Abstract
After the surface silylation with 3-methacryloxypropyltrimethoxysilane, silica nanoparticles were further modified by 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO). The immobilization of DOPO on silica nanoparticles was confirmed by Fourier transform infrared spectroscopy, UV–visible spectroscopy, magic angle spinning nuclear magnetic resonance, and thermogravimetric analysis. By incorporating the DOPO-immobilized silica nanoparticles (5 wt%) into polypropylene matrix, the thermal oxidative stability exhibited an improvement of 62 °C for the half weight loss temperature, while that was only 26 °C increment with incorporation of virgin silica nanoparticles (5 wt%). Apparent activation energies of the polymer nanocomposites were estimated via Flynn–Wall–Ozawa method. It was found that the incorporation of DOPO-immobilized silica nanoparticles improved activation energies of the degradation reaction. Based on the results, it was speculated that DOPO-immobilized silica nanoparticles could inhibit the degradation of polypropylene and catalyze the formation of carbonaceous char on the surface. Thus, thermal stability was significantly improved.
Keywords: Polypropylene, DOPO, Silica, Nanocomposites, Kinetic
Introduction
Silica nanoparticles are considered probably one of the most valuable nanomaterials as they demonstrated excellent performance in many important applications, such as composites, coatings, binders, catalysis, and so on [1–4]. Despite the wide utilization achieved nowadays, two major issues play continuous difficulties in realizing the full potential of the silica nanoparticles: one is the aggregation of the nanoparticles themselves and the other is the lack of strong interactions with matrix. So, various strategies for the surface modification have been explored during the last decade [5–23].
Dispersion of silica nanoparticle is not easily achieved because the silanol groups residing on adjacent nanoparticles could form hydrogen bonds and lead to aggregations. Surface modification of silica nanoparticle is usually carried out by grafting polymer chains for a better dispersion [5–8]. As the representative example, surface-initiated polymerization has been applied to a number of polymers such as polystyrene [5], polypropylene [6], poly(methyl methacrylate) [7], and poly (ethylene terepthalate) [8] to graft polymer chains on the surface of silica nanoparticles. As the polymer coating formed, the surface of silica nanoparticle is altered from hydrophilic to hydrophobic and therefore, the aggregations of silica nanoparticles are dramatically decreased. Surface modification with silane coupling agents has also been actively investigated to decrease the aggregation of the nanoparticles as well as improve interaction between filler and matrix [5, 9–18]. It was found that surface modification with vinylsilanes could decrease the secondary agglomerate structures. And the increasing amounts of silane used for the silica modification could further improve the dispersion of silica nanoparticles [13]. Methacryloxypropyltrimethoxysilane was used to improve the interaction of the silica nanoparticles and matrix. The mechanical strength of filled resins could be enhanced by advance preparation of silica fillers with silane coupling agents followed by electron beam irradiation [18].
It was also reported that silane coupling agents could be used to immobilize specific functional groups on the surface of silica nanoparticle to further enhance the interaction between the filler and matrix [11, 12, 14–17]. Immobilizing suitable compound on the surface of silica nanoparticles could not only decrease the aggregation of the nanoparticles but also provide unique opportunity to engineer the interfacial properties. Enzymes lactate dehydrogenase and glutamate dehydrogenase were successfully anchored on silica nanoparticles. The resultant hybrid materials were proved to be of excellent enzymatic activities and detection capacity [12]. The immobilization of formylsalicylic acid on the surface of silica nanoparticle was reported for selective extraction of iron from a mixture containing several other metal ions [15]. The nickel-immobilized silica nanoparticle was found to be an effective recyclable heterogeneous catalyst in a room temperature cross-coupling reaction [17].
In recent years, there is a growing interest to incorporate specific-compound-immobilized silica nanoparticles to prepare functional polymer nanocomposites [19–23]. Brancatelli et al [19, 20] very recently reported that the phosphorus-doped silica could enhance the thermal stability of cotton fabrics. The treated silica nanoparticles could distribute uniformly in the matrix. Moreover, our recent work [21–23] indicated that when antioxidant compound was immobilized on the surface of silica, the antioxidant behavior to polyolefin was significantly improved. For example, by incorporating antioxidant compound-immobilized silica nanoparticles into polypropylene (PP) matrix, tensile strength of the nanocomposites only reduced from 31.6 to 22.6 MPa even after 6 weeks of thermal aging. While for PP with antioxidant compound, the tensile strength dramatically reduced from 31.9 to 2.5 MPa after only 1 week thermal aging [21].
