Majority of patients with AML achieve complete remission (CR) after induction therapy. Despite intensive consolidation regimens, less than one third of adult patients with AML are cured, mainly due to a high incidence of relapse.1 In a recently published randomized trial, treatment with azacitidine increased overall survival relative to supportive care in patients with myelodysplastic syndrome (MDS).2 Therapy with decitabine (DAC) also improves outcomes in MDS, including time to AML transformation or death.3 DAC is also active in AML and produces CRs in a number of patients.4
We and others have reported that DNA methylation increases at relapse in patients with AML and may predict the occurrence of relapse, suggesting its involvement in disease progression and providing a rationale for the use of epigenetic agents in maintenance.5, 6 In this study, we compared DAC to conventional care (CC), including low dose subcutaneous cytarabine, prolonged intensive chemotherapy, or observation in patients with AML in their first or subsequent CR.
Inclusion criteria included age ≥ 18 years, verified CR or CRp (CR without platelet recovery to > 100x109/L); adequate organ function (creatinine ≤ 2.0 mg/dL, bilirubin ≤ 3.5 mg/dL, ALT and AST ≤3 times upper limit of normal); and ECOG performance status of 0–2. Patients eligible for an allogeneic SCT were allowed to participate. Exclusion criteria were active and uncontrolled disease or infection, HIV, pregnancy, lactation. The study was conducted between August 2006 and October 2009.
Fifty patients (median age 57 years, range 24–79) were enrolled, of which 45 (19 male and 26 female) patients (including 35 in first CR and 10 in subsequent CR) were evaluable. Twenty seven patients were ≤ 60 years of age and 18 > 60 years. Seven patients had treatment-related AML and one had secondary AML. Median follow-up was 44.9 months. Among the 45 evaluable patients, 20 received DAC 20 mg/m2 IV daily x 5 every 4–8 weeks; DAC was then continued for a total of 12 cycles, until unacceptable grade 3 or above toxicity developed or until disease relapsed. Twenty five patients were randomized to CC, including 6 (24%), 9 (36%), and 10 (40%) who were assigned by to observation, low dose subcutaneous ara-C (LDAC), and intensive chemotherapy (IC), respectively. The length of CC treatment was at the discretion of the treating physicians. Primary endpoint was incidence of relapse at one year. We monitored minimal residual disease (MRD) by multi-parameter flow cytometry (MFC) and studied DNA methylation as a predictor of outcome.
Baseline characteristics were relatively balanced between patients treated with DAC or CC (Table 1A). Patients receiving DAC were older [median age, 62 years (range, 24–79)] than those in the CC arm [median age, 53 years (range 31–79)](p=0.11). Fifty five percent of patients were > 60 years in the DAC group versus 28% patients in the CC group. All patients in CC group who received IC were ≤ 60 years. Overall, 10 (22%) of patients had an unfavorable karyotype (including -5/-7/complex, 11q/complex, isolated -5 or -7), including 25% in the DAC and 20% in CC arm. Thirty four (76%) patients had an intermediate karyotype (DAC, 75%; CC, 76%); one relapsed patient with favorable cytogenetics [inv(16)] received CC.
Table 1A.
Patients characteristics
| Characteristics | Decitabine (n=20) | Conventional Care (n=25) | p-value |
|---|---|---|---|
| Age, median [range], years | 62, [24–79] | 53 [31–72] | 0.10 |
| ≤ 60 | 9 (45) | 18 (72) | 0.12 |
| > 60 | 11 (55) | 7 (28) | |
|
| |||
| CR1 | 14 (70) [1CRp] | 21 (83) [1CRp] | 0.26 |
| CR2 | 6 (30) [1CRp] | 3 (12) | |
| CR3 | 0 | 1 (4) | |
|
| |||
| Induction regimen | 0.20 | ||
| SDAC + Anthracycline (3+7) | 7 (35) | 3 (12) | |
| HiDAC + Anthracycline | 11 (55) | 18 (72) | |
| Single agent (e.g. Clofarabine) | 2 (10) | 4 (16) | |
|
| |||
| No. of consolidation cycles | 0.19 | ||
| ≤ 1 | 8 (40) | 5 (20) | |
| > 1 | 12 (60) | 20 (80) | |
|
| |||
| Cytogenetics | 0.99 | ||
| Intermediate | 15 (75) | 19 (76) | |
| Unfavorable | 5 (25) | 5 (20) | |
| Favorable (relapsed) | 0 | 1 (4) | |
The median number of cycles of DAC administered was 4.5 (range, 1–12). For the CC group, the median number of LDAC cycles was 5 (range, 1–14), and the median number of IC cycles was 1 (range, 1– 2). Two patients underwent SCT in CC arm and one is alive to date; 3 patients underwent SCT in the DAC arm, and one is alive. Toxicity in the DAC arm was limited; grades 3 and 4 events are shown in Table 1B.
