Abstract
The effects of the antidepressant venlafaxine (VEN-225 mg daily) and transdiagnostic cognitive behavioral treatment (CBT) alone and in combination on alcohol intake in subjects with co-morbid alcohol use disorders (AUDs) and anxiety disorders were compared. Drinking outcomes and anxiety were assessed for 81 subjects treated for 11 weeks with one of 4 conditions:1) VEN-CBT, 2)VEN-Progressive Muscle Relaxation therapy (PMR),3) Placebo (PLC)-CBT and 4) a comparison group of PLC-PMR. For subjects who reported taking at least one dose of study medication, the Time × Group interaction was significant for percent days of heavy drinking and drinks consumed per day. For the measure of percent days heavy drinking, the paired comparison of PLC-CBT versus PLC-PMR group indicated that the PLC-CBT group had greater drinking reductions, whereas other groups were not superior to the comparison group. In Week 11, the proportion of subjects in the PLC-CBT group that had a 50% reduction from baseline in percent days heavy drinking was significantly greater than those in the comparison group. Of the 3 “active treatment” groups only the PLC-CBT group had significantly decreased heavy drinking when contrasted to the comparison group. This finding suggests that the transdiagnostic CBT approach of Barlow and colleagues may have value in the management of heavy drinking in individuals with co-morbid alcoholism and anxiety.
Keywords: anxiety, alcoholism, antidepressants, cognitive behavioral therapy
INTRODUCTION
Alcohol use disorders (AUDs) have been reported to occur with a 12-month prevalence of almost 9% of the adult population in the United States (Grant et al., 2004; Hasin, Stinson, Ogburn, & Grant, 2007). In excess of 33% of treatment seeking individuals with an AUD may have at least one concurrent independent anxiety disorder (Grant et al., 2004). Panic and social phobia are both predictors of later alcohol use problems in adolescents and young adults (Zimmermann et al., 2003). Recent work suggests increases in the number of anxiety and other internalizing disorders, including depression and dysthymia, are directly related to increases in the prevalence of alcohol dependence (Kushner et al., 2012).
The results of several studies suggest that the existence of a co-morbid anxiety disorder can have a significant influence on the outcome of treatment of AUDs. In one study, social anxiety limited the willingness of individuals with AUDs to seek some forms of treatment for their drinking problems (Book et al., 2009). In another investigation, the severity of anxiety symptoms predicted recurrence of alcohol dependence in remitted patients (Boschloo et al., 2012). Phobic anxiety disorders also have been reported to predict shorter duration of treatment and discontinuation of treatment (Haver & Gjestad, 2005). Finally, there is evidence that social phobia is a predictor of return drinking after completion of treatment and panic disorder is a predictor of post-treatment alcohol dependence (Kushner et al., 2005).
Based on this empirical and theoretical literature, treatments for alcohol dependence that target anxiety as a mediator of treatment gains and/or relapse are appealing. Empirical evaluations of anxiety-focused treatments among substance-dependent patients (including alcoholics) have yielded mixed results. Several studies have called into question the utility of this approach (Bowen, D’Arcy, Keegan, Senthilselvan, 2000; Ormrod & Budd, 1991; Schadé et al., 2005). In contrast, other researchers have reported that addressing symptoms of anxiety could be important for the treatment of alcoholism (Fals-Stewart & Schafer, 1992; Modesto-Lowe & Kranzler, 1999).
In addition to behavioral therapies some investigators have examined the use of selective serotonin reuptake inhibitors (SSRIs), which have anxiolytic effects, in the treatment of alcohol use disorders in individuals with co-morbid anxiety disorders. In one study, although anxiety symptoms improved in subjects treated with the SSRI paroxetine, alcohol consumption was not meaningfully altered (Thomas, Randall, Book, & Randall, 2008). In another investigation, the addition of the SSRI fluvoxamine to the treatment regime of cognitive behavioral treatment (CBT ) in abstinent subjects who also received an intensive psychological relapse prevention program did not lead to better outcomes with respect to reduction in either alcohol intake or severity of anxiety (Schadé et al., 2005).
The role played by noradrenergic systems in both AUD and anxiety remains to be more fully delineated. There is evidence, however, of the involvement of these systems in both AUDs (Kash et al., 2012; Smith and Aston-Jones; 2008) and anxiety disorders (Bremner et al., 1996; Dell’Oso 2010; Rasmussen et al., 2006). Thus, agents that modify the activity of brain noradrenergic systems may be value in the management of co-morbid AUD and anxiety disorders. The present study examined the use of the NSRI (norepinephrine serotonin reuptake inhibitor) antidepressant venlafaxine for this purpose. Venlafaxine and its active metabolite, O-desmethylvenlafaxine (ODV), are potent inhibitors of both norepinephrine and serotonin uptake and weak inhibitors of dopamine reuptake. It is of particular interest that the chronic administration of venlafaxine does not alter basal levels of norepinephrine within the prefrontal cortex, but does significantly lower the amount of this neurotransmitter released by a stressor such as foot shock (Dazzi et al., 2002). Chronic administration of venlafaxine has also been found to desensitize frontal cortical β-adrenoreceptor-coupled adenylase cyclase systems in animals selectively depleted of serotonin (Nalepa et al., 1998). These results indicate that treatment with venlafaxine can reduce the activity of noradrenergic receptor systems in certain conditions.
