Abstract
Background
The 3-gene signature of periostin, chloride channel accessory 1 (CLCA1), and Serpin β2 (SERPINB2) in airway epithelial brushings is used to classify asthma into TH2-high and TH2-low endotypes. Little is known about the utility of gene profiling in sputum as a molecular phenotyping method.
Objective
We sought to determine whether gene profiling in sputum cells can identify TH2-high and TH2-low subtypes of asthma.
Methods
In induced sputum cell pellets from 37 asthmatic patients and 15 healthy control subjects, PCR was used to profile gene expression of the epithelial cell signature of IL-13 activation (periostin, CLCA1, and SERPINB2), TH2 genes (IL4, IL5, and IL13), and other genes associated with airway TH2 inflammation.
Results
Gene expression levels of CLCA1 and periostin, but not SerpinB2, were significantly higher than normal in sputum cells from asthmatic subjects. Expression levels of IL-4, IL-5, and IL-13 were also significantly increased in asthmatic patients and highly correlated within individual subjects. By combining the expression levels of IL-4, IL-5, and IL-13 in a single quantitative metric (“TH2 gene mean”), 26 (70%) of the 37 asthmatic patients had TH2-high asthma, which was characterized by more severe measures of asthma and increased blood and sputum eosinophilia. TH2 gene mean values tended to be stable when initial values were very high or very low but fluctuated above or below the TH2-high cutoff when initial values were intermediate.
Conclusion
IL-4, IL-5, and IL-13 transcripts are easily detected in sputum cells from asthmatic patients, and their expression levels can be used to classify asthma into TH2-high and TH2-low endotypes.
Keywords: Asthma, phenotypes, TH2 cell, mast cells, eotaxin, inflammation, sputum, cytokines, eosinophils, IL-4, IL-5, IL-13, IL-17
Asthma is characterized by airway TH2 inflammation,1 and drugs targeting TH2 cytokines (IL-4, IL-5, and IL-13) are in development.2-4 Gene transcripts for IL4, IL5, and IL13 are minimally expressed in the airway epithelium, but we previously reported that periostin, chloride channel accessory 1 (CLCA1), and Serpin β2 (SerpinB2) are specifically upregulated by IL-13 in airway epithelial cells,5,6 and their expression profile allows classification of asthma as TH2 high or TH2 low, with each subtype having specific inflammatory, remodeling, and treatment response characteristics.6
Molecular phenotyping methods that require bronchoscopic samples have limited applicability, and we considered whether methods using sputum induction would be more broadly applicable. Although gene transcripts for IL4, IL5, and IL13 are known to be expressed in induced sputum cells,7 it is not known whether RNA from sputum cells is high quality or whether sputum cell gene profiling can identify TH2-high and TH2-low endotypes. To address these issues, we collected induced sputum from asthmatic patients and healthy control subjects and measured the RNA quality and expression of genes related to TH2 inflammation.
METHODS
Subjects and clinical samples
We studied induced sputum samples stored in the Airway Tissue Bank at the University of California, San Francisco (UCSF). The biological samples were collected in multiple research studies at UCSF from 2009-2013 in which the characterization studies and biospecimen collections had all been collected according to standardized and uniform protocols (NCT00917787, NCT01073410, NCT00595153, and NCT01508078). Asthmatic patients had a prior physician's diagnosis of asthma and airway hyperresponsiveness (defined as a methacholine PC20 <8.0 mg/mL while not taking steroids or <16 mg/mL while taking steroids) or reversible airflow obstruction (defined as a postbronchodilator increase in FEV1 of ≥12%) in subjects whose FEV1 percent predicted value was less than 60%. For asthmatic patients who had been taking long-acting bronchodilators (long-acting β-agonist [LABA]) with or without inhaled corticosteroids, the protocols included an initial visit to determine the safety of withholding LABAs before the main characterization visit. Asthmatic patients assessed by a study physician to have stable disease were asked to discontinue LABAs for 48 hours before characterization studies; others with unstable or severe asthma did not have LABAs discontinued. Characterization studies included a physician-directed history, asthma characterization questionnaire, Asthma Control Test, spirometry, methacholine challenge,6 complete blood count with cell differential, and serum IgE measurement. Subjects also underwent sputum induction and measurements of nitric oxide in exhaled breath (2 subjects had fraction of exhaled nitric oxide [Feno] measurements excluded from analysis for lack of measurement reproducibility).8
Healthy control subjects had no lifetime history of pulmonary disease and lacked airway hyperresponsiveness. Both asthmatic patients and healthy control subjects were excluded if they had a history of any lung disease other than asthma, had any history of an upper or lower respiratory tract infection in the 4 weeks preceding the study, were pregnant or breast-feeding, were taking β-blocker medication, were actively smoking or previously smoked more than 5 cigarettes per month, and had a total pack-year history of greater than 10 years.
All subjects had provided informed consent for the study in which they originally participated and had also provided consent for their biospecimens to be placed in the UCSF Airway Tissue Bank for studies in addition to the original protocol. All studies and UCSF Airway Tissue Bank procedures were reviewed and approved by the UCSF Committee on Human Research.
Sputum induction and processing
Sputum induction and processing were done similarly in all protocols that contributed induced sputum samples to the tissue bank. Subjects inhaled nebulized 3% saline through a mouthpiece for 12 minutes, as previously described.9 Subjects interrupted inhalation at 2-minute intervals to spit saliva into a saliva cup and induced sputum into a sputum cup. Saliva was discarded, and induced sputum was processed within 1 hour. A 10% solution of Sputolysin (EMD Millipore, Temecula, Calif) was added at a 1:1 g/mL (sputum weight/Sputolysin) ratio to the induced sputum, mixed with a serologic pipette, and placed in a 37°C shaking water bath for 15 minutes. Samples were removed at 5-, 10-, and 15-minute intervals for additional mixing with the pipette, and a portion of this sample was used to determine total and differential cell counts, as previously described.9 The sample was then centrifuged under cold (4°C) conditions at 2000 rpm for 10 minutes. The remainder of the cell pellet was then resuspended in one of 3 types of solution: (1) 200 to 1000 μL of PBS (n = 52); (2) 600 μL of a mixture of RLT lysis buffer (Qiagen, Valencia, Calif) and 1% β-mercaptoethanol (BME; n = 41); or (3) 1 mL of Qiagen RNAprotect Saliva Reagent (n = 45). All pellets were stored at 280°C.
