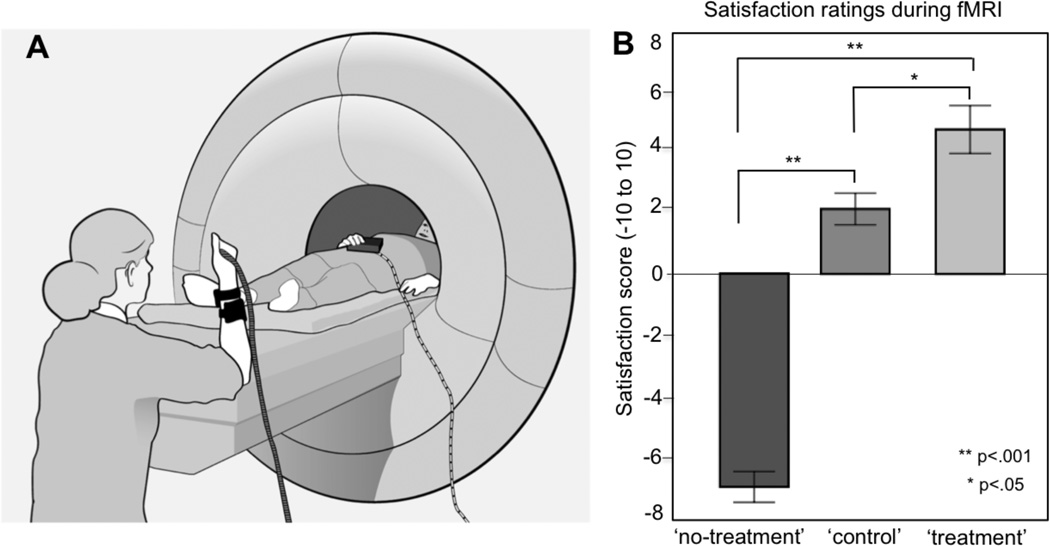
Figure 1.
Experimental setup and physicians’ satisfaction ratings during the three experimental conditions. (A) Illustration of the setup for the fMRI experiment. The physician is lying down in the scanner and the patient is placed opposite the physician, sitting on a chair. A heat pain stimulator is strapped onto the patient’s arm and a sham analgesic device is attached adjacent to the heat stimulator. The physician holds a button box that allows for pressing a ‘pain relief button’, a ‘control button’ and performing self-ratings on a visual analogue scale. The physician and the patient are positioned so that they can have constant eye contact and the physician can see the patient from the waistline and up. Treatment instructions for the physician are displayed on a screen. (B) Results from physicians’ self-ratings during fMRI scanning. After each experimental task, physicians were prompted to answer the question “How do you feel?”. The physicians responded by moving a cursor on a horizontal visual analogue scale anchored by −10 “completely dissatisfied” and +10 “completely satisfied”. A within-subject statistical analysis of the physicians’ ratings (ANOVA) validated that the three conditions ‘treatment’, ‘no-treatment’ and ‘control’ were associated with significantly different feelings.
Parameters of fMRI data acquisition
Measurements of brain activity were performed using a 3 Tesla Siemens MRI System equipped for Echo Planar Imaging (EPI). Physicians were also scanned with a high-resolution MPRAGE sequence for a high-resolution anatomical image. One functional scan was performed during physician pain (192 volumes) and three scans were performed during patient-physician interaction (215 volumes each). Thirty axial interleaved slices (4 mm thick with 1 mm skip) parallel to the anterior and posterior commissure covering the whole brain were acquired with TR=2000 ms, TE=40 ms, flip angle= 90°, and a 3.13 * 3.13 mm in-plane spatial resolution. Visual presentation was performed using E-prime 2.0 software (Psychology Software Tools, USA).
Statistical analyses
All statistical analyses of behavioral data were performed in SPSS 20.0. A statistical significance threshold of p<.05 was considered and all tests were two-tailed. The difference in physicians’ ratings between the three conditions, ‘treatment’, ‘no-treatment’ and ‘control’, was analyzed using a repeated-measures ANOVA. Correlation analyses were performed using Pearson’s r.