Several studies support the efficacy of venlafaxine in generalized anxiety disorder (Allgulander, Hackett, & Salinas, 2001; Gelenberg et al., 2000; Rickels, Pollack, Sheehan, & Haskins, 2000), panic disorder (Kjernisted & McIntosh D, 2009; Liebowitz, Asnis, Mangano, & Tzanis, 2009), social phobia (Stein et al., 2005), and obsessive-compulsive disorder (Denys, van der Wee, van Megen, & Westenberg 2003; Grossman & Hollander, 1996).
The objective of the present investigation was to determine whether venlafaxine administered alone or in combination with CBT using the Unified Protocol for the Transdiagnostic Treatment of Emotional Disorders (UP) developed by Barlow et al. (Barlow, Allen, & Choate, 2004; Barlow et al., 2011) would reduce alcohol intake and anxiety compared to either CBT in combination with placebo (PLC) or PLC in combination with a “control” behavioral therapy, i.e. progressive muscle relaxation (PMR). For safety reasons all subjects were provided with an initial platform treatment of motivational enhancement therapy (MET), and were required to achieve four days of abstinence prior to randomization to reduce the potential for alcohol-venlafaxine interactions. The primary study hypothesis was that in comparison to the PLC-PMR treatment condition, the other treatment combinations would produce greater decreases during the treatment period in both alcohol consumption and anxiety. It was also hypothesized that the combination of venlafaxine and CBT would show greater improvements in decreasing alcohol consumption and anxiety than did these treatments when they were administered alone.
METHODS
Men and women were recruited into an outpatient anxiety treatment program via radio, web, and newspaper advertisements. Telephone screenings determined initial eligibility, and potential research participants were invited to the Center for Anxiety and Related Disorders at Boston University for a more extensive assessment of alcohol use and emotional symptoms.
Inclusion criteria for subject eligibility included: 1) DSM-IV diagnosis of alcohol abuse or dependence (alcohol use disorder: AUD) and a diagnosis of anxiety disorder (panic disorder, social phobia, or generalized anxiety disorder); 2) minimum age of 18 years; and 3) expressed desire to stop drinking alcohol completely or to reduce alcohol consumption with the possible long-term goal of abstinence. Exclusion criteria were: 1) DSM-IV diagnosis of bipolar disorder, schizophrenia, bulimia/anorexia, dementia, or other substance dependence, with the exception of nicotine, marijuana, and caffeine dependence; 2) medical contraindication to the use of venlafaxine; 3) currently taking anti-craving agents, anti-depressant medications, or medication known to reduce anxiety or alcohol consumption; 4) ongoing concurrent treatment for alcohol problems; 5) currently taking medication that has significant interactions with venlafaxine; 6) previously received venlafaxine; 7) currently prescribed medication with known abuse potential; and 8) having experienced severe depression or suicidal behaviors in the past 30 days.
The objective of this study was to compare the efficacy and safety of using venlafaxine and CBT to facilitate abstinence from alcohol consumption in individuals with co-morbid AUDs and anxiety disorders, as compared to combined treatment with placebo and PMR, the control treatment condition. The flow diagram for this study is presented in Figure 1. This study, with respect to medication therapy, followed a double-blind, randomized, placebo-controlled study design.
Fig.1.
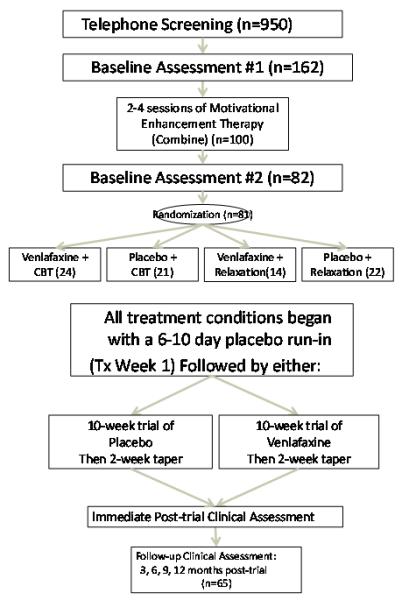
Study Flow Diagram
All procedures used in this study were approved by the Boston University and Boston University Medical Center’s Institutional Review Boards (IRBs), as well as the Central Texas Veterans Health Care System IRB. Subjects provided informed consent in accordance with IRB requirements. Participants who were unable to continue the drug treatment due to adverse effects were discontinued from the medication but continued to attend clinic for psychological treatment and assessment.
Following telephone screening, subjects completed an in-clinic baseline assessment to determine eligibility for inclusion in the study. Eligible subjects were assigned to a counselor who provided MET (Miller, 2004) to aid the subject in achieving an initial period of four days of complete abstinence from alcohol (as a safety precaution due to 50% chance of receiving venlafaxine). MET feedback was given based on normative data published in the Combined Behavioral Intervention Manual.