RNA extraction
RNA was extracted from sputum cells with RNeasy Qiagen kits (Qiagen). In addition, 26 of the 86 samples that had been stored in an RNA protection buffer were resuspended in RLT lysis buffer plus 1% BME and underwent an initial DNA elimination step with a Qiagen gDNA elimination column before the RNA extraction step. RNA quality was measured with the Agilent 2100 bioanalyzer (Biogen, Weston, Mass), which performs electrophoretic separations according to molecular weight. Each sample was assigned an RNA integrity number (RIN) based on the extent of RNA degradation. An RIN of 10 indicates intact RNA, whereas an RIN of 1 indicates totally degraded RNA.10 RIN values of greater than 5 are generally considered adequate for gene expression profiling.11,12 Purified RNAwas placed in aliquots and stored at 280°C.
Gene expression analyses
By using real-time TaqMan-based quantitative PCR methods,6 induced sputum cells from 37 asthmatic patients and 15 healthy control subjects with RIN values of greater than 5 were analyzed for expression of 14 genes relevant to airway inflammation in asthmatic patients. The expressions of 4 housekeeping genes (GAPDH, PPIA, YWHAZ, and PSMB2) were also measured. One sample with housekeeping gene cycle threshold values of greater than 35 was excluded. Some reactions yielded no cycle threshold value, and here we assigned a gene expression value equal to the minimum gene expression detected in other samples for that gene. Details of the primers and probes are listed in Table E1 in this article's Online Repository at www.jacionline.org.
TABLE E1.
Subjects’ characteristics between clinical trials
|
NCT01073410 |
||||||
|---|---|---|---|---|---|---|
|
NCT00917787 |
NCT01508078 |
NCT00595153 |
||||
| Healthy subjects | Asthmatic patients | Healthy subjects | Asthmatic patients | Healthy subjects | Asthmatic patients | |
| No. | 10 | 14 | 3 | 20 | 2 | 3 |
| Age (y) | 36.1 ± 12.4 | 35.9 ± 15.2 | 28.3 ± 6.0 | 37.7 ± 12.3 | 40.5 ± 7.8 | 26.3 ± 1.5 |
| Female sex, no. (%) | 7 (50) | 7 (50) | 1 (33) | 14 (70) | 2 (100) | 2 (67) |
| FEV1 (%) | 101.2 ± 12.5 | 84.9 ± 15.8 | 111.6 ± 12.9 | 75.6 ± 18.0 | 104.2 ± 14.6 | 75.6 ± 18.7 |
| IgE* (IU/mL) | 10 ± 39.2 | 239 ± 175.6 | 242 ± 137 | 495 ± 606 | 13.5 ± 2.1 | 214 ± 211 |
| Feno† (ppb) | 18.2 ± 11.6 | 40.5 ± 33.4 | 18.8 ± 14.63 | 46 ± 20.22 | 12.8 ± 2.9 | 36.4 ± 35.9 |
| BMI (kg/m2) | 25.2 ± 5.65 | 27.9 ± 4.76 | 25.7 ± 4.1 | 29.5 ± 8.4 | 26.2 ± 4.2 | 28.2 ± 6.3 |
| Blood eosinophils (cells/μL) | 84 ± 56 | 245 ± 125 | 100 ± 56 | 364 ± 309 | 75 ± 21 | 156 ± 103 |
| ACT score‡ | 18.2 ± 4.5 | 18.7 ± 3.6 | 20 ± 0 | |||
| PC20 | 1.54 ± 2.0 | 2.0 ± 2.4 | ||||
| Taking ICSs, no. (%) | 8 (57) | 12 (60) | 3 (100) | |||
| Sputum cells (%) | ||||||
| Macrophages | 59.9 (10.0-80.2) | 49.2 (8.4-75.2) | 40.9 (31.5-76.7) | 42.9 (14.5-73.9) | 23.5 (15.3-31.6) | 53.8 (17.8-62.5) |
| Epithelial cells | 6.7 (1.75-40.2) | 15.5 (1.2-27.9) | 32.2 (14.7-34.3) | 21.3 (0-72.8) | 15.0 (14.8-15.1) | 15.1 (4.0-26.0) |
| Neutrophils | 33.4 (4.4-70.5) | 35.1 (8.6-69.4) | 23.8 (6.9-34.9) | 31.4 (2.7-68.0) | 61.3 (53.4-69.2) | 26.1 (19.6-65.1) |
| Lymphocytes | 0.84 (0-5.1) | 0.4 (0-1.8) | 1.0 (1-1.5) | 0.5 (0-2.4) | 0.1 (0-0.2) | 0 (0-0.4) |
| Eosinophils | 0 (0-1.2) | 1.2 (0-11.0) | 0 (0-0.3) | 1.1 (0-12.3) | 0 (0-0.4) | 2.0 (0.4-7.5) |
Data are frequencies (percentages), means ± SDs, or medians (ranges).
ACT, Asthma Control Test; ICS, inhaled corticosteroids.
One healthy control subject in NCT01508078 did not have an IgE measurement performed.
One asthmatic patient in NCT01073410 and NCT00917787 did not have reproducible Feno results and was excluded.
One asthmatic patient in NCT01073410 and NCT00595153 did not have an Asthma Control Test performed.
Statistical methods
Statistical analyses were performed with the JMP 10 software package (SAS Institute, Cary, NC) and Stata 12.0 software (StataCorp, College Station, Tex). Continuous variables are presented as means ± SDs, and sputum cell counts are presented as median (ranges). Categorical variables are presented as frequencies and percentages. Correlation was performed with the Pearson rank order correlation. P values of less than .05 were taken as statistically significant, and 2-group comparisons were made with the Wilcoxon rank sum (Mann-Whitney) test. A receiver operating characteristic curve analysis was used to select cutoff values for the percentages of sputum eosinophilia, peripheral blood eosinophilia, and Feno that maximized the sensitivity and specificity for predicting TH2-high asthma.