Pre-processing and analyses of imaging data were performed using the Statistical Parametric Mapping8 (SPM8) software (Wellcome Trust Centre for Neuroimaging, London, UK) and Matlab7.4 (Mathworks). All functional brain volumes were realigned to the first volume, spatially normalized to a standard EPI template, and finally smoothed using an 8 mm full-width at half-maximum isotropic Gaussian kernel. High-pass filtering of fMRI data (cutoff 128s) and correction for temporal autocorrelations using AR(1) were also done. The univariate data analysis was performed using the general linear model (GLM). The individual design matrix for each physician (first-level) included a total of 15 regressors, including physicians’ own pain and patient-physician interaction. A file containing the movement parameters for each individual (3 translation, 3 rotation axes) was obtained from the realignment step and saved for inclusion in the model. Regression coefficients were estimated using least squares within SPM8. Specific effects were tested by creating contrasts of the parameter estimates, resulting in a t-statistic for each voxel. After the individual first-level estimations, a second-level analysis was performed using a one-way within-subject ANOVA with 3 contrasts: (1) ‘treatment’ versus ‘control’, (2) ‘no-treatment’ versus ‘control’ and (3) ‘treatment’ versus ‘no-treatment’. The contrast between ‘treatment’ and ‘control’ was balanced since it compared two conditions where the patient was not in pain and had a neutral facial expression.
The physicians’ brain activations during the initial pain scan ([painful stimulation-baseline]) was determined by a one-sample t-test and used as a mask for the patient-physician contrasts. A masking procedure is a conservative test for commonly or uniquely activated networks between two conditions, using an inclusive or exclusive mask. All analyses were performed using an initial image threshold of p<.005 (uncorrected) with a spatial extent threshold of 30 contiguous voxels, and all reported results were FWE-corrected at the cluster level p<.05. Extraction of parameter estimates was performed by extracting a 3 mm sphere around the peak voxel of a significant cluster.
Results
Physician behavioral data
The demonstration of the sham analgesic device led to a significant decrease in physicians’ ratings of experimental pain, t(17)=7.5, p<.001. When the experimenter indicated that the device was turned off, physicians rated the painful experience on average 53 (SD=20) on a 0–100 Numeric Response Scale, compared to 30 (SD=14) when the analgesic device was “turned on”. Physicians’ expectancy of the device was high; they rated that they had on average 75% (SD=5) confidence that the device would relieve the patient’s pain, rated on a 0–100 scale where 0 represented no confidence and 100 complete confidence.
The three different conditions during the fMRI experiment, ‘treatment’, ‘no-treatment’ and ‘control’, gave rise to significantly different ratings on the −10 to +10 satisfaction scale, F(2, 30)=67, p<.001, representing strong feelings of dissatisfaction during ‘no-treatment’ (M= −7, SEM=0.5), neutral/positive feelings during ‘control’ (M=2, SEM=0.5) and high satisfaction during ‘treatment’ (M=5, SEM=0.8) All pairwise comparisons were significant, validating that the three conditions represented significantly different subjective states in the physicians: ‘no-treatment’/’control’ (p<.001); ‘treatment’/’control’ (p<.05); ‘no-treatment’/’ treatment’ (p<.001), see Figure 1B.
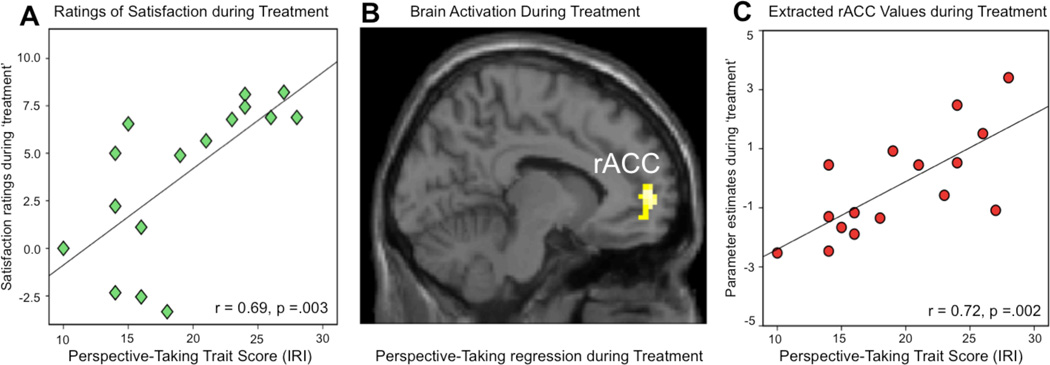
The physicians’ confidence in the analgesic device, based on their 0–100% rating, was significantly correlated with ratings of satisfaction during the ‘treatment’ condition during the fMRI experiment, (r=.65, p<.01, two tailed). Moreover, physicians with high perspective-taking scores reported significantly higher satisfaction during the ‘treatment’ condition, indicated by a significant correlation (r=.69, p<.005, two-tailed), see Figure 4A.
Figure 4.