The target dose of MET varied from two to four sessions depending upon the client’s desire and ability to achieve the required period of initial abstinence. These sessions were completed within two to four weeks on average. Once subjects had achieved the four day period of abstinence they completed a second baseline assessment in which eligibility was re-assessed. Eligible subjects were then randomized to receive one of four treatment conditions: 1) Venlafaxine plus individualized CBT (VEN-CBT); 2) Placebo medication plus CBT (PLC-CBT); 3) Venlafaxine plus PMR (VEN-PMR); or 4) Placebo medication plus PMR (PLC-PMR). Each treatment condition included a 7 day placebo run-in, and a 10 week trial of venlafaxine or placebo followed by a 2-week taper. Individual CBT was conducted in a standardized fashion, following the Unified Protocol for the Transdiagnostic Treatment of Emotional Disorders (UP) developed by Barlow and colleagues (Barlow, Allen, & Choate, 2004; Barlow et al., 2011). The UP is a transdiagnostic, emotion-focused CBT that emphasizes the adaptive, functional nature of emotions, and seeks to identify and correct maladaptive attempts to regulate emotional experiences. Development of this protocol began with the distillation of key principles from traditional empirically-supported CBT treatments integrated with innovations from the field of emotion science, incorporating and addressing deficits in emotion regulation common in emotional disorders (for discussion on the development of the UP, see Wilamowska et al., 2010). In the PMR condition, participants were told that PMR would be taught in order to help them decrease stress and negative emotions. The PMR conditions consisted of training in full-body muscle relaxation, as well as cue-controlled muscle relaxation. Eleven sessions of either CBT or PMR were administered, each of which was approximately 50-60 minutes long.
Subjects received either a sustained release formulation of venlafaxine (Effexor XR) or matched placebo capsules. Active medication capsules each contained a 75 mg dose of venlafaxine. Medications were supplied in identical appearing capsules. A maintenance dose of 225 mg of venlafaxine was set as the target for subjects selected to receive active medication. This dose has been shown to have efficacy in clinical trials of several anxiety disorders including general anxiety disorder (Rickels et al., 2000), panic disorder (Pollack et al., 2007), and social anxiety disorder (Liebowitz et al., 2005). In vitro evidence suggests that venlafaxine has greater affinity for the serotonin as opposed to the norepinephrine neuronal transporter (NET) proteins (Roseboom and Kaslin, 2000). These results indicate a dose if venlafaxine in the range of 225 mg would be needed to elevate brain concentrations of this drug and its active metabolite O-des-methylvenlafaxine to levels sufficient to produce significant inhibition of NETs.
Subjects received one placebo capsule daily during the first study week. In the second study week, one capsule of either venlafaxine or placebo was provided daily to subjects. In Week 3 two medication capsules were administered to subjects daily, and the maintenance of 225 mg of venlafaxine or 3 placebo capsules were to be taken daily thereafter. The study psychiatrists were allowed to decrease the dose of medications received by subjects when it was needed to reduce adverse drug effects. Pill counts were conducted at each visit to encourage medication adherence. After completion of Study Week 11, subjects’ medications were reduced over a 2-week period by weekly reductions to a dose of two then one capsule daily.
Assessments and Measures
Anxiety and depression were measured using 5 instruments. The clinician-administered Anxiety Disorders Interview Schedule-IV: Lifetime Version (ADIS-IV-L; DiNardo, Brown, & Barlow, 1994) was used to assess anxiety disorders, depressive disorders, and AUD. The Anxiety Sensitivity Index (ASI; Reiss, Peterson, Gursky, & McNally, 1986) was used to assess fears that anxiety symptoms would have negative somatic, mental, or social consequences (Reiss, 1991). The Depression Anxiety Stress Scales (DASS; Lovibond & Lovibond, 1995) assessed depression, anxiety and stress responses. Finally, the Hamilton Anxiety Rating Scale (Hamilton, 1959) and Hamilton Depression Rating Scale (Hamilton, 1960) were clinician-administered to assess symptoms of anxiety and depression, respectively.
Three measures were used to assess alcohol use and craving. The Alcohol Timeline Followback (TLFB; Sobell & Sobell, 1992) was utilized to estimate participants’ daily drinking during the 90 day period that preceded screening and at the beginning of each psychotherapy session. The Alcohol Dependence Scale (ADS; Skinner & Allen, 1982) was used to assess the severity of alcohol dependence within the last 12 months, and the Craving Desire Scale (CDS) is a 3-item scale (namely, “1. I do want to drink now”, “2. I crave a drink right now”, 3. “I have a desire for a drink right now”) that was used to identify the degree of current alcohol craving, with responses provided on a Likert-scale. A maximum score of 21 could be obtained on this scale.
Participants were given a breathalyzer test and a blood pressure test at psychotherapy sessions. CDS, DASS and TLFB data were obtained prior to every treatment session. HAM-D and HAM-A data were collected for behavioral treatment Sessions 1, 4, and 8. Treatment sessions typically occurred during the corresponding study week, but in some cases, because of missed appointments behavioral treatment sessions took place in subsequent weeks.
Follow-up assessment sessions similar to the baseline assessment sessions were conducted by a research assistant one week after the completion of the treatment protocol, then subsequently at three, six, nine, and 12 months.