Table 1B.
Toxicities in the DAC arm
| Toxicity (Grade 3 and 4) | Patients (n=20) |
|---|---|
|
| |
| Thrombocytopenia, reversible | 14 |
|
| |
| Neutropenia, reversible | 19 |
|
| |
| Non-hematological Infections | |
| Line-related | 1 |
| Cellulitis (finger) | 1 |
| Cellulitis (scrotum) | 1 |
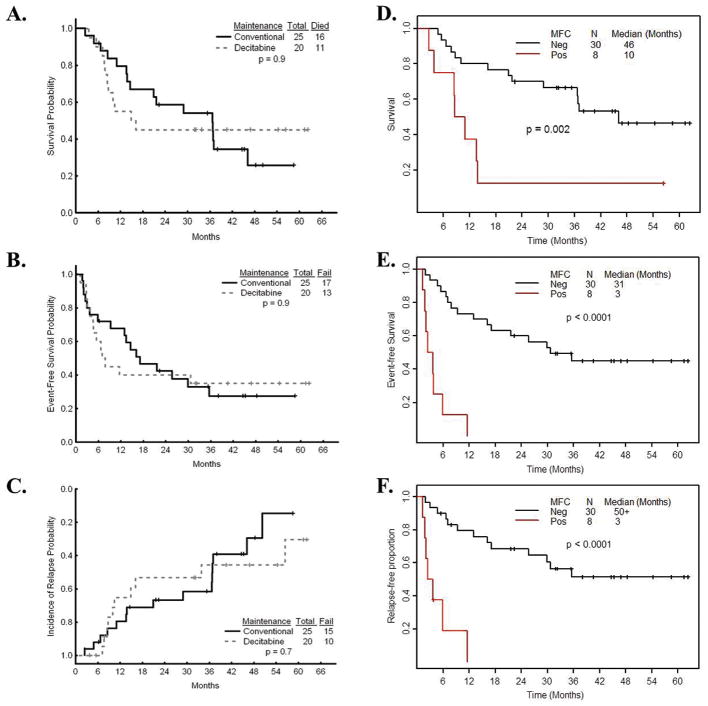
The study was terminated early by the institutional DSMB due to the higher incidence of relapse at 1 year in the DAC arm (Figure 1, A–C). At a median follow-up time of 44.9 months (range, 6.3 – 62.3), 10 patients on the DAC arm and 10 patients on the CC arm have remained in remission, and 10 of 20 (50%) on DAC versus 15 of 25 (60%) on CC arm have relapsed (p = 0.7) (Figure 1C). No significant difference in overall survival (OS) or event-free survival (EFS) was observed, with OS rate 45% on DAC versus 36% on CC group (p=0.9) and the EFS rate 35% on DAC versus 32% on CC group (p=0.9) (Figure 1, A–B). None of the 7 patients who received maximum number of cycles (11–12 cycles of DAC) have relapsed, and 6 of them are alive.
Figure 1.
(A–C) Kaplan-Meier estimates for 45 patients, including 25 patients on the CC arm and 20 patients on the DAC arm of (A) OS, (B) EFS and (C) incidence of relapse are shown. There was no significant difference noted between the group (P-values of 0.9, 0.9 and 0.7, respectively). (D–F) Kaplan-Meier estimates for MFC for 38 patients, including 30 patients with negative and 8 patients with positive MFC at the time of study entry. (D) OS, (E) EFS and (F) Cumulative incidence of relapse are shown. There was significant difference between the groups (P-values of p = 0.002, p < 0.0001 and p < 0.0001, respectively, by log-rank test).
MRD assays by MFC were performed at enrollment and at approximately 3–6 month intervals while on the study on bone marrows of 38 patients, including 16 patients on the DAC and 22 on the CC arm. MFC identified MRD in 9 of 20 (45%) patients with subsequent relapse, at levels from 0.04%–1% of total cells. In 14 patients remaining alive in CR, no MRD was identified. Relapses occurred 1.5 to 10 months after the first positive MFC.