Similarly, phosphorus compounds, such as ammonium polyphosphate, bis(diphenyl phosphate), and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) derivatives have been proved to be good candidates to improve the thermal and flame retardancy properties for polymeric materials [24, 25]. Therefore, this study aims to immobilize phosphorus compound onto silica nanoparticles and incorporate such modified silica within PP. DOPO was chosen because of the versatility of DOPO in organic synthesis [25, 26]. It was hypothesized that such modification could not only improve the dispersion of silica nanoparticles but also could increase the thermal stability of PP.
DOPO was chemically immobilized onto the surface of silica nanoparticle through 3-methacryloxypropyltrimethoxysilane. The functionalized silica nanoparticle was characterized by thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), UV–visible (UV–vis) spectroscopy, and cross-polarization magic angle spinning nuclear magnetic resonance (CP MAS-NMR). Polypropylene/silica (PP/SiO2) nanocomposites were prepared through melt compounding. TGA was used to characterize the thermal oxidative degradation behavior and investigate the degradation kinetics.
Experimental part
Materials
PP (T30S; melt flow rate, 3.0 g/10 min) was supplied by Petro China Daqing Petrochemical Company, China. Aerosil-200 fumed SiO2 (surface area, 200±25 m2/g; primary particle size, 12 nm) was obtained from Degussa-Hüls and dried in a vacuum oven overnight at 110 °C prior to use. 3-Methacryloxypropyltrimethoxysilane (WD70) was obtained from Hubei Wuhan University Silicon New Material Co. Ltd, Hubei, China. DOPO was supplied by H&G Chemical Co. Ltd, Jiangsu, China. Toluene and triethylamine, both of analytical grades, were purchased from Beijing Chemical Factory and used without further purification.
Preparation of functionalized SiO2 nanoparticle
The silylation reaction was performed under reflux using a suspension of 5 g fumed SiO2 nanoparticle and 2.5 g WD70 in 180 mL toluene with continuous stirring under nitrogen atmosphere for 24 h at 110 °C. The product was filtered and then washed with toluene for five times (200 mL for each time). The WD70-functionalized SiO2 (SiO2–WD70) was dried in a vacuum oven overnight at 110 °C.
DOPO (2.5 g) was introduced into the suspension of SiO2–WD70 (5.0 g) in toluene (180 mL). Added dropwise into the suspension is anhydrous triethylamine (0.5 g), and the mixture was under reflux with continuous stirring under nitrogen atmosphere for 24 h at 110 °C [27]. The product was filtered and washed several times with toluene (200 mL, five times) for ensuring that any absorbed DOPO was completely removed. The DOPO-immobilized SiO2 nanoparticles (SiO2–WD70–DOPO) were dried in a vacuum oven overnight at 110 °C. The amount of surface-grafted compound was determined by TGA.
Preparation of PP/SiO2 nanocomposites
Prior to melt processing, the neat SiO2 nanoparticles and functionalized SiO2 nanoparticles were dried in a vacuum oven overnight at 110 °C. The PP/SiO2 nanocomposites were prepared by melt compounding using Haake Polylab OS RheoDrive 7 (Germany) at 180 °C with a screw speed of 50 rpm for the first 2 min then with a screw speed of 100 rpm for another 5 min. The PP/DOPO/SiO2 nanocomposites were prepared at varied weight ratio according to Table 1. The obtained nanocomposites were used to characterize the thermal property.
Table 1.