Data Analysis
An intent-to-treat approach to analysis was used for this study. A second analysis was performed for alcohol drinking variables for subjects who reported taking at least one dose of study medication. Demographic and diagnostic data are presented as means (± standard deviations), subject numbers, or as frequencies for each treatment group. Treatment group means were also determined for other data used to characterize subjects including the ADS. The total number of subjects meeting diagnostic criteria for AUD and anxiety disorders was compiled. Baseline values for data with continuous variables for each group were compared using one-way analysis of variance (ANOVA). Comparisons of frequency data for the groups were performed using chi-square tests. Adverse effects were analyzed by pooling data into two groups based on whether a subject received venlafaxine or placebo. Between group comparisons were performed for moderate to severe adverse effects that were rated as being medication related. The Fisher’s exact test was used for group comparisons of adverse effects.
The patterns of subjects’ alcohol consumption were determined using TLFB derived values for percent days drinking, percent days heavy drinking, and mean drinks consumed per day. Weekly means were determined for these variables for the treatment and post-treatment periods, while a single mean for these variables was found for the 90 day-long pre-screening period. The percentage of subjects for each treatment group reporting complete abstinence during the last 5 weeks of the treatment period i.e. Study Weeks 7-11, was determined based on the available reported data from each subject. It was assumed that subjects for whom TLFB was not available for any Study Week during this period were not abstinent.
Treatment group drinking variables for both the treatment and post-treatment data sets were analyzed using a two-way repeated measures mixed models analysis (SAS version 9.2) with prescreening values used as covariates where appropriate. Study week served as the within subject factor, while treatment condition was the between group factor. Drinking data for the drug treatment period were analyzed using data for the placebo run-in week and for the last 5 weeks of the treatment period. The assessment period using the last 5 weeks of medication administration was determined a priori and is consistent with current NIAAA studies of medications that are expected to have a delayed onset of action in alcoholism (Litten RA, personal communication). A second analysis was conducted for a comparison of the placebo run-in week drinking data with data obtained for the first week of the follow-up period. Values for percentages of abstinent subjects in each group were compared using chi square tests.
Repeated measures mixed models analyses were also used to evaluate all treatment period data obtained for the CDS, DASS, HAM-A and HAM-D. Behavioral treatment Session, i.e. the particular therapy session prior to which assessments were administered, was used as the within subject factor for these analyses. When needed, within group time related changes between behavioral treatment Session 1 and Session 11 values were compared using paired t-tests for model generated least squares means. An α value of less than 0.05 was considered to be significant. When several comparisons were made the appropriate significance level was determined based on the Dunn-Sidak procedure. For 3 comparisons an unadjusted α value of less than 0.017 was regarded as being significant.
RESULTS
Subjects and Subject Compliance and Motivation
The number of subjects assessed in each step of the study are shown in Figure 1. Not every subject completed the full course of behavioral therapy during the 12 week medication treatment period. These individuals received the balance of this therapy following the medication treatment period. Follow-up data was obtained after the completion of both medication and behavioral treatments.
Subjects’ characteristics including mean ages, race, alcohol use and anxiety diagnoses are presented in Table 1. There were no significant differences between the treatment groups with respect to any of the variables presented in the table. Significant differences amongst groups were not found for baseline ASI, HAM-A, HAM-D scores, the DASS subscale scores, or baseline measures of alcohol consumption.
Table 1.
Subject description
| VEN-CBT | PLC-CBT | VEN-PMR | PLC-PMR | |
|---|---|---|---|---|
| N | 24 | 21 | 14 | 22 |
| Females | 6 | 4 | 3 | 5 |
| African American | 2 | 1 | 0 | 2 |
| White | 21 | 18 | 12 | 19 |
| Other | 1 | 2 | 2 | 1 |
| Alcohol | 23 | 16 | 14 | 20 |
| Dependence | ||||
| Alcohol Abuse | 1 | 5 | 0 | 2 |
| Panic Disorder | 2 | 2 | 3 | 1 |
| GAD | 18 | 14 | 8 | 15 |
| Social Phobia | 11 | 10 | 7 | 13 |
Medication compliance based on pill count was not significantly different among the four treatment groups. Patients in three of the treatment groups had medication compliance rates above 95%:VEN-CBT: 96.89% [7.15]; PLC-CBT: 97.06% [7.41]; PLC-PMR: 95.39% [9.62]while the VENPMR group had a compliance rate of 88.70% [24.41]). Three subjects who, although randomized, reported not taking even a single dose of study medication.
Alcohol Consumption
The mean weekly percent days heavy drinking are presented in Figure 2 for PLC-CBT and PLC-PMR treatment groups. 2. In the intent to treat analysis the Time × Group interaction was found to be significant for the number of drinks consumed per day [F(15, 37)=1.73; p=0.044] and approached significance for the percent days heavy drinking F(15, 337 )=1.7; p=0.058]. In a second analysis, in which data for three subjects who reported no use of any study medication or placebo were excluded, the Time × Group interaction was significant for both number of drinks consumed per day [F(15, 327)=1.85; p=0.027] and the percent days heavy drinking F( 15, 327)=1.7; p=0.047]. For the three paired comparisons between each “active” treatment group and the PLC-PMR group for percent days heavy drinking, only the comparison with the PLC-CBT group was found to have a significant Time × Group interaction (p=0.01) after correction for multiple comparisons in both analyses. None of the other alcohol outcome measures differed significantly between groups in the mixed models analysis. In a post-hoc analysis the proportion of subjects with a 50% reduction from prescreening values in the percent days heavy drinking in week 11 were significant (p=0.01) only for the comparison between the PLC-CBT group (90%) and PLC-PMR (53%). The number of subjects who were abstinent during the entire last half of the drug treatment period were two for the VEN-CBT group, four for the PLC-CBT group, three for the VEN-PMR group, and one for the PLC-PMR group. Pair-wise comparisons were not significant for comparisons of the proportion abstinent subjects in the groups between the PLC-PMR group and any of the three other groups.