RESULTS
RNA quality in cell pellets from induced sputum
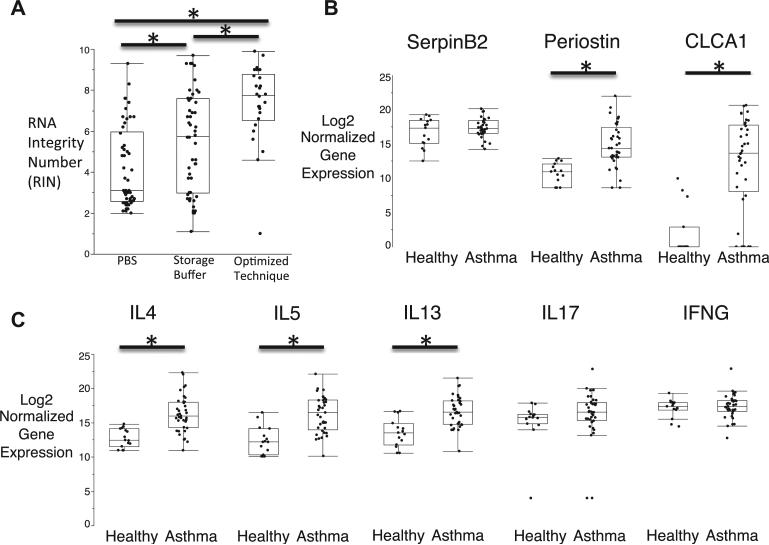
Storage buffer and DNA extraction before RNA extraction had marked effects on the RIN values of sputum cell RNA (Fig 1, A). Specifically, 32% of the 52 sputum cell pellets stored in PBS had RIN values of greater than 5, 46% of the 60 sputum cell pellets stored in RNA protection buffer (RLT/BME or the Qiagen RNAprotect Saliva Reagent) had RIN values of greater than 5, and 92% of 26 sputum cell pellets stored in RLT/BME or RNA Saliva Reagent and processed with a DNA elimination column before RNA extraction had RIN values of greater than 5 (Fig 1, A).
FIG 1.
A, RIN values are highest in sputum cells stored and processed with optimized methods. *Significant difference between methods (P < .01). B, Expression of periostin and CLCA1 is higher than normal in asthmatic patients, but expression of SerpinB2 is not. C, IL4, IL5, and IL13 expression is higher than normal in asthmatic patients, but expression of IL17 and IFNG is not. *Significant difference from healthy control subjects (P < .0001).
Gene profiling in sputum cell RNA
Induced sputum cells from 37 asthmatic patients and 15 healthy control subjects with high-quality RNA (RIN values > 5) were analyzed for expression of 14 genes relevant to airway inflammation in asthmatic patients. The clinical characteristics of the asthmatic patients are summarized in Table I and Table E1.
TABLE I.
Subjects’ characteristics
| Healthy subjects | Asthmatic patients | |
|---|---|---|
| No. | 15 | 37 |
| Age (y) | 35.1 ± 11.1 | 36.1 ± 13.1 |
| Female sex, no. (%) | 8 (53) | 23 (62) |
| FEV1 (%) | 103.7 ± 12.5 | 79.1 ± 17.4* |
| IgE (IU/mL) | 64.5 ± 97.5 | 375.6 ± 474.4* |
| Feno (ppb) | 17.6 ± 11.0 | 43.4 ± 25.9* |
| BMI (kg/m2) | 25.4 ± 4.9 | 28.7 ± 6.9 |
| Blood eosinophils (cells/μL) | 86 ± 13.1 | 302 ± 40.8* |
| Smoking (pack years) | 0.1 ± 0.4 | 0.9 ± 2.1 |
| ACT score | 18.5 ± 3.8 | |
| PC20 | 1.8 ± 2.6 | |
| Taking ICSs, no. (%) | 20 (54) | |
| Sputum cells (%) | ||
| Macrophages | 54.9 (10.0-79.4) | 46.6 (8.4-75.2) |
| Epithelial cells | 14.7 (1.8-40.2) | 17.8 (0-72.8) |
| Neutrophils | 34.2 (4.5-70.5) | 31.5 (2.7-69.4) |
| Lymphocytes | 0.9 (0-5.1) | 0.4 (0-2.4) |
| Eosinophils | 0.0 (0-1.2) | 1.1 (0-12.3)* |
Data are shown as frequencies (percentages), means ± SDs, or medians (ranges).
ACT, Asthma Control Test; ICS, inhaled corticosteroid.
Significant difference from healthy subjects (P < .05).
Epithelial cell signature of TH2 inflammation
The gene expression of periostin and CLCA1 in asthmatic patients was higher than in healthy control subjects. In contrast, the expression of SerpinB2 was similar in asthmatic patients and healthy subjects (Fig 1, B).
TH2 cytokines
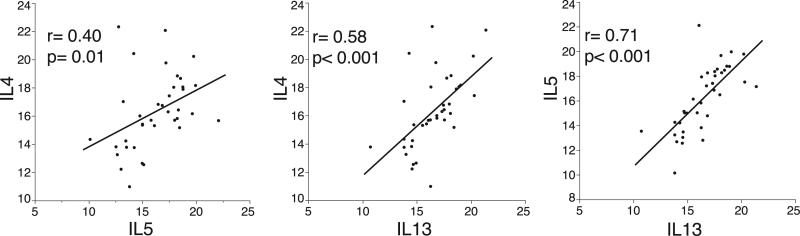
Unlike IFN-γ or IL17 gene expression levels, which were similar in asthmatic patients and healthy subjects, the gene expression levels of IL-4, IL-5, and IL-13 were significantly higher in asthmatic patients compared with those seen in healthy control subjects (Fig 1, C). The difference from normal values in the expression of TH2 genes in asthmatic patients was driven by subgroups with high gene expression because some asthmatic patients had expression levels in the normal range (Fig 1, C), which is consistent with the previously identified TH2-high and TH2-low subsets of asthma.6 Also consistent with the TH2-high and TH2-low asthma subsets is the finding that the gene expression of IL-4, IL-5, and IL-13 was highly correlated among asthmatic patients (see Fig E1 in this article's Online Repository at www.jacionline.org).
FIG E1.
Gene expression for IL4, IL5, and IL13 in sputum cells is highly correlated among asthmatic patients. Data are log2-normalized gene expression.