PP compounds and their compositions
| Samples | Type of additives | Content of additives (wt%) |
|---|---|---|
| Pure PP | – | – |
| PP/DOPO 0.32 wt% | DOPO | 0.32 |
| PP/DOPO 5 wt% | DOPO | 5 |
| PP/SiO2 5 wt% | SiO2 | 5 |
| PP/SiO2 10 wt% | SiO2 | 10 |
| PP/SiO2–WD70 5 wt% | SiO2–WD70 | 5 |
| PP/SiO2–WD70–DOPO 5 wt% | SiO2–WD70–DOPO | 5 |
| PP/(SiO2+DOPO) 5 wt%a | SiO2+DOPO | 5 |
PP/(SiO2+DOPO) 5 wt% means that SiO2 and DOPO were physically mixed by the weight ratio of 96.8:3.2 (WSiO2/WDOPO) before compounding with PP
Characterization
FTIR spectrometry was performed on KBr pellets using a Thermo Nicolet 6700 FTIR spectrum analyzer in the wave numbers range of 4,000 to 650 cm−1. Spectra were signal averaged over 16 scans at a resolution of 4 cm−1. UV–vis measurement was carried out on a UV–vis spectroscopy (UV-2450, Shimadzu, Japan) at a resolution of 1 nm. The absorption spectra were recorded at room temperature on a diode array spectrophotometer. The amount of surface-grafted compound was determined by TGA (Perkin-Elmer, Pyris 1) in nitrogen atmosphere from 100 to 700 °C at a heating rate of 20 °C/min. Perkin-Elmer TGA-7 was used to characterize the thermal oxidative stability of the nanocomposites under air atmosphere with airflow of 80 mL/min. The dynamic experiments were performed from 100 to 500 °C at a heating rate of 20 °C/min with about 3 mg of sample for each time. Isothermal experiments (200 °C) were carried out with an initial heating rate of 100 °C/min. A scanning electron microscope (SEM; JSM-6700F field emission SEM) was used to characterize the morphology of the nanocomposites. For SEM observation, the nanocomposite samples were submerged in liquid nitrogen for 30 min, broken to expose the cross-sections and successively sputtered with gold. Transmission electron microscopy (TEM) images were obtained by a digital camera equipped on a Hitachi H-800 instrument operated at an accelerating voltage of 100 kV. The ultra thin (50–100 nm) TEM specimens were obtained using Leica Ultracut UCT ultra microtome and a diamond knife at −100 °C. Solid 29Si CP MAS and 31P MAS-NMR studies were performed via Bruker Avanve III 400 NMR spectrometer with a magic angle spinning technique. Magic angle spinning was performed at 79.3 MHz with 5 kHz spin rate for 29Si study and at 161.58 MHz with 10 kHz spin rate for 31P study.
Results and discussion
Immobilization of DOPO on the surface of SiO2 nanoparticles
Because modification of DOPO onto SiO2 nanoparticles by using direct reaction between the silanol groups on SiO2 nanoparticles and P–H bond in DOPO is relatively difficult [26–31], bifunctional coupling agents could be used as linkers to fulfill such modification. Geminal silanols and free silanols on the surface of SiO2 allow various modifications with suitable chemical species, e.g., silanes [32, 33] particularly those silane coupling agents with functional groups (vinyl, isocyanate, epoxy, etc.) [26, 31]. Therefore, in the present work, WD70 was chosen to introduce C=C groups onto the surface of SiO2 nanoparticles. Then, DOPO was immobilized on the surface of SiO2 nanoparticles through the reaction with C=C bonds [31, 34]. The related chemical reactions are shown in Scheme 1.