Fig. 2.
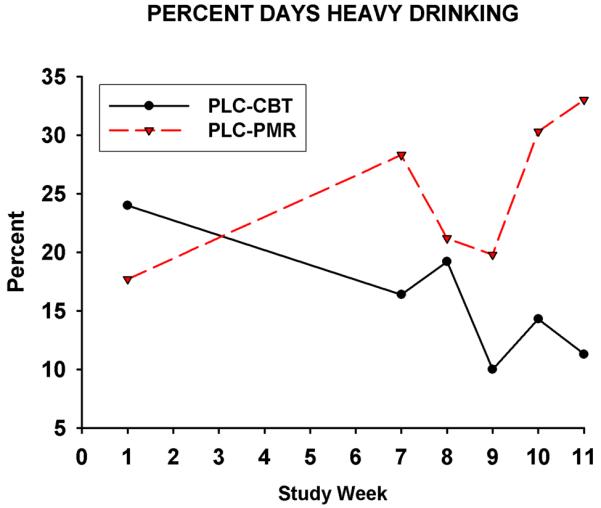
Intent to treat data for weekly mean percent days heavy drinking for PLC-CBT and PLC-PMR treatment groups for study weeks included in the analysis, i.e Week 1 (the Placebo Run-In Week) and Weeks 5-12. p=0.01 for the Time × Group interaction for paired comparisons in both the intent to treat analysis and the analysis of data for subjects who used at least one dose of medication.
Anxiety, Depression, Craving Measures
Time effects, but not the Group or the Time × Group effects, were significant for the anxiety [F(10, 242)=5.01; p<0.0001] (see Figure 3), depression [F(9, 211)=4.7; p<0.0001], and stress [F(9,209)=12.2; p , 0.0001] subscales of the DASS obtained for the treatment period [see Fiq 3.]. For the DASS anxiety subscale, model generated least squares mean values for the PLC-CBT, VEN-PMR and PLCPMR groups were significantly lower for behavioral treatment Session 11 as compared to Session 1 (See Figure 3). Values for the stress subscale of the DASS were significantly less for Session 11 versus Session 1 for VEN-CBT [from 16.7 (7.6) to 8.1 (4.6)], PLC-CBT [from 19.2 (9.1) to 9.6 (7.9)], VEN-PMR [from 21.5 (11.3) to 4.1 (7.9)] and PLC-PMR [from 16.6 (8.2) to 11.2 (11.1)] groups. The Session 1-Session 11 comparisons were significant for the depression subscale of the DASS only for PLC-CBT [from 12.8 (10.7) to 6.6 (7.6)] and VEN-PMR [ from 16.3 (12.5) to 6.1 (10.0)] groups, but again, as indicated above, the Time × Group interaction was not significant for this measure.
Fig.3.
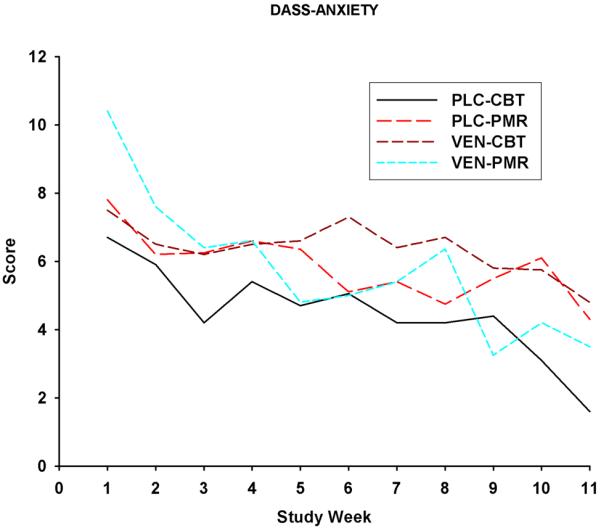
Mean DASS-A anxiety (subscale scores obtained for Study Weeks 1-11 for each of the treatment groups. VEN-CBT PLC-CBT VEN-PMR PLC-PMR
For the HAM-A scale, the Time effects, but not the Group or the Time × Group effects, were found to be significant [F(2, 90)= 16.4; p<0.0001]. The least squares means for the HAM-A scale declined significantly from Session 1 to Session 8 for the PLC-CBT [from 14.2 (7.3) to 9.1 (5.5)] and VEN-PMR [from 16.5 (6.8) to 7.9 (5.5)] treatment groups, but not for the VEN-CBT [from 13.5 (5.9) to 9.8 (6.9)] or the PLC-PMR [from 12.9 (4.1) to 9.1 (4.2)] groups.. The Time effect was also significant for the HAM-D [F(2, 80.4)=8.8; p=0.0003]. Session 1 to Session 8 comparisons for HAM-D least squares means were significant for the VEN-PMR group [ from 13.0 (7.2) to 5.9 (5.60)], but not the VEN-CBT [from 12.0 (5.7) to 8.8 (6.6)], PLC-CBT [from 11.7 (6.0) to 8.5 (4.6)], or PLC-PMR [from 13.6 (5.0) to 10.0 (6.2)] groups. Group effects and Time × Group interactions were not significant for the CDS measure of craving obtained during the treatment period. The Time effect was significant for this analysis [F(10, 271)= 3.9; p<0.0001]. The decrease in CDS least squares mean values from Session 1 to Session 11 was significant only for the VEN-CBT group [p=0.011; from 8.6 (6.0) to 4.2 (1.9)] and for the PLC-PMR group (p=0.004), with scores declining from 7.5 (5.1) to 4.3 (2.7).