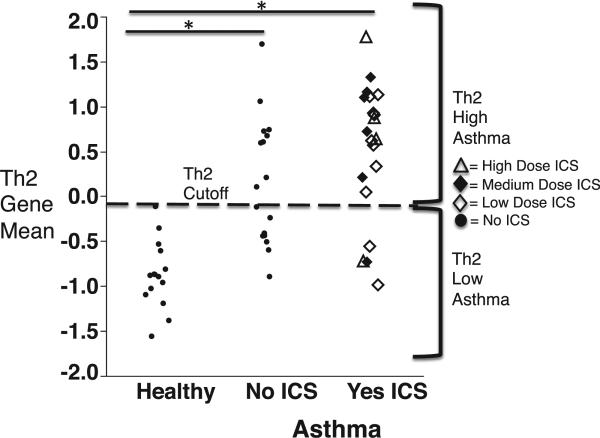
To provide a simplified metric of TH2 gene expression, we first centered and scaled the gene expression for each TH2 cytokine (IL-4, IL-5, and IL-13). Thereby generating standardized gene expression variables for IL-4, IL-5, and IL-13. We then calculated a mean of these standardized variables to generate a single value representing the mean expression of the TH2 cytokines that we call the “TH2 gene mean.” By adding 2 SDs to the mean TH2 gene mean value obtained from healthy subjects, we classified asthmatic patients as TH2 high and TH2 low (Fig 2). In this way we found that 26 (70%) of the 37 asthmatic patients had TH2-high asthma.
FIG 2.
The TH2 gene mean as a scaled and centered mean expression value for IL-4, IL-5, and IL-13. The TH2 gene mean data for the healthy subjects show the cutoff value that represents 2 SDs greater than the mean value. ICS, Inhaled corticosteroids. *Significant difference between asthmatics on ICS and not on ICS compared to healthy controls (P < .01).
Reproducibility of the TH2 gene mean
To establish the within-sample repeatability of the TH2 gene mean, we repeated measures of gene expression for TH2 cytokine genes in sputum cell RNA aliquots from the same sputum sample in 48 of the original 52 subjects. As shown in Fig 3, A, the within-sample repeatability for the TH2 gene metric was excellent (Pearson correlation 5 0.94; mean difference in gene expression between repeated measurements = –0.0006).
FIG 3.
A, The TH2 gene mean is highly reproducible in RNA aliquots of induced sputum from asthmatic patients. B, The TH2 gene mean in 13 asthmatic patients at repeat visits. Red lines, Persistent TH2-high asthma; black lines, intermittent TH2-high asthma; blue lines, persistent TH2-low asthma. Triangles represent subjects taking inhaled corticosteroids, and circles represent subjects not taking inhaled corticosteroids.
To determine the reproducibility of the TH2 gene mean in the same subjects at different time points, we measured TH2 cytokine levels and the TH2 gene mean in 2 different sputum cell pellets from each of 13 asthmatic patients who had repeat sputum inductions subsequent to the index induction (see Table E2 in this article's Online Repository at www.jacionline.org for the clinical characteristics of these subjects). We found that 5 of the 13 subjects had a persistently high TH2 phenotype, 5 of the 13 subjects had an intermittently high TH2 phenotype, and 3 of the 13 subjects had a persistently low TH2 phenotype (Fig 3, B). The subgroup of 5 subjects with an intermittent phenotype tended to have TH2 gene mean values close to the cutoff value. Fig E2 in this article's Online Repository at www.jacionline.org provides additional data for how time from the index induction influences the repeated measures of the TH2 gene mean.
TABLE E2.
Gene primers and probes
| Gene primers | Sequence |
|---|---|
| PPIA-outer forward | ATGAGAACTTCATCCTAAAGCATACG |
| PPIA-outer reverse | TTGGCAGTGCAGATGAAAAACT |
| PPIA-inner forward | ACGGGTCCTGGCATCTTGT |
| PPIA-probe | ATGGCAAATGCTGGACCCAACACA |
| PPIA-inner reverse | GCAGATGAAAAACTGGGAACCA |
| GAPDH-outer forward | CAATGACCCCTTCATTGACCTC |
| GAPDH-outer reverse | CTCGCTCCTGGAAGATGGTGAT |
| GAPDH-inner forward | GATTCCACCCATGGCAAATTC |
| GAPDH-probe | CGTTCTCAGCCTTGACGGTGCCA |
| GAPDH-inner reverse | GGGATTTCCATTGATGACAAGC |
| YWHAZ-outer forward | CTTCTGTCTTGTCACCAACCATTC |
| YWHAZ-outer reverse | CAACTAAGGAGAGATTTGCTGCAG |
| YWHAZ-inner forward | TGGAAAAAGGCCGCATGAT |
| YWHAZ-probe | TGGCTCCACTCAGTGTCTAAGGCACCCT |
| YWHAZ-inner reverse | TCTGTGGGATGCAAGCAAAG |
| PSMB2-outer forward | CCATATCATGTGAACCTCCTCCT |
| PSMB2-outer reverse | GTCGAGGATACTGAGAGTCAGGAA |
| PSMB2-inner forward | TCCTCCTGGCTGGCTATGAT |
| PSMB2-probe | ACAGCGCTGGCCCTTCATGCTC |
| PSMB2-inner reverse | GGCTGCCAGGTAGTCCATGT |
| CLCA1-outer forward | CCAGGCATTGCTAAGGTTGG |
| CLCA1-outer reverse | ACTGGCCCTGAGAATTGGG |
| CLCA1-inner forward | CCTTGACCCTGACTGTCACGT |
| CLCA1-probe | TGCGTCCAATGCTACCCTGCCTC |
| CLCA1-inner reverse | TTGTTCGTTTTGGAAGTCACTGTAA |
| SERPINB2-outer forward | CTGAAGTGTTCCACCAAGCCA |