Scheme 1.
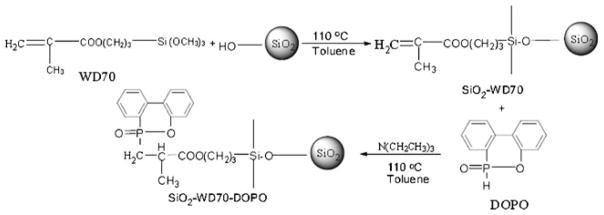
Preparation of SiO2–WD70–DOPO
FTIR and UV–vis analysis of DOPO-immobilized SiO2 nanoparticle
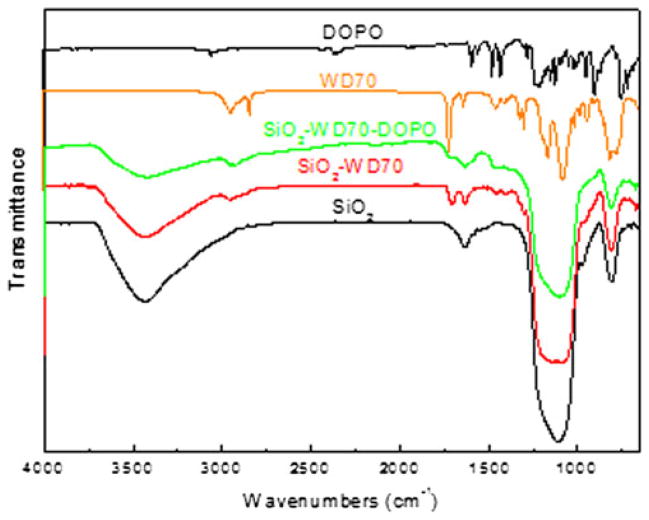
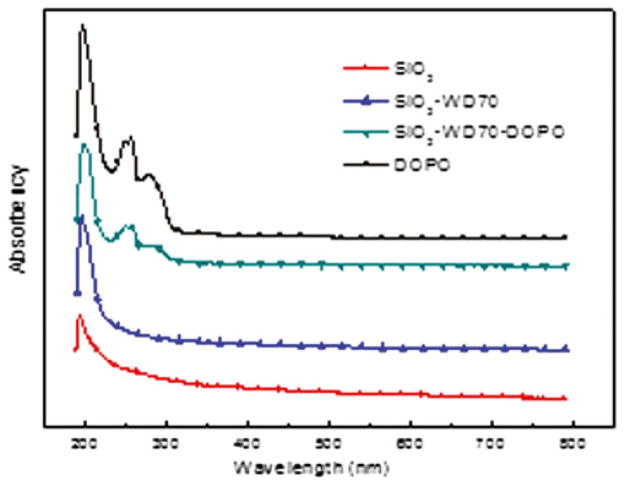
The immobilization of WD70 on the surface of SiO2 nanoparticle was characterized by the FTIR spectra. As shown in Fig. 1, for neat SiO2 nanoparticle, the broad bands around 3,700–3,200 cm−1 are assigned to the OH stretching vibration of silanol hydroxyls and adsorbed water, while the band at about 1,670–1,570 cm−1 is ascribed to OH bending of adsorbed water. For SiO2–WD70, the bands that appeared around 2,935 and 2,870 cm−1 correspond to CH2 asymmetric and symmetric stretch. The emerged bands at 1,705 and 1,635 cm−1 are attributed to the C=O and C=C groups of WD70, respectively. Clearly, WD70 was anchored on the surface of SiO2 nanoparticle. The bands at 1,705 and 1,635 cm−1 becomes weak after introducing DOPO and suggests that DOPO was immobilized on the surface of SiO2 nanoparticle through WD70, via the reaction between P–H and C=C (Scheme 1). Because of the band overlapping between DOPO and WD70 in FTIR spectra, strong signal change from FTIR spectra after immobilizing DOPO on the surface of nano-SiO2 can hardly be detected. UV–vis spectrum confirmed the immobilization of DOPO on the surface of SiO2 nanoparticle (Fig. 2). There was no absorbance for neat SiO2 and SiO2–WD70, but clear absorbance peaks between 230 and 300 nm were observed for SiO2– WD70–DOPO, similar to the pristine DOPO.
Fig. 1.
FTIR spectra for DOPO, WD70, SiO2, and functionalized SiO2
Fig. 2.
UV–vis spectra for DOPO, SiO2, SiO2-WD70, and SiO2–WD70–DOPO
Solid-state NMR analysis of DOPO-immobilized SiO2 nanoparticle
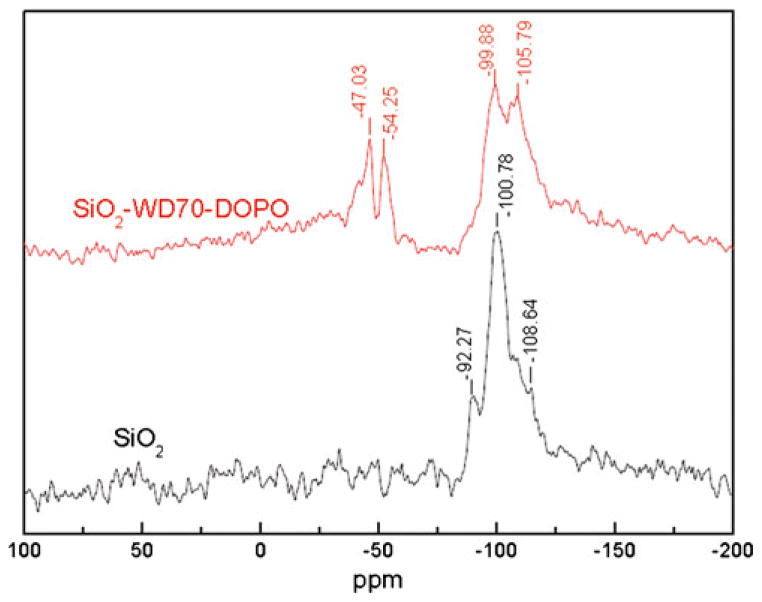
The 29Si CP MAS-NMR spectrum of the untreated SiO2 nanoparticle (Fig. 3) shows three signals at −92.27 (Q2), −100.78 (Q3), and −108.64 (Q4)ppm, which are usually assigned to geminal silanols, free silanols, and siloxane groups, respectively [32, 33]. As expected, the grafting process reduced the intensities of the signals of geminal and free silanol groups in comparison with those of the siloxane groups (Fig. 3). It was reported that the geminal silanol is more chemically active than free silanol and siloxane groups [1, 33, 35]. Thus, the peaks assigned to geminal silanes almost disappeared in Fig. 3. For SiO2–WD70–DOPO, two additional peaks were found at −47.03 and −54.25 ppm, which represented bidental and monodental structures, respectively [32]. The newly observed peaks proved that one or two methoxy groups of WD70 reacted with silanols of SiO2. It was suggested that WD70 was successfully immobilized on the surface of SiO2 nanoparticle.