Perspective-taking skills during patient-physician interaction. Perspective-taking skills were associated with the physician’s satisfaction during treatment and increased activation of the rACC. (A) The physicians’ perspective-taking scores (IRI) correlated significantly to ratings of satisfaction during the ‘treatment’ condition. With higher perspective-taking skills, physicians felt more treatment-related satisfaction (r=.69, p=.003, two-tailed). (B) A regression analysis for the contrast ‘treatment’ versus ‘control’, using the physicians perspective-taking scores as covariate, demonstrated a significant increase of rACC activity with increased perspective-taking scores ([−12, 56, −2]). The initial statistical threshold was p<.005 with 30 contiguous voxels. (C) Illustration of the data points from the perspective-taking regression analysis (shown in panel B). A scatterplot of the extracted rACC parameter estimates and the physicians’ perspective-taking scores was performed for illustrative reasons but should not be used for statistical inference since it would infer circularity.
Neuroimaging data
The initial fMRI scan, in which calibrated thermal pain was administered to the physicians, resulted in activation of several regions of the cerebral pain network; including the bilateral insulae, cingulum and secondary somatosensory cortex (S2) (Table 1).
Table 1.
Physicians’ brain activations in response to thermal pain. Randomized blocks of calibrated thermal pain (12 s duration) were administered to the physicians’ left volar forearm. The pain main effect contrast was created by comparing the signal intensity during pain, compared to baseline. One significant cluster comprised more than 3000 voxels, encompassing several different significant sub-clusters, indicated by italics in the “Cluster size” column. Coordinates (x, y, z) correspond to the anatomical space as defined in the MNI standard brain atlas. All reported clusters are FWE-corrected at the cluster level.
| Pain > baseline | MNI x | MNI y | MNI z | Cluster size (voxels) |
Z-score | p-value corrected cluster |
|---|---|---|---|---|---|---|
| R. Anterior Insula | 33 | 5 | 10 | 3598 | 5.33 | 0.001 |
| R. Lateral prefrontal cortex | 42 | 44 | 19 | 3598 | 5.23 | 0.001 |
| R. Posterior Insula | 36 | 5 | −8 | 3598 | 5.19 | 0.001 |
| R. S2 | 57 | −22 | 28 | 3598 | 4.25 | 0.001 |
| R. Cingulate cortex | 6 | 11 | 43 | 142 | 3.75 | 0.050 |
| L. Anterior Insula | −33 | 5 | 10 | 344 | 3.69 | 0.048 |
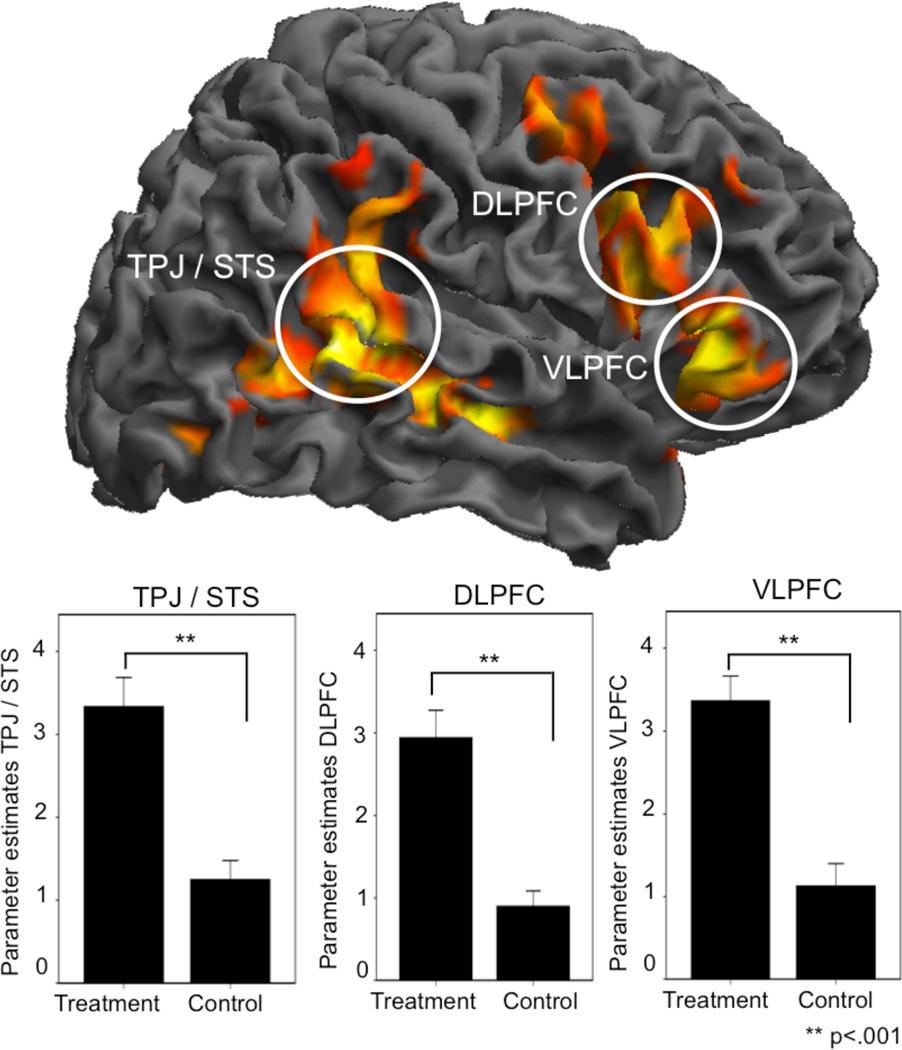
During patient-physician interaction, the balanced contrast between ‘treatment’ versus ‘control’, resulted in increased activations in five different brain regions ([MNI coordinates]); the right inferior frontal gyrus, including the VLPFC ([48, 29, 1]) and dorsolateral prefrontal cortex (DLPFC) ([48, 20, 28]), right temporoparietal junction (TPJ)/posterior superior temporal sulcus (pSTS) ([63, −46, 10]), right ventral striatum ([15, 2, 10]) and cerebellum ([−15, −76, −38)]. Moreover there was a deactivation in the