| SERPINB2-outer reverse | CAAACTGTGGGCCTCCATGT |
| SERPINB2-inner forward | GTGAATGAGGAGGGCACTGAA |
| SERPINB2-probe | TAACACCTCCTGTGCCAGCGGCTG |
| SERPINB2-inner reverse | CCATGTCCAGTTCTCCCTGTC |
| Periostin-outer forward | GCAAACCACCTTCACGGATCT |
| Periostin-outer reverse | TTATTCACAGGTGCCAGCAAAG |
| Periostin-inner forward | CGGATCTTGTGGCCCAATT |
| Periostin-probe | CTTGGCATCTGCTCTGAGGCC |
| Periostin-inner reverse | AGGTGCCAGCAAAGTGTATTCTC |
| IL4-outer forward | GGGTCTCACCTCCCAACTGC |
| IL4-outer reverse | TGTCTGTTACGGTCAACTCGGT |
| IL4-inner forward | GCTTCCCCCTCTGTTCTTCCT |
| IL4-probe | TCCACGGACACAAGTGCGATATCACC |
| IL4-inner reverse | GCTCTGTGAGGCTGTTCAAAGTT |
| IL5-outer forward | GCCATGAGGATGCTTCTGCA |
| IL5-outer reverse | GAATCCTCAGAGTCTCATTGGCTATC |
| IL5-inner forward | AGCTGCCTACGTGTATGCCA |
| IL5-probe | CCCCACAGAAATTCCCACAAGTGCA |
| IL5-inner reverse | GTGCCAAGGTCTCTTTCACCA |
| IL13-outer forward | GGATGCTGAGCGGATTCTG |
| IL13-outer reverse | CCCTCGCGAAAAAGTTTCTT |
| IL13-inner forward | AAGGTCTCAGCTGGGCAGTTT |
| IL13-probe | CCAGCTTGCATGTCCGAGACACCA |
| IL13-inner reverse | AAACTGGGCCACCTCGATT |
| IL13-outer forward | ACTGCTACTGCTGCTGAGCCT |
| IL13-outer reverse | GGTGAGGTGGATCGGTTGTAGT |
| IL17-inner forward | CAATCCCACGAAATCCAGGA |
| IL17-probe | CCCAAATTCTGAGGACAAGAACTTCCCC |
| IL17-inner reverse | TTCAGGTTGACCATCACAGTCC |
| IFNG-outer forward | GTAACTGACTTGAATGTCCAACGC |
| IFNG-outer reverse | GACAACCATTACTGGGATGCTC |
| IFNG-inner forward | CCAACGCAAAGCAATACATGA |
| IFNG-probe | TCCAAGTGATGGCTGAACTGTCGCC |
| IFNG-inner reverse | TTTTCGCTTCCCTGTTTTAGCT |
| CCL11-outer forward | CCAGAGCCTAAGAACTGCTTGATT |
| CCL11-outer reverse | GGAACTACATGAAGCCAAGTCCTT |
| CCL11-inner forward | GAGCCTAAGAACTGCTTGATTCCT |
| CCL11-probe | TCCCTCAGAGCACGTCTTAGGAAAG |
| CCL11-inner reverse | TGGGCGACTGGTGCTGATA |
| CCL24-outer forward | GATGACCATAGTAACCAGCCTTCTG |
| CCL24-outer reverse | GCAGCAGGGAGAGGGTATGAC |
| CCL24-inner forward | AGCCTTCTGTTCCTTGGTGTCT |
| CCL24-probe | CCCACCACATCATCCCTACGGGCT |
| CCL24-inner reverse | GCAGCAGGGAGAGGGTATGA |
| CCL26-outer forward | AAGACCTGCTGCTTCCAATACAG |
| CCL26-outer reverse | TGCCTCTTTTGGTAGTGAATATCACA |
| CCL26-inner forward | CTGCTTCCAATACAGCCACAAG |
| CCL26-probe | CTTCCCTGGACCTGGGTGCGAA |
| CCL26-inner reverse | GAGCAGCTGTTACTGGTGAATTCA |
| Tryptase-outer forward | GCCATTTCCTCTGAAGCAGGT |
| Tryptase-outer reverse | GCATGTCGTCACGGACGAT |
| Tryptase-inner forward | GGTCCCCATAATGGAAAACCA |
| Tryptase-probe | TTGTGACGCAAAATACCACCTTGGCG |
| Tryptase-inner reverse | GGACGTCGTCTCCCGTGTA |
| Chymase-outer forward | GGCCCAGGGCATCGTATC |
| Chymase-outer reverse | CAGGATTAATTTGCCTGCAGG |
| Chymase-inner forward | TATGGACGGTCGGATGCAA |
| Chymase-probe | CCCTGCTGTCTTCACCCGAATCTCC |
| Chymase-inner reverse | TTGATCCAGGGCCGGTAAT |
| CPA3-outer forward | AGGATGAAAAACAAGCAGACATCA |
| CPA3-outer reverse | CAGACTGGATGGCTTGGGATT |
| CPA3-inner forward | CAAAACCAATGAGCTTGACTTCTG |
| CPA3-probe | TCCAGGTGCCACCCACCACGTA |
| CPA3-inner reverse | CGGAAATCCACCATCATATTAGC |
FIG E2.
Influence of the time interval between sputum induction on the reproducibility of TH2 classification. Red lines, Persistent TH2-high asthma; black lines, intermittent TH2 asthma; blue lines, persistent TH2-low asthma. Triangles represent subjects taking inhaled corticosteroids, and circles represent subjects not taking inhaled corticosteroids.
Clinical characteristics of TH2-high asthma as classified by sputum cell gene profiling
Compared with TH2-low asthma, TH2-high asthma is characterized by higher levels of airway nitric oxide and higher airway and peripheral blood eosinophil counts (Table II). For prednisone-requiring asthma exacerbations, the TH2-high subgroup had an exacerbation rate in the past 2 years of 0.42 exacerbations per year; the rate in the TH2-low subgroup was 0.36 (Table II). In addition, we found that the TH2 gene mean value was inversely related to FEV1 in the asthmatic patients and that it was highest in asthmatic patients with poor asthma control (Fig 4). We found no such relationships between sputum cell IL17 gene expression and measures of asthma control or FEV1 (Fig 4).
TABLE II.