A multivariate Cox regression model was used to assess simultaneously the effect of two or more variables on OS or EFS (Table 1C). Adjusting for treatment, age and cytogenetics, positive MFC at study entry was associated with short survival [hazard ratio (HR)=5.24; p=0.0004]. There was no significant difference on survival by treatment groups (HR=0.63; p=0.32), age (HR=1.013; p=0.51), and cytogenetics (HR=2.32, p=0.104). After adjusting for treatment, age and cytogenetics, positive MFC was also associated with short EFS [hazard ratio (HR)=14.24; p<0.0001]. There was no significant difference on EFS by treatment groups (HR= 0.7; p=0.4), age (HR=1.003; p=0.85), and cytogenetics (HR=1.75, p=0.23).
Table 1C.
Multivariate Cox regression model for OS and EFS with FC as a time-change covariate adjusting for treatment, age and cytogenetics.
| OS | Estimate | SE | Hazard Ratio | p-value |
|---|---|---|---|---|
| Age | 0.013 | 0.019 | 1.013 | 0.510 |
| Poor Cytogenetics | 0.841 | 0.517 | 2.318 | 0.104 |
| Positive FC | 1.657 | 0.471 | 5.243 | 0.0004 |
| Rx = DAC | −0.468 | 0.475 | 0.626 | 0.324 |
| EFS | ||||
| Age | 0.003 | 0.018 | 1.003 | 0.849 |
| Poor Cytogenetics | 0.557 | 0.461 | 1.746 | 0.227 |
| Positive FC | 2.656 | 0.536 | 14.236 | <0.0001 |
| Rx = DAC | −0.363 | 0.433 | 0.695 | 0.402 |
Eight patients had positive MFC (5 in the CC arm and 3 in the DAC arm) at baseline. One patient on the DAC arm changed the MFC status from negative to positive at 3 months after treatment; two patients on the CC arm changed the MFC status from negative to positive at 4 months and 18 months, respectively. Therefore, we included MFC as a time-dependent variable in the Cox regression model. Median survival was 46 months for patients with negative MFC and 10 months for patients with positive MFC at the time of enrollment (Figure 1D). The OS at 3, 6, 9 and 12 months was 88%, 75%, 50% and 38% in patients with positive MFC, in comparison with 100%, 93%, 83%, and 80% in patients with negative MFC at enrollment (p=0.002, log-rank test). Median EFS was 31 months for patients with negative MFC and 3 months for patients with positive MFC at enrollment (Figure 1E). The EFS at 3, 6, 9 and 12 months was 50%, 13%, 13% and 0% in patients with positive MFC at enrollment, in comparison with 93%, 87%, 77%, and 73% in patients with negative MFC (p < 0.0001, log-rank test). Median RFS was greater than 50 months for patients with negative MFC and 3 months for patients with positive MFC at enrollment (Figure 1F). The RFS at 3, 6, 9 and 12 months was 50%, 19%, 19% and 0% in patients with positive MFC, in comparison with 93%, 90%, 83%, and 79% in patients with negative MFC at enrollment (p<0.0001, log-rank test). No significant differences were found in OS, EFS and RFS for patients who were MFC positive or negative at the time of study entry between the two treatment arms.
DNA methylation was studied in patients receiving DAC, for LINE-1 global methylation marker, 2 genes (CDH13 and PDLIM4) and one microRNA (Mir124a) (previously reported to be hypermethylated in AML with prognostic impact).5, 7–9 There was a statistically significant higher LINE-1 methylation at baseline between the relapsed patients and those remaining in CR (78% vs. 73%, P=0.02), whereas no differences were observed for methylation of CDH13 (10% vs. 12%, P=0.93) and Mir124a-1 (9% vs. 9%, P=0.98). No statistically significant differences were observed for LINE-1, CDH13, Mir124a-1 and PDLIM4 methylation levels measured at cycles 1– 5 for available samples.
In summary, this randomized trial demonstrated safety and feasibility of maintenance treatment with DAC for patients with AML in first or subsequent CR. Treatment with standard dose DAC was well tolerated with the most common adverse events being uncomplicated grade 3 neutropenia and/or thrombocytopenia. After a median follow-up of 44.9 months, fewer patients in the DAC arm relapsed (50% vs. 60%) and OS rate was 45% on DAC vs. 36% on CC group; these differences were not statistically significant. Using multivariate Cox regression model, after adjusting for treatment, age and cytogenetics, positive MFC was associated with shortened EFS and OS survival, as has been reported by others10–12.