right primary somatosensory cortex (S1) ([21, −37, 79]), contralateral to the previously applied heat stimuli during the physician pain-scan (see Table 2). When analyzing ‘treatment’ versus ‘control’ by using the physicians’ own pain as an inclusive mask, there was overlapping activity in the right anterior insula (AI), bordering the inferior frontal gyrus ([48, 26, 1]; voxels 179; z-score 3.65); indicating involvement of a region previously implicated in empathy-for-pain tasks. When using the physicians’ pain as an exclusive mask, all other findings of the ‘treatment’ versus ‘control’ contrast survived, emphasizing the independence of treatment-related brain activations, compared to overlapping regions of treatment and pain, reflected in the AI.
Table 2.
Physicians’ brain activations during doctor-patient interaction. All contrasts are derived from a one-way within-subject ANOVA, including 3 conditions: ‘treatment’, ‘notreatment’, and ‘control’. Coordinates (x, y, z) correspond to the anatomical space as defined in the MNI standard brain atlas. All reported clusters are FWE-corrected at the cluster level.
| Treatment > Control | MNI x |
MNI y |
MNI z |
Cluster size (voxels) |
Z-score | p-value cluster corrected |
|---|---|---|---|---|---|---|
| R. TPJ / pSTS | 63 | −46 | 10 | 1013 | 5.52 | 0.001 |
| L. TPJ | −48 | −46 | 10 | 301 | 4.69 | 0.010 |
| R. Inf frontal gyrus (VLPFC, DLPFC) | 48 | 29 | 1 | 1191 | 4.55 | 0.001 |
| L. Cerebellum | −15 | −76 | −38 | 245 | 4.10 | 0.042 |
| Control > Treatment | ||||||
| L. Parahippocampal gyrus/PCC | −33 | −31 | −14 | 323 | 4.19 | 0.038 |
| R. S1 and parietal cortex | 21 | −37 | 79 | 1048 | 4.16 | 0.001 |
| No-treatment > Control | ||||||
| R. TPJ | 48 | −46 | 10 | 756 | 4.76 | 0.001 |
| R. Anterior Insula | 48 | 29 | 1 | 195 | 3.84 | 0.018 |
| Control > No-treatment | ||||||
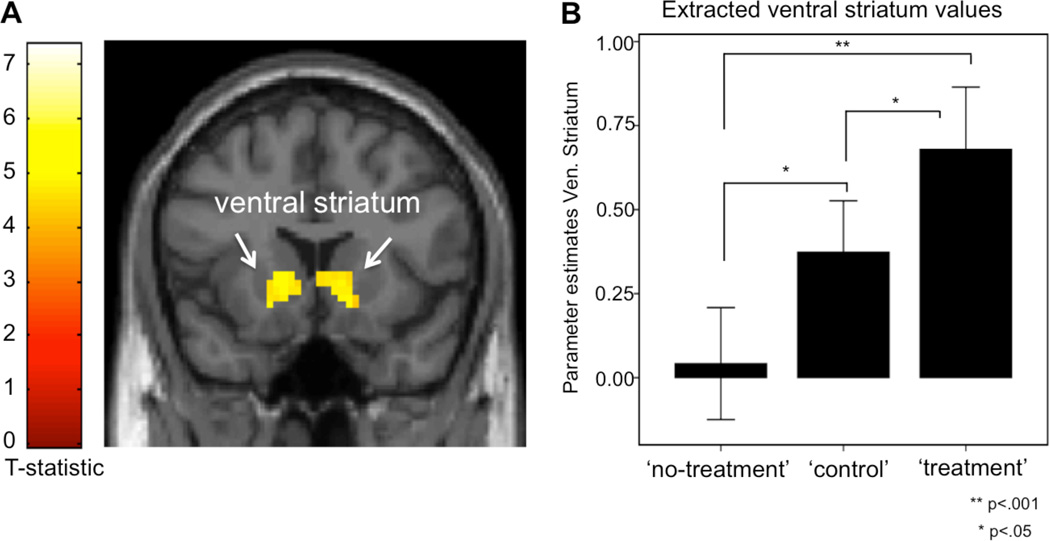
| R. Ventral striatum | 9 | 26 | 1 | 426 | 3.56 | 0.012 |
| Treatment > No-treatment | ||||||
| R. Inferior parietal cortex | 39 | −49 | 61 | 621 | 4.08 | 0.001 |
| R. Inf frontal gyrus (VLPFC, DLPFC) | 36 | 41 | 37 | 302 | 3.33 | 0.022 |
| No-treatment > Treatment | ||||||
| No clusters | ||||||
A regression analysis, using the ‘treatment’ versus ‘control’ contrast and the physicians’ perspective-taking scores as covariate, confirmed our hypothesis about the impact of perspective-taking skills on brain activations during treatment. A positive regression contrast revealed that higher perspective-taking scores were associated with increased activity in the rACC during treatment ([−12, 56, −2]; voxels 183; z-score 3.41) (Figure 4B).