Clinical characteristics of patients with TH2-high and those with TH2-low asthma
| TH2-low asthma | TH2-high asthma | P value | |
|---|---|---|---|
| No. | 11 | 26 | |
| Age (y) | 32.6 ± 13.3 | 37.6 ± 13.1 | .30 |
| Female sex, no. (%) | 6 (55) | 17 (65) | .53 |
| FEV1 (%) | 88.6 ± 14.0 | 75.1 ± 17.3* | <.05 |
| Sputum eosinophils (%) | 0.33 (0.59) | 3.4 (3.4)* | <.01 |
| Blood eosinophils (cells/μL) | 139 ± 371 | 371 ± 268* | <.01 |
| IgE (IU/mL) | 312 ± 404 | 402 ± 506 | .60 |
| Feno (ppb) | 28.1 ± 17.6 | 49.6 ± 26.4* | <.05 |
| BMI (kg/m2) | 27.5 ± 5.6 | 29.3 ± 7.4 | .48 |
| ACT score | 20.5 ± 3.7 | 17.8 ± 3.7* | <.05 |
| logPC20 | 0.14 ± 0.47 | –0.22 ± 59 | .08 |
| Exacerbation rate (no./y) | 0.36 ± 0.78 | 0.42 ± 1.20 | .88 |
| Taking ICSs, no. (%) | 4 (36) | 16 (62) | .16 |
| Periostin | 13.2 ± 3.4 | 15.4 ± 2.9* | <.05 |
| SerpinB2 | 17.0 ± 1.3 | 17.6 ± 1.5 | .21 |
| CLCA1 | 8.1 ± 6.9 | 14.0 ± 6.1* | <.01 |
| IL17 | 17.0 ± 2.1 | 15.8 ± 4.0 | .34 |
| IFN-γ | 17.8 ± 0.9 | 17.1 ± 2.0 | .23 |
Gene expression is normalized and log2 transformed. TH2 inflammation is determined based on the scaled and centered mean gene expression of IL-4, IL-5, and IL-13.
ACT, Asthma Control Test; ICS, inhaled corticosteroids.
Significant difference from patients with TH2-low asthma (P < .05).
FIG 4.
The TH2 gene mean is significantly higher in patients with poorly controlled asthma (Asthma Control Test [ACT] score <20 or prebronchodilator FEV1 percent predicted <80%), whereas IL17 gene expression is not. The TH2 gene mean is inversely correlated with prebronchodilator FEV1 percent predicted, but it is not correlated with IL17 gene expression. *Significant difference from healthy control subjects (P < .01).
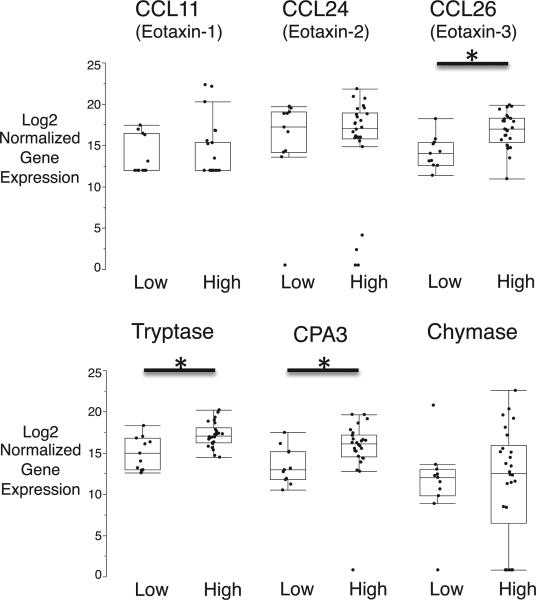
Mast cell genes and eotaxins in TH2 subgroups of asthma
We reported previously that mast cell numbers are increased in the airway epithelium in patients with TH2-high asthma.13 Here we explored mast cell gene expression in sputum cell pellets and found that the expression of mast cell genes (tryptase and carboxypeptidase A3 [CPA3]) was increased in patients with TH2-high asthma (Fig 5). Because eosinophils are prominent in induced sputum in patients with TH2-high asthma, we measured the expression of eotaxin family members and found that the expression of CCL26 (but not CCL11 or CCL24) was increased in patients with TH2-high asthma (Fig 5).
FIG 5.
Gene expression for eotaxin genes (CCL11, CCL24, and CCL26) and mast cell genes (tryptase, CPA3, and chymase) in sputum cells from patients with TH2-high and TH2-low asthma. *Significant difference from healthy control subjects (P < .01).
Sputum eosinophils, blood eosinophils, and Feno values as tests of airway TH2 inflammation
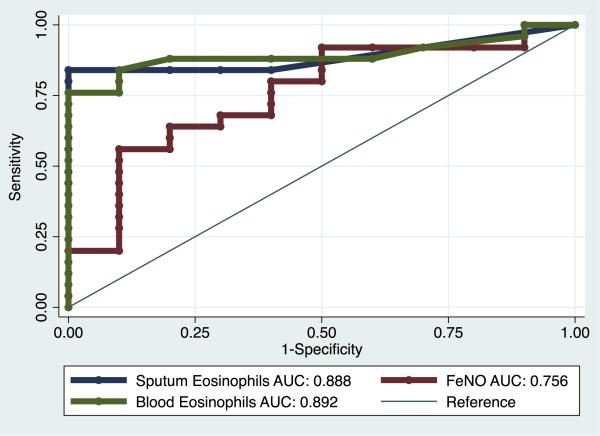
Sputum eosinophilia, usually defined as greater than 2% sputum eosinophils, is used as a noninvasive marker for TH2 inflammation.14,15 We found here that 14 (38%) of 37 asthmatic patients had sputum eosinophilia, and all of these were also classified as TH2 high based on results of IL-4, IL-5, and IL-13 gene profiling. This indicates that sputum eosinophilia of greater than 2% is 100% specific for airway TH2 inflammation. However, among the 26 asthmatic patients classified as having TH2-high asthma by using gene expression profiling, only 14 had sputum eosinophilia of greater than 2%. This indicates that sputum eosinophilia of greater than 2% has a sensitivity of only 54% for airway TH2 inflammation. Using a receiver operating characteristic curve analysis to determine the optimal cutoff of sputum eosinophilia as a test for predicting TH2-high asthma, we found that a lower cutoff of 0.8% for sputum eosinophils had a much better combination of sensitivity and specificity (84% and 100%, respectively; Fig 6).
FIG 6.