Fig. 3.
29Si CP MAS-NMR spectra for SiO2 and SiO2–WD70–DOPO
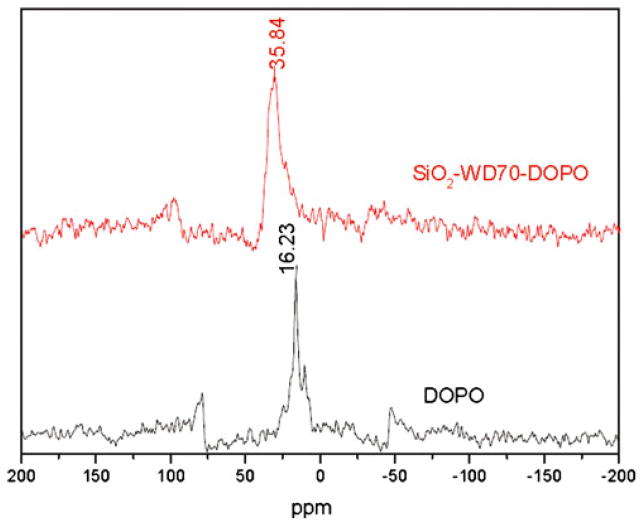
According to the literature, there is an active hydrogen atom bonded to phosphorus atom (–P(O)–H) in DOPO, which could easily react with vinyl groups [27, 29, 31, 34]. As shown in the 31P MAS-NMR spectrum of SiO2– WD70–DOPO (Fig. 4), the typical resonance peak shifts from 16.23 ppm for DOPO to 35.84 ppm for the immobilized DOPO [27, 34]. It was suggested that DOPO molecules reacted with the vinyl groups in WD70 and thus chemically anchored on surface of SiO2 nanoparticle. This result is in good agreement with FTIR and UV–vis spectra.
Fig. 4.
31P CP MAS-NMR spectra for DOPO and SiO2–WD70–DOPO
TGA analysis of DOPO-immobilized SiO2 nanoparticle
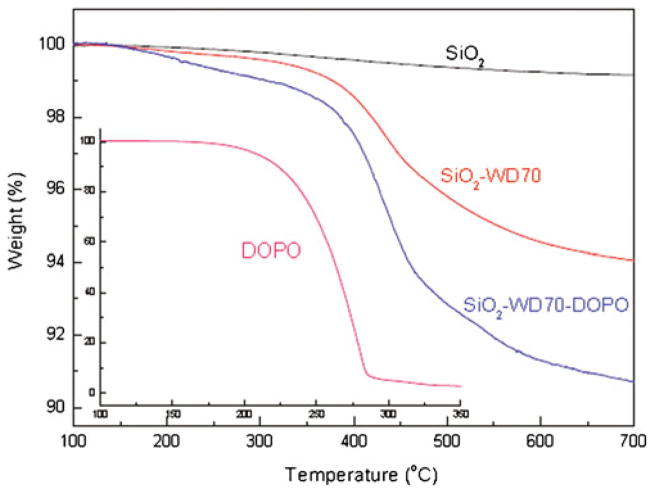
In addition to the above qualitative evidence by FTIR, UV– vis, and NMR for the immobilization of DOPO on SiO2, TGA was used to characterize the quantity of DOPO bonded on the SiO2 nanoparticles [35]. It was assumed that there was no residue left for WD70 after TGA test. As shown in Fig. 5, there was no weight loss for all the compounds at the onset temperature (100 °C). The weight percent at the end temperature (700 °C) were 99.2, 94.0, 90.5, and 0.3 % for SiO2, SiO2–WD70, SiO2–WD70–DOPO, and DOPO, respectively. It was easy to calculate the amount of grafted compound from the difference of weight loss at the onset temperature and the end temperature. Thus, the amount of WD70 grafted on the surface of silica nanoparticles was 5.2 wt% and it was 3.2 wt% for DOPO immobilized on the surface of silica nanoparticles.