Two clusters were significantly activated during ‘no-treatment’, compared to the control condition: the right TPJ ([48, −46, 10]) and the right AI ([48, 29, 1]). The opposite contrast, indicating higher activity during the control condition, compared to ‘no-treatment’, revealed significant activations in the bilateral ventral striatum ([9, 26, 1]) (see Figure 3). As a validation of previous findings of brain activations associated with empathy-for-pain tasks, we used the physicians’ own pain activations as an inclusive mask and found overlapping right AI activations for the physicians’ pain and activations during the ‘no-treatment’ task ([45, 20, 10]; voxels 107; z-score 3.57). The use of the physicians’ pain matrix as an exclusive mask for the ‘no-treatment’ contrast resulted in significant activations in the right TPJ ([48, −46, 10]; voxels 756; z-score 4.76), and left TPJ ([−27, −88, −8]; voxels 312; z-score 4.11), indicating that the activation of the TPJ was independent from the physicians own pain processing regions.
Figure 3.
Activation of the ventral striatum during patient-physician interaction. The activity of the reward-related circuitry was significantly increased during the ‘treatment’ condition compared to ‘control’, represented in the right ventral striatum. The same effect was found for the ‘control’ versus ‘no treatment’ contrast, represented in the bilateral ventral striatum, shown here. The extraction of the parameter estimates from the peak coordinate (3 mm sphere) in the right ventral striatum ([9, 26, 1]) indicate a dose effect of the physicians’ positive feelings during patient-physician interaction, i.e. the ‘no-treatment’ condition was associated with little or no activation of the ventral striatum, whereas the ‘treatment’ condition was associated with most robust increased activations in this region. The parameter estimates are represented in the bar-plot (± 1 Standard Error). The initial statistical image threshold was p<.005 with 30 contiguous voxels.
An exploratory analysis between ‘no-treatment’ AI activations ([45, 20, 10]) and ‘treatment’-related activations, revealed a significant partial correlation (controlling for parameter estimates during the common control condition) between the AI and the VLPFC ([48, 29, 1]), r=.66, p<.05, Bonferroni corrected. There were no similar correlations between the AI and TPJ (r=.41, p=.15) or AI and ventral striatum (r=.03, p=.91).
Discussion
The present data provides the first description of the neural correlates of the physician component of the clinical dyad. We found that physicians, while treating patients, activate the right VLPFC. Among other functions, this region has been implicated in placebo responses. For example, in experiments on placebo effects in volunteers, the orbitofrontal cortex and right VLPFC have repeatedly been activated during top-down modulation of pain and negative affect 9, 11, 15, 17, 29, suggesting a cognitive mechanism for endogenous control of a variety of symptoms. It has been suggested that the VLPFC does not directly modulate incoming nociceptive signals. Instead, this region may represent expectancy for relief by exerting control over brain circuitries with neurochemical resources to modulate pain 12, 17, 33. Herein, we speculate that physicians activated similar regions, during treatment of a patient, suggesting a model of the patient-physician relationship that includes two dimensions of expectancy processing.