Receiver operating characteristic (ROC) analysis of peripheral blood eosinophil counts, sputum eosinophil counts, and Feno levels as biomarkers of airway TH2 inflammation, as assessed by using the sputum cell TH2 gene mean. Green line, ROC curve for peripheral blood eosinophil counts; blue line, ROC for sputum eosinophil counts; red line, ROC for Feno values. AUC, Area under the curve.
Because measurement of sputum eosinophil counts is not a simple clinical test, we evaluated peripheral blood eosinophil counts and measures of Feno as more easily measured biomarkers of TH2 inflammation. By using receiver operating characteristics, the highest combination of sensitivity and specificity for peripheral blood eosinophil counts was reached at a derived threshold of 230 cells/μL, a value that had similar sensitivity and specificity as sputum eosinophilia (76% and 100%, respectively) for airway TH2-high asthma (Fig 6). In contrast, Feno did not perform as well as a biomarker: the highest combination of sensitivity and specificity for Feno was reached at a derived threshold of 51 ppb, a value with a good specificity of 90% but a sensitivity of only 56% for airway TH2-high asthma (Fig 6).
DISCUSSION
We describe optimized methods for extracting high-quality RNA from cells in induced sputum, and we show that measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. Our data suggest that measures of gene expression in sputum cells could be applied in molecular phenotyping studies of asthma, including studies to investigate the unknown molecular abnormalities occurring in patients with TH2-low disease.
In initial studies we set out to examine the quality of RNA in cell pellets from induced sputum that had been stored under different conditions in our tissue bank. These studies revealed the importance of RNA protection buffer and DNA removal, without which the RNA quality from stored cells was suboptimal. To our knowledge, this is the first assessment of RNA quality in induced sputum cell pellets, and we describe optimized techniques that yield high-quality RNA. Prior investigators have used sputum RNA to detect differences in gene expression profiling in asthmatic patients, but these investigations did not provide any analysis of RNA quality.7,16 Our optimized methods yielded RNA with an RIN of greater than 5 in more than 80% of samples. This RIN threshold of 5 as a determination of high-quality RNA has been advocated by other investigators and has been validated for the specific purposes of RT-PCR gene expression analysis.11 Moreover, our RT-PCR method produces small amplicons (100-250 bp), and small amplicons are known to be more tolerant of partial RNA degradation.11,12
A recent study used an unsupervised approach to cluster asthmatic patients into 3 groups based on gene expression profiles in induced sputum cells.16 The differentially expressed genes in each cluster were shown to correlate with inflammatory cell types and some clinical characteristics. Here, we take a different approach, with a supervised analysis that focused on genes related to TH2 inflammation. By converting the expression of IL-4, IL-5, and IL-13 into a single TH2 gene mean value, we found that we could classify asthmatic patients as having TH2-high or TH2-low disease. Specifically, two thirds of the asthmatic patients had a TH2 gene mean value higher than a cutoff value that was based on the mean value plus 2 SDs in the healthy subgroup. The subgroup of asthma with a high TH2 gene mean represents the TH2-high subgroup previously described,6 and we propose here that sputum TH2 gene profiling can be applied to identify TH2-high and TH2-low subgroups of asthma. In a subgroup of asthmatics, we were able to measure the TH2 gene mean in a second sample of sputum cell RNA collected at varying time points after the initial sample. Using this approach, we found evidence for asthmatic patients with a persistently high TH2 phenotype, an intermittently high TH2 phenotype, and a persistently low TH2 phenotype. These findings mirror our previous findings for eosinophilic and noneosinophilic asthma.14
Notably, the sputum cell TH2 gene mean value was high in many asthmatic patients being treated with inhaled corticosteroids, and a subgroup of steroid-treated asthmatic patients demonstrated persistently increased TH2 inflammation when airway secretions were repeated at subsequent visits. Previously, we have found that the airway epithelial cell gene signature of TH2 inflammation is highly sensitive to repression by steroid treatment.5,6 Therefore an advantage of sputum cell profiling over other molecular phenotyping methods might be that it reveals TH2-high asthma, even when subjects are receiving steroid treatment. Moreover, these findings suggest that TH2 inflammation persists in some asthmatic patients taking inhaled corticosteroids. It is possible that this subgroup could benefit from adjunctive treatment with anti-TH2 cytokine therapy.
Unlike gene expression measures in bronchial epithelial brushings in which the expression levels of TH2 cytokines are low, we show here that sputum cell samples have relatively high levels of TH2 cytokine transcripts and that airway TH2 status can be determined by using the expression levels of IL-4, IL-5, and IL-13. The sputum gene expression of periostin and CLCA1 is differentially expressed in asthmatic patients, which is consistent with our prior findings in bronchial brushings, but the gene expression of SerpinB2 is not differentially expressed in sputum cells. Unlike CLCA1 and periostin, the gene expression of which is likely restricted to epithelial cells, SerpinB2 is also expressed in inflammatory cells, namely macrophages and neutrophils,17 and the lack of differential expression of SerpinB2 in sputum cells from asthmatic patients might be because expression in macrophages and neutrophils masks increased expression in epithelial cells.
Previously, using gene expression measures in airway epithelial brushings from asthmatic patients with mild-to-moderate asthma, we reported that TH2-high asthma occurs in 50% of subjects. The prevalence of TH2-high asthma in the cohort reported here is greater at 70%, but the asthmatic patients we studied here had more severe disease, and TH2 inflammation is an important driver of asthma disease severity.15 Indeed, we found here that the TH2 gene mean value was highest in asthmatic patients with more severe airflow obstruction and in those with poor asthma control. We did not detect any difference in rates of asthma exacerbations between the TH2-high or TH2-low asthmatic patients, but our study was not large enough for a robust analysis of the effects of TH2 status on this outcome.
Relevant here is how well sputum eosinophil counts and blood eosinophil counts perform as biomarkers of TH2-high asthma based on the sputum TH2 gene mean. Using receiver operating characteristics, we found that we could identify threshold values for sputum and blood eosinophil counts that had very good sensitivity and specificity for TH2-high asthma, but even the best threshold value for Feno had a sensitivity of only 56%.