Adverse Effects
The proportion of subjects experiencing dry mouth was significantly greater (p=0.005) in the venlafaxine group (39.5%) than in the placebo group (11.6%). Complaints of sexual dysfunction occurred with significantly greater frequency (p=0.001) in the venlafaxine (34.2%) than in the placebo group (4.7%). Of note is that suicidal ideation occurred in one subject in the venlafaxine group and in none of the placebo patients. Although not significantly different, the proportion of subjects experiencing anorexia, insomnia, and restlessness was twice as high in the venlafaxine group (as compared to the placebo group).
DISCUSSION
In the present study, subjects in the CBT-PLC group had greater reductions in percent days heavy drinking in contrast to the PLC-PMR comparison group. In contrast, to the hypotheses that venlafaxine alone or CBT and active medication would significantly decrease alcohol consumption neither VEN-CBT nor the VEN-PMR was superior to the placebo group on any alcohol outcome measure. The actions of venlafaxine alone on alcohol consumption were similar to those of the SSRI’s paroxetine (Schade et al., 2005) and fluvoxamine (Thomas, et al., 2008) in the treatment of AUDs in individuals with co-morbid anxiety disorders in previous studies.
Anxiety, as measured by the anxiety subscale of the DASS, decreased significantly over the treatment period in the PLC-CBT, VEN-PMR, and PLC-PMR groups. For each group, scores for DASS stress subscales declined significantly across the treatment period, with no group differences being detected. As measured by the HAM-A, anxiety also decreased significantly from treatment session one to eight in both the PLC-CBT and VEN-PMR groups (but not PLC-PMR) with no differences between groups. Drinking was only decreased in the PLC-CBT group, suggesting a possible dissociation between changes in anxiety and in drinking. The finding that anxiety was not significantly reduced in the VEN-CBT group compared to the PLC-PMR group is consistent with other evidence that the addition of venlafaxine to CBT did not enhance the effects of CBT alone for the treatment of generalized anxiety disorder (Crits-Christoph et al., 2011).
One limitation of the present study is the small size of the treatment groups. A major consequence of this deficiency was that subject sample size became diminished during the follow-up period to the point where it may have precluded meaningful between group comparisons in this period. A second major limitation was the failure of our randomization procedure to lead to the placement of equivalent numbers of subjects into each treatment group. This resulted from our use of pre-determined randomization of subjects based on their original assigned subject number and an unexpected disparity in percentage of individuals who were able to progress from MET therapy to the treatment phase of the study amongst the subjects assigned to the difference treatment groups.
The results of this study indicate venlafaxine is not superior to placebo in its effects on alcohol consumption or anxiety in subjects with co-morbid AUD and anxiety disorders. In contrast, CBT alone may be of value in assisting individuals with co-morbid AUD and anxiety disorders in reducing heavy drinking.. We observed a significant reduction in anxiety in the absence of a similar finding for alcohol consumption. This finding contradicts the notion that lessening anxiety symptoms necessarily leads to better control of drinking behavior. The goal of the Unified Protocol in this study was not to directly treat the mood symptoms of each disorder, but rather teach broad skills that address emotional regulation problems that underlie a cluster of internalizing disorders. This trans-diagnostic approach is in part intended to reduce maladaptive responses to intense affect without specifically reducing or eliminating this affect. The present findings suggest transdiagnostic treatment that addresses emotional regulation problems may be of value as a tool for the management of heavy drinking. Future studies are necessary to replicate our findings, and should involve larger samples sizes and comparisons with medications that decrease anxiety and alcohol consumption.
Highlights (for review).
The effects on alcohol consumption in subjects with a co-morbid anxiety-alcohol-use disorder (AUD) of treatment with antidepressant venlafaxine (VEN) alone and in combination with transdiagnostic cognitive behavioral therapy (CBT) were compared in this study to those of placebo plus CBT (PLC-CBT) and to a control comparison condition PLC plus progressive muscle relaxation therapy (PLC-PMR).
For paired comparisons between treatments only the Time × Group interaction for the comparison of CBT-PLC and PLC-PMR was found to be significant for the measure of percent days of heavy drinking, with the CBT-Group showing reduced drinking over the treatment period.
A significantly greater portion of subjects in the PLC-CBT group had a 50% reduction in the percent days of heavy drinking during the last week of drug treatment than did those in the PLC-PMR group.
Anxiety as measured by the Depression Anxiety Stress Scale decreased significantly between the first and last weeks of treatment in the PLC-CBT, VEN-PMR, and PLC-PMR groups, with no groups showing a superior effect to the PLC-PMR group.
The results of this study support the efficacy of transdiagnostic CBT alone in the reduction of heavy drinking in individuals with co-morbid anxiety and alcoholism.