In line with our hypothesis, the physicians’ perspective-taking skills were correlated to brain activations and subjective ratings during the treatment condition. The perspective-taking score is an independent measure of the ability to imagine how things look from another person’s perspective 31, often referred to as the cognitive aspect of empathy 34. High perspective-taking scores have previously been associated with greater somatosensory activations during observations of touch in others 35 and greater recruitment of brain regions involved in social cognition regions during a social belief task 36. In line with previous validations of the relevance of perspective-taking skills in social interactions and clinical expertise 37, 38, the present data suggest that physicians with high perspective-taking skills were more likely to activate the rACC during ‘treatment’ and, if our hypothesis is true, simulate the patient’s pain relief. The rACC is a key region in a placebo associated network, often activated in combination with the prefrontal cortex 7–10, 15, and further validated in studies of opioid receptor function 12, 33. The rACC is also implicated in the coding of value 39, 40 and might therefore be a correlate of the physicians motivation to treat during the treating task. Future studies will have to verify if the ability of physicians to activate brain regions for pain control and subjective value during administration of treatment is related to measurable clinical outcomes in patients.
Physicians had increased neural activity in the DLPFC during treatment, a region involved in several higher functions such as sequencing, planning, attention and working memory. Recent studies have demonstrated that the executive functions of the DLPFC are also applicable to social cognition 41, 42, where the DLPFC may facilitate complex social reasoning and store social schemata used for familiar social interaction 43. In the present study, the treatment condition was a highly directed social interaction of adhering to an experimental protocol with strict requirements that may have required more DLPFC involvement to sustain the social scheme. Also, it is likely that the treatment task required increased attention on the patient, a process that could contribute to increased DLPFC activity 44. Also, the bilateral TPJ and the pSTS were activated during treatment, two regions well known for their role in social interaction 45. Activity in the right TPJ/pSTS may be predicted by social stimuli that describe a person’s intentions 46, 47. A possible role within this context is therefore that it represents the physician’s increased reading of the patient’s response during treatment. Along these lines, the DLPFC and the TPJ/pSTS may be crucial for reciprocal and efficient patient-physician interactions, however, these regions are activated by many types of social interactions, and may not have a specific role in relieving the pain of others.
The ‘no-treatment’ condition was comparable to previous neuroimaging studies that used empathy-for-pain paradigms 27, 28, meaning that the physicians were watching the patient in pain without giving any pain relief. The contrast ‘no-treatment’ versus ‘control’ represented two significantly different facial expressions in the patient: ‘no-treatment’ was associated with a high pain facial expression and the ‘control’ condition was associated with a neutral face. Our data display a functional overlap in the AI for the ‘no-treatment’ condition and the physicians’ own pain, possibly reflecting a previously described empathy-for-pain function reflected in the AI 27, 28. However, the anterior insula is a structure with many functions that might reflect a broader type of emotional and homeostatic mapping and regulation 48. The significant correlation between AI activations during ‘no-treatment’ and VLPFC activations during ‘treatment’ points towards a reciprocal relationship between the experience of other’s pain and the ability to simulate the patients’ pain relief. Successful social interactions depend heavily on predictions of the thoughts and intentions of others 45 and it is possible that AI activations during an empathy-for-pain task provides learning for predictions of the patient’s reactions during treatment.
The increased activation of the ventral striatum during the ‘control’ task, compared to ‘no-treatment’, may indicate a relative feeling of relief/reward since no heat stimuli were given to the patient. The ventral striatum is a key region for dopamine-related reward processing49 and has been also been observed in placebo analgesia 15, 18, 30. It is possible that the activation of the ventral striatum reflects the physicians’ subjective level of well being during the experiment without necessarily representing the interaction with the patient. The activation of the reward circuitry during treatment may represent a motivational aspect of relieving the patient’s pain, similar to the suggestions by Decety and colleagues who found increased involvement of the ventral striatum during imagination of relieving the suffering of others 50. The correlation between ratings of satisfaction and high perspective-taking scores, and between ratings of satisfaction and increased activation of the rACC during ‘treatment’, might be related to the activity of ventral striatum, based on the known interaction between opioid- and dopamine-related reward processing in the brain 51.
One limitation of our study is that we did not measure the physicians’ neural response to expectations for their own pain relief. We plan to do this in a future experiment.
In summary, understanding the neural underpinnings of the clinician component of the clinical dyad may be important for the understanding and improvement of treatment efficacy. We propose that a complex set of brain events, including deep understanding of the patient’s state, close monitoring and feedback of the patient’s expressions, possibly in combination with the physician’s own expectations of relief and feelings of reward, may be involved in successful treatment interactions. Previous behavioral research imply that physicians’ expectancies modulate clinical outcomes 23, 24 and further research is warranted to see whether their activations of expectancy and reward-related brain regions are related to clinical outcomes.