It has previously been shown that TH2-high asthma is associated with airway eosinophilia, increased expression of eotaxin 3, and increased airway epithelial mast cell counts,6,13,18 findings that we confirm here. We show that eotaxin 3 (CCL26) is specifically upregulated in sputum cells in patients with TH2-high asthma, and we show that gene expression for the mast cell genes tryptase and CPA3 is also increased in sputum cell pellets from patients with TH2-high asthma. We also show that these luminal mast cells also have the same unusual protease phenotype (tryptase and CPA3 high and chymase low) that we previously have described in the airway epithelial layer in patients with TH2-high asthma.13
Although we identify increased TH2 inflammation in 70% of asthmatic patients, 30% of our cohort did not have evidence of airway TH2 inflammation. TH17 cells and IL-17 are implicated as an alternative mediator of asthma inflammation and severity,19,20 but we found no evidence in support of an IL-17 subtype of asthma here. Additional work is needed to identify the mechanisms operating in TH2-low asthma.
In summary, we have optimized methods for ensuring high-quality RNA from cells in induced sputum, and we show measures of gene expression for TH2 cytokines in sputum cells can be used to identify asthmatic patients with TH2-high asthma. Measures of gene expression in sputum cells therefore represent a relatively noninvasive method to identify molecular phenotypes of asthma in large studies of treatment or disease mechanisms. In addition, unbiased gene profiling methods could also be applied in sputum cell expression studies in future research to help reveal the non-TH2 molecular mechanisms of asthma that operate in relatively large subgroups of patients.
Key messages.
Measures of gene expression in induced sputum cells is feasible, and the expression levels of IL-4, IL-5, and IL-13 can be used to classify asthma into TH2-high and TH2-low endotypes.
Increased TH2 airway inflammation is characterized by poor asthma control and more severe airflow obstruction.
TABLE E3.
Subjects’ characteristics with repeat sputum induction
| Asthmatic patients | |
|---|---|
| No. | 13 |
| Age (y) | 42.1 ± 12.7 |
| Female sex, no. (%) | 9 (69) |
| FEV1 (%) | 77.4 ± 14.6 |
| IgE (IU/mL) | 408.7 ± 465.3 |
| Feno (ppb) | 44.1 ± 24.4 |
| BMI (kg/m2) | 30.5 ± 8.2 |
| Blood eosinophils (cells/μL) | 398.5 ± 437.5 |
| ACT score | 17.1 ± 3.3 |
| PC20 | 0.84 ± 0.89 |
| Taking ICSs, no. (%) | 8 (62) |
| Sputum cells (%) | |
| Macrophages | 30.7 (12.1-70.5) |
| Epithelial cells | 12.3 (1.9-45.9) |
| Neutrophils | 50.3 (2.7-77.4) |
| Lymphocytes | 0.2 (0-2.4) |
| Eosinophils | 1.0 (0-19.2) |
Data are frequencies (percentages), means ± SDs, or medians (ranges).
ACT, Asthma Control Test; ICS, inhaled corticosteroid.
Acknowledgments
Supported in part by a research grant from Genentech, and National Institutes of Health grants P01HL107202 and T32HL007185.
M. C. Peters has received grants from the National Institutes of Health (NIH; T325T32HL007185). N. R. Bhakta has received grants from the NIH (F321F32HL110720 and NIH T325T32HL007185). P. G. Wood-ruff has received grants from Genentech; has consultant arrangements with Genentech, MedImmune, Astra Zeneca, Boehringer Ingelheim, Merck, and Kalobios; has grants/grants pending with Genentech and Pfizer; and has a patent application for asthma diagnostics. J. V. Fahy has received research grants from the NIH; has received consulting fees or honoraria from Merck, Regeneron, Boehringer Ingelheim, Pathway Therapeutics, Cytokinetics, Amgen, and the University of Calgary; has received support for travel to meetings for study or other purposes from Boehringer Ingelheim to the Transatlantic Airway Conference; has received fees for participation in review activities, such as data monitoring boards, statistical analysis, end point committees, and the like from the NIH; and has patents planned, pending, or issued for a patent describing biomarkers of TH2-high asthma.
Abbreviations used
- BME
β-Mercaptoethanol
- CLCA1
Chloride channel accessory 1
- CPA3
Carboxypeptidase A3
- Feno
Fraction of exhaled nitric oxide
- LABA
Long-acting β-agonist
- RIN
RNA integrity number
- SerpinB2
Serpin β2
- UCSF
University of California, San Francisco
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 2.Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181:788–96. doi: 10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- 3.Kips JC, O'Connor BJ, Langley SJ, Woodcock A, Kerstjens HA, Postma DS, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 2003;167:1655–9. doi: 10.1164/rccm.200206-525OC. [DOI] [PubMed] [Google Scholar]
- 4.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 5.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–63. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–95. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truyen E, Coteur L, Dilissen E, Overbergh L, Dupont LJ, Ceuppens JL, et al. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax. 2006;61:202–8. doi: 10.1136/thx.2005.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Thoracic Society European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 9.Gershman NH, Wong HH, Liu JT, Mahlmeister MJ, Fahy JV. Comparison of two methods of collecting induced sputum in asthmatic subjects. Eur Respir J. 1996;9:2448–53. doi: 10.1183/09031936.96.09122448. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27:126–39. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Fleige S, Walf V, Huch S, Prgomet C, Sehm J, Pfaffl MW. Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR. Biotechnol Lett. 2006;28:1601–13. doi: 10.1007/s10529-006-9127-2. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J Allergy Clin Immunol. 2010;125:1046–53. e8. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185:612–9. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–54. e10. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J Allergy Clin Immunol. 2011;127:153–60. e1–9. doi: 10.1016/j.jaci.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Abbas AR, Baldwin D, Ma Y, Ouyang W, Gurney A, Martin F, et al. Immune response in silico (IRIS): immune-specific genes identified from a compendium of microarray expression data. Genes Immun. 2005;6:319–31. doi: 10.1038/sj.gene.6364173. [DOI] [PubMed] [Google Scholar]
- 18.Choy DF, Modrek B, Abbas AR, Kummerfeld S, Clark HF, Wu LC, et al. Gene expression patterns of Th2 inflammation and intercellular communication in asthmatic airways. J Immunol. 2011;186:1861–9. doi: 10.4049/jimmunol.1002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–8. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 20.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–7. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]