We have previously reported that high levels of LINE correlate with high levels of methylation genome-wide as reflected by increased cumulative number of methylated genes and by CpG island methylator phenotype (CIMP)13. Thus, patients that relapse may have higher global methylation as reflected by higher LINE-1 levels. Indeed, we and others have previously reported that higher methylation levels associate with poor prognosis in MDS and AML7, 14. Whether LINE-1 DNA methylation levels at baseline truly predicts relapse remains to be studied and confirmed in larger trials. The small size of our study prohibits us from making more definitive conclusions. However, these data support the need for larger randomized studies with hypomethylating agents in the maintenance setting in patients with AML.
Acknowledgments
Supported in part by the Leukemia SPORE, CA100632 and by a grant from Eisai, Pharmaceuticals.
Footnotes
Authorship Contributions and Disclosure of Conflicts of Interest:
Contribution: F.R. designed the research; R.C. performed the experiments and J.J. performed flow cytometry; and Y.B., H.K, J.J, J.A., S.F., G.G.M, G.B, E.J, Z.E., J.C., J.P.I. and F.R. performed the research, analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: Consultant or Advisory role: Jean-Pierre Issa, GSK, Syndax; Farhad Ravandi, Eisai and Johnson and Johnson; Honoraria: Jean-Pierre Issa, Celgene, Novartis, Johnson and Johnson; Farhad Ravandi, Eisai and Johnson and Johsnon; Research Funding: Jean-Pierre Issa, MERC, Eisai, Celgene; Stephan Faderl, Eisai; Gautam Borthakur, Eisai; Jorge Cortes, Eisai; Hagop Kantarjian, Celgene; Farhad Ravandi, Eisai and Johnson and Johsnon.
References
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–62. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 4.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556–61. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 5.Kroeger H, Jelinek J, Estecio MR, He R, Kondo K, Chung W, et al. Aberrant CpG island methylation in acute myeloid leukemia is accentuated at relapse. Blood. 2008;112(4):1366–73. doi: 10.1182/blood-2007-11-126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal S, Unterberg M, Koschmieder S, zur Stadt U, Brunnberg U, Verbeek W, et al. DNA methylation of tumor suppressor genes in clinical remission predicts the relapse risk in acute myeloid leukemia. Cancer Res. 2007;67(3):1370–7. doi: 10.1158/0008-5472.CAN-06-1681. [DOI] [PubMed] [Google Scholar]
- 7.Shen L, Kantarjian H, Guo Y, Lin E, Shan J, Huang X, et al. DNA methylation predicts survival and response to therapy in patients with myelodysplastic syndromes. J Clin Oncol. 2010;28(4):605–13. doi: 10.1200/JCO.2009.23.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boumber YA, Kondo Y, Chen X, Shen L, Gharibyan V, Konishi K, et al. RIL, a LIM gene on 5q31, is silenced by methylation in cancer and sensitizes cancer cells to apoptosis. Cancer Res. 2007;67(5):1997–2005. doi: 10.1158/0008-5472.CAN-06-3093. [DOI] [PubMed] [Google Scholar]
- 9.Wong KY, So CC, Loong F, Chung LP, Lam WW, Liang R, et al. Epigenetic inactivation of the miR-124-1 in haematological malignancies. PLoS One. 2011;6(4):e19027. doi: 10.1371/journal.pone.0019027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.San Miguel JF, Vidriales MB, Lopez-Berges C, Diaz-Mediavilla J, Gutierrez N, Canizo C, et al. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood. 2001;98(6):1746–51. doi: 10.1182/blood.v98.6.1746. [DOI] [PubMed] [Google Scholar]
- 11.Stone RM. Should the presence of minimal residual disease (MRD) in morphologic complete remission alter post-remission strategy in AML? Best Pract Res Clin Haematol. 2011;24(4):509–14. doi: 10.1016/j.beha.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Buccisano F, Maurillo L, Del Principe MI, Del Poeta G, Sconocchia G, Lo-Coco F, et al. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood. 2012;119(2):332–41. doi: 10.1182/blood-2011-08-363291. [DOI] [PubMed] [Google Scholar]
- 13.Estecio MR, Gharibyan V, Shen L, Ibrahim AE, Doshi K, He R, et al. LINE-1 hypomethylation in cancer is highly variable and inversely correlated with microsatellite instability. PLoS One. 2007;2(5):e399. doi: 10.1371/journal.pone.0000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullinger L, Ehrich M, Dohner K, Schlenk RF, Dohner H, Nelson MR, et al. Quantitative DNA methylation predicts survival in adult acute myeloid leukemia. Blood. 2010;115(3):636–42. doi: 10.1182/blood-2009-03-211003. [DOI] [PubMed] [Google Scholar]