Figure 2.
Physician brain activations during treatment of a patient. The ‘treatment’ condition, compared to the ‘control’ condition, was associated with significantly increased brain activity in four clusters: right DLPFC ([48, 20, 28]), VLPFC ([48, 29, 1]), TPJ/pSTS ([63, −46, 10]) and the cerebellum ([−15, −76, −38]), as illustrated by the rendered brain in this figure. The initial statistical image threshold was p<.005 with 30 contiguous voxels and all results were FWE-corrected at the cluster level. The contrast ‘treatment’ versus ‘control’ was balanced since the physicians got identical visual inputs during both conditions; the patient was not in pain and kept a neutral face during both conditions. The only difference was the physicians’ knowledge that he/she had relieved the patient’s pain during ‘treatment’ whereas the ‘control’ condition did not include any pain application in the first place. The extracted parameter estimates from the peak activations (3 mm sphere) during ‘treatment’ and ‘control’ are represented in the three bar-plots (± 1 Standard Error). A complete list of the significant areas can be found in table 2.
Acknowledgements
The authors wish to thank Dr. Jonathan Berrebi and Dr. Chantal Berna who provided technical and clinical expertise for this study. We also wish to thank Prof. Martin Ingvar for the valuable theoretical contributions to the study design and Magnus Gyllenswärd for help with graphic illustrations. The work is supported by the Swedish Society for Medical Research (SSMF) and the Swedish Council for Working Life and Social Research to K. Jensen, K24AT004095 (NCCAM) and P01 AT006663 (NCCAM) to T. Kaptchuk, KO1AT003883 (NCCAM), R21AT004497 (NCCAM), R03AT218317 (NIDA), R01AT006364 (NCCAM) to J. Kong, R01AT005280 (NCCAM) to R. Gollub, M01-RR-01066 and UL1 RR025758-01 for Clinical Research Center Biomedical Imaging Core from National Center for Research Resources (NCRR), and P41RR14075 for Center for Functional Neuroimaging Technologies from NCRR.
Footnotes
Conflicts of interest
The authors report no conflicts of interest.
References
- 1.Finniss DG, Benedetti F. Mechanisms of the placebo response and their impact on clinical trials and clinical practice. Pain. 2005;114(1-2):3–6. doi: 10.1016/j.pain.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336(7651):999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93(1):77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 4.Rief W, Nestoriuc Y, Weiss S, Welzel E, Barsky AJ, Hofmann SG. Metaanalysis of the placebo response in antidepressant trials. J Affect Disord. 2009;118(3):1–8. doi: 10.1016/j.jad.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Wechsler ME, Kelley JM, Boyd IO, Dutile S, Marigowda G, Kirsch I, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365(2):119–126. doi: 10.1056/NEJMoa1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKay KM, Imel ZE, Wampold BE. Psychiatrist effects in the psychopharmacological treatment of depression. J Affect Disord. 2006;92(2-3):287–290. doi: 10.1016/j.jad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, et al. Activation of the Opioidergic Descending Pain Control System Underlies Placebo Analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, et al. Brain Activity Associated with Expectancy-Enhanced Placebo Analgesia as Measured by Functional Magnetic Resonance Imaging. J Neurosci. 2006;26(2):381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and Opioid Analgesia-- Imaging a Shared Neuronal Network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 11.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-Induced Changes in fMRI in the Anticipation and Experience of Pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 12.Zubieta J-K, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo Effects Mediated by Endogenous Opioid Activity on {micro}-Opioid Receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leuchter AF, Cook IA, Witte EA, Morgan M, Abrams M. Changes in brain function of depressed subjects during treatment with placebo. The American journal of psychiatry. 2002;159(1):122–129. doi: 10.1176/appi.ajp.159.1.122. [DOI] [PubMed] [Google Scholar]
- 14.Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, et al. The functional neuroanatomy of the placebo effect. The American journal of psychiatry. 2002;159(5):728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 15.Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in Emotional Processing-- Induced Expectations of Anxiety Relief Activate a Generalized Modulatory Network. Neuron. 2005;46(6):957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 16.de la Fuente-Fernandez R, Ruth T, Sossi V, Schulzer M, Calne D, Stoessl A. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 17.Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. A prefrontal non-opioid mechanism in placebo analgesia. Pain. 2010;150(1):59–65. doi: 10.1016/j.pain.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta J-K. Individual Differences in Reward Responding Explain Placebo-Induced Expectations and Effects. Neuron. 2009;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3(70) doi: 10.1126/scitranslmed.3001244. 70ra14. [DOI] [PubMed] [Google Scholar]