Table 2.
Means (SD) obtained for ADS scores and percent days drinking, percent days heavy drinking, and mean drinks per day for the 90 day-long pre-screening period.
| VEN-CBT | PLC-CBT | VEN-PMR | PLC-PMR | |
|---|---|---|---|---|
| ADS Scores | 15.0 (7.6) | 15.8 (7.7) | 16.6 (6.6) | 16.1 (5.9) |
| Percent Days Drinking | 72.0 (24.5) | 76.0 (25.6) | 88.1 (12.7) | 76.5 (25.8) |
| Percent Days Heavy Drinking | 63.1 (28.0) | 59.1 (33.7) | 76.9 (19.1) | 67.2 (28.6) |
| Drinks Per Day | 8.4(6.5) | 7.0 (4.6) | 9.6 (4.3) | 9.7 (7.5) |
Acknowledgements
This work was supported by a grant R01-AA013727-awarded to DAC, K23-DA016376 awarded to SBM, and K23DA016138 awarded to BWK. The authors would like to thank Ann Marie Ciraulo RN for her critical review of the data and Ms. Michele Procida for preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allgulander C, Hackett D, Salinas E. Venlafaxine extended release (ER) in the treatment of generalised anxiety disorder: twenty-four-week placebo-controlled dose-ranging study. Br J Psychiatry. 2001;179:15–22. doi: 10.1192/bjp.179.1.15. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders. Behaviour Therapy. 2004;35:205–230. doi: 10.1016/j.beth.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Farchione TJ, Fairholme CP, Ellard KK, Boisseau CL, Allen LB, Ehrenreich-May J. The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders: Therapist Guide. Oxford University Press; New York: 2011. [Google Scholar]
- Book SW, Thomas SE, Dempsey JP, Randall PK, Randall CL. Social anxiety impacts willingness to participate in addiction treatment. Addict Behav. 2009;34(5):474–6. doi: 10.1016/j.addbeh.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, van den Brink W, Smit JH, Beekman AT, Penninx BW. Predictors of the 2-year recurrence and persistence of alcohol dependence. Addiction. 2012 doi: 10.1111/j.1360-0443.2012.03860.x. [DOI] [PubMed] [Google Scholar]
- Bowen RC, D’Arcy C, Keegan D, Senthilselvan A. A controlled trial of cognitive behavioral treatment of panic in alcoholic inpatients with coco-morbid panic disorder. Addict Behav. 2000;25(4):593. doi: 10.1016/s0306-4603(99)00017-9. 7. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety Preclinical studies. Synapse. 1996;23(1):28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Newman MG, Rickels K, Gallop R, Gibbons MB, Hamilton JL, Ring-Kurtz S, Pastva AM. Combined medication and cognitive therapy for generalized anxiety disorder. Pharmacogenomics J. 2011;25:1087–1095. doi: 10.1016/j.janxdis.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzi L, Vignone V, Seu E, Ladu S, Vacca G, Biggio G. Inhibition by venlafaxine of the increase in norepinephrine output in rat prefrontal cortex elicited by acute stress or by the anxiogenic drug FG 7142. J Psychopharmacol. 2002;16(2):125–31. doi: 10.1177/026988110201600202. [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Buoli M, Baldwin DS, Altamura AC. Serotonin norepinephrine reuptake inhibitors (SNRIs) in anxiety disorders: a comprehensive review of their clinical efficacy. Hum Psychopharmacol. 2010;25(1):17–29. doi: 10.1002/hup.1074. [DOI] [PubMed] [Google Scholar]
- Denys D, van der Wee N, van Megen HJ, Westenberg HG. A double blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. J Clin Psychopharmacol. 2003;23(6):568–75. doi: 10.1097/01.jcp.0000095342.32154.54. [DOI] [PubMed] [Google Scholar]
- DiNardo PA, Brown TA, Barlow DH. Anxiety disorders interview schedule for DSM IV: Lifetime version (ADIS-IV-L) Oxford University Press; New York: 1994. [Google Scholar]
- Fals-Stewart W, Schafer J. The treatment of substance abusers diagnosed with obsessive-compulsive disorder: an outcome study. J Subst Abuse Treat. 1992;9(4):365–70. doi: 10.1016/0740-5472(92)90032-j. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807–16. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, Lydiard RB, Rudolph RL, Aguiar L, Haskins JT, Salinas E. Efficacy of venlafaxine extended-release capsules in nondepressed outpatients with generalized anxiety disorder: A 6-month randomized controlled trial. JAMA. 2000;283(23):3082–8. doi: 10.1001/jama.283.23.3082. [DOI] [PubMed] [Google Scholar]
- Grossman R, Hollander E. Treatment of obsessive-compulsive disorder with venlafaxine. Am J Psychiatry. 1996;153(4):576–7. doi: 10.1176/ajp.153.4.576b. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. 1959. [DOI] [PubMed] [Google Scholar]
- Hamilton MA. Rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and coco-morbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Haver B, Gjestad R. Phobic anxiety and depression as predictor variables for treatment outcome. A LISREL analysis on treated female alcoholics. Nord J Psychiatry. 2005;59(1):25–30. doi: 10.1080/08039480510018797. [DOI] [PubMed] [Google Scholar]