- 20.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious Expectation and Unconscious Conditioning in Analgesic, Motor, and Hormonal Placebo/Nocebo Responses. J Neurosci. 2003;23(10):4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124(1-2):126–133. doi: 10.1016/j.pain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients: An empirical investigation. Pain. 2003;105(1-2):17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 23.Gracely R, Dubner R, Deeter W, Wolskee P. Clinicians' expectations influence placebo analgesia. Lancet. 1985;1(8419):43. doi: 10.1016/s0140-6736(85)90984-5. [DOI] [PubMed] [Google Scholar]
- 24.Levine JD, Gordon NC. Influence of the method of drug administration on analgesic response. Nature. 1984;312:755–756. doi: 10.1038/312755a0. [DOI] [PubMed] [Google Scholar]
- 25.Nummenmaa L, Glerean E, Viinikainen M, Jaaskelainen IP, Hari R, Sams M. Emotions promote social interaction by synchronizing brain activity across individuals. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1206095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens G, Silbert L, Hasson U. Speaker-listener neural coupling underlies successful communication. Proc Natl Acad Sci U S A. 2012;109(27):9599–9604. doi: 10.1073/pnas.1008662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochsner KN, Zaki J, Hanelin J, Ludlow DH, Knierim K, Ramachandran T, et al. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cogn Affect Neurosci. 2008;3(2):144–160. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for Pain Involves the Affective but not Sensory Components of Pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, et al. The neural correlates of placebo effects: a disruption account. NeuroImage. 2004;22(1):447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 30.Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. The Journal of neuroscience. 2009;29(15):4882–4887. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980;10(85) [Google Scholar]
- 32.Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–126. [Google Scholar]
- 33.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamm C, Batson CD, Decety J. The Neural Substrate of Human Empathy: Effects of Perspective-taking and Cognitive Appraisal. Journal of Cognitive Neuroscience. 2007;19(1):42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- 35.Schaefer M, Heinze H-J, Rotte M. Embodied empathy for tactile events: Interindividual differences and vicarious somatosensory responses during touch observation. NeuroImage. 2012 doi: 10.1016/j.neuroimage.2012.01.112. In Press. [DOI] [PubMed] [Google Scholar]
- 36.Falk EB, Spunt RP, Lieberman MD. Ascribing beliefs to ingroup and outgroup political candidates: neural correlates of perspective-taking, issue importance and days until the election. Philos Trans R Soc Lond B Biol Sci. 2012;367(1589):731–743. doi: 10.1098/rstb.2011.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decety J, Yang C-Y, Cheng Y. Physicians down-regulate their pain empathy response: An event-related brain potential study. NeuroImage. 2011;50(4):1676–1682. doi: 10.1016/j.neuroimage.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, Hung D, et al. Expertise modulates the perception of pain in others. Curr Biol. 2007;17(19):1708–1713. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature neuroscience. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrovic P, Pleger B, Seymour B, Kloppel S, De Martino B, Critchley H, et al. Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. J Neurosci. 2008;28(42):10509–10516. doi: 10.1523/JNEUROSCI.2807-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nature neuroscience. 2011;14(11):1468–1474. doi: 10.1038/nn.2933. [DOI] [PubMed] [Google Scholar]
- 42.Buckholtz JW, Asplund CL, Dux PE, Zald DH, Gore JC, Jones OD, et al. The neural correlates of third-party punishment. Neuron. 2008;60(5):930–940. doi: 10.1016/j.neuron.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Rankin KP. The Human Frontal Lobes: Functions and Disorders. vol. 2. New York: The Guilford Press; 2007. [Google Scholar]
- 44.Posner MI. Attention: the mechanisms of conciousness. Proc Natl Acad Sci USA. 1994;2(91):7390–7403. doi: 10.1073/pnas.91.16.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frith CD. The social brain? Philos Trans R Soc Lond B Biol Sci. 2007;362(1480):671–678. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young L, Dodell-Feder D, Saxe R. What gets the attention of the temporoparietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia. 2010;48(9):2658–2664. doi: 10.1016/j.neuropsychologia.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Saxe R, Xiao DK, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42(11):1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- 49.Schultz W. Behavioral theories and the neurophysiology of reward. Annual review of psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 50.Decety J, Porges EC. Imagining being the agent of actions that carry different moral consequences: an fMRI study. Neuropsychologia. 2011;49(11):2994–3001. doi: 10.1016/j.neuropsychologia.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 51.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of general psychiatry. 2008;65(2):220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]