- Kash TL. The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol. 2012 Jun;46(4):303–8. doi: 10.1016/j.alcohol.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjernisted K, McIntosh D. Venlafaxine extended release (XR) in the treatment of panic disorder. Ther Clin Risk Manag. 2007;3(1):59–69. doi: 10.2147/tcrm.2007.3.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Thuras P, Hanson KL, Brekke M, Sletten S. Follow-up study of anxiety disorder and alcohol dependence in coco-morbid alcoholism treatment patients. Alcohol Clin Exp Res. 2005;29(8):1432–43. doi: 10.1097/01.alc.0000175072.17623.f8. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Wall MM, Krueger RF, Sher KJ, Maurer E, Thuras P, Lee S. Alcohol dependence is related to overall internalizing psychopathology load rather than to particular internalizing disorders: evidence from a national sample. Alcohol Clin Exp Res. 2012;36(2):325–31. doi: 10.1111/j.1530-0277.2011.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR, Asnis G, Mangano R, Tzanis E. A double-blind, placebo-controlled, parallel-group, flexible-dose study of venlafaxine extended release capsules in adult outpatients with panic disorder. J Clin Psychiatry. 2009;70(4):550–61. doi: 10.4088/jcp.08m04238. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Gelenberg AJ, Munjack D. Venlafaxine extended release vs placebo and paroxetine in social anxiety disorder. Arch Gen Psychiatry. 2005;62(2):190–8. doi: 10.1001/archpsyc.62.2.190. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression, Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- Miller WR, editor. COMBINE Monograph Series. Vol.1. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2004. Combined Behavioral Intervention manual: A clinical research guide for therapists treating people with alcohol abuse and dependence. DHHS No. 04-5288. [Google Scholar]
- Modesto-Lowe V, Kranzler HR. Diagnosis and treatment of alcohol-dependent patients with coco-morbid psychiatric disorders. Alcohol Res Health. 1999;23(2):144–9. [PMC free article] [PubMed] [Google Scholar]
- Nalepa I, Manier DH, Gillespie DD, Rossby SP, Schmidt DE, Sulser F. Lack of beta adrenoceptor desensitization in brain following the dual noradrenaline and serotonin reuptake inhibitor venlafaxine. Eur Neuropsychopharmacol. 1998;8(3):227–32. doi: 10.1016/s0924-977x(97)00078-3. [DOI] [PubMed] [Google Scholar]
- Ormrod J, Budd R. A comparison of two treatment interventions aimed at lowering anxiety levels and alcohol consumption amongst alcohol abusers. Drug Alcohol Depend. 1991;27(3):233–43. doi: 10.1016/0376-8716(91)90006-k. [DOI] [PubMed] [Google Scholar]
- Pollack M, Mangano R, Entsuah R, Tzanis E, Simon NM, Zhang Y. A randomized controlled trial of venlafaxine ER and paroxetine in the treatment of outpatients with panic disorder. Psychopharmacology (Berl) 2007;194(2):233–42. doi: 10.1007/s00213-007-0821-0. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during “abstinence”. Alcohol. 2006;38(3):173–7. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. 1986. [DOI] [PubMed] [Google Scholar]
- Rickels K, Pollack MH, Sheehan DV, Haskins JT. Efficacy of extended-release venlafaxine in nondepressed outpatients with generalized anxiety disorder. Am J Psychiatry. 2000;157(6):968–74. doi: 10.1176/appi.ajp.157.6.968. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Kalin NH. Neuropharmacology of venlafaxine. Depress Anxiety. 2000;12(Suppl 1):20–9. doi: 10.1002/1520-6394(2000)12:1+<20::AID-DA3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Schadé A, Marquenie LA, van Balkom AJ, Koeter MW, de Beurs E, van den Brink W, van Dyck R. The effectiveness of anxiety treatment on alcohol-dependent patients with a co-morbid phobic disorder: a randomized controlled trial. Alcohol Clin Exp Res. 2005;29(5):794–800. doi: 10.1097/01.alc.0000163511.24583.33. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Littenand RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biological methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91(3):199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213(1-2):43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Pollack MH, Bystritsky A, Kelsey JE, Mangano RM. Efficacy of low and higher dose extended-release venlafaxine in generalized social anxiety disorder: a 6-month randomized controlled trial. Psychopharmacology (Berl) 2005;177(3):280–8. doi: 10.1007/s00213-004-1957-9. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Randall PK, Book SW, Randall CL. A complex relationship between co-occurring social anxiety and alcohol use disorders: what effect does treating social anxiety have on drinking? Alcohol Clin Exp Res. 2008 Jan;32(1):77–84. doi: 10.1111/j.1530-0277.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- Wilamowska ZA, Thompson-Hollands J, Fairholme CP, Ellard KK, Farchione TJ, Barlow DH. Conceptual background, development, and preliminary data from the unified protocol for transdiagnostic treatment of emotional disorders. Depression and Anxiety. 2010;27:882–890. doi: 10.1002/da.20735. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Wittchen HU, Höfler M, Pfister H, Kessler RC, Lieb R. Primary anxiety disorders and the development of subsequent alcohol use disorders: a 4-year community study of adolescents and young adults. Psychol Med. 2003;33(7):1211–22. doi: 10.1017/s0033291703008158. [DOI] [PubMed] [Google Scholar]