Fig. 5.
TGA curves for SiO2, functionalized SiO2 and DOPO
Morphology
The phase morphology and interfacial properties could significantly influence the properties of the nanocomposites owing to the formed nanocomposites that exhibited high interfacial areas. Electron microscopes were used to characterize the dispersion of the nanofillers. As shown in Fig. 6a, there were large agglomerations of SiO2 nanoparticles in the PP/SiO2 nanocomposite because of the hydrogen bonds of adjacent SiO2 nanoparticles. After the silylation, SiO2–WD70 nanoparticles could disperse in the matrix to a better state (Fig. 6b) compared with virgin SiO2 nanoparticles (Fig. 6a). The significantly increased hydrophobicity of the nanoparticles due to the presence of WD70 was beneficial for a good interface between the nanoparticles and the matrix; even though, a completely uniform distribution of the nanoparticles cannot be achieved during the compounding process [36, 37]. There was no considerable difference of the dispersion for the SiO2–WD70–DOPO nanoparticles (Fig. 6c) and SiO2–WD70 nanoparticles (Fig. 6b). It was suggested that the DOPO-immobilized SiO2 could also disperse uniformly in the matrix.
Fig. 6.
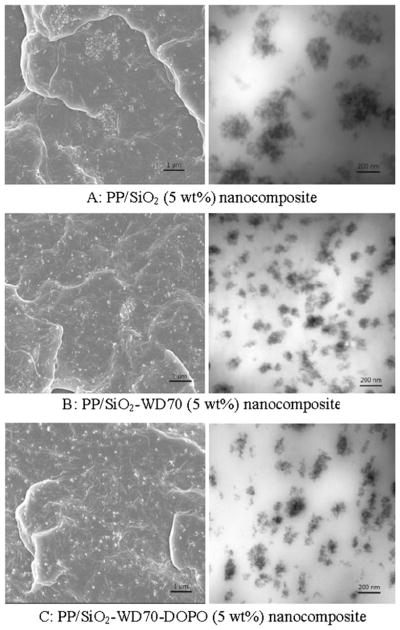
Electron microscope for PP/SiO2 nanocomposites. Left SEM, right TEM
Thermal property
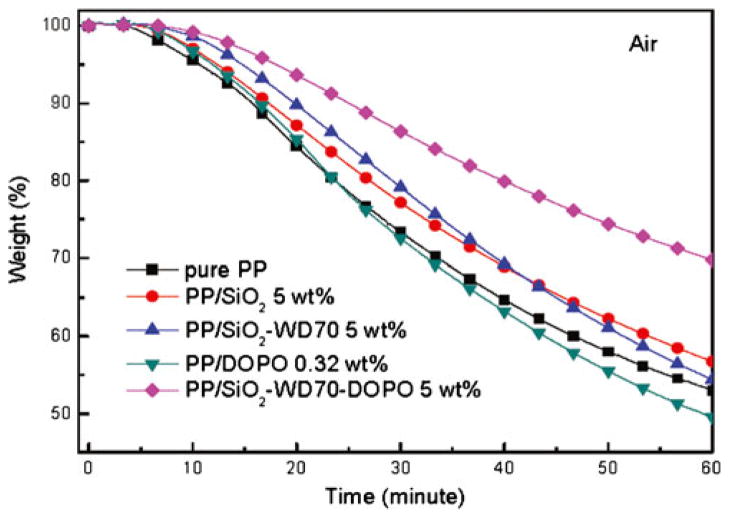
In order to investigate the influence of different SiO2 nanoparticles on the thermal oxidative property of the nanocomposites, isothermal TGA was performed to characterize the weight loss at 200 °C in air atmosphere (Fig. 7). The PP/SiO2–WD70–DOPO (5 wt%) nanocomposite volatilized (decomposed) at the slowest rate compared with that of the pure PP and other polymer nanocomposites. For example, the pure PP sample lost 30 % of weight in a time as short as 33 min, while the PP/SiO2–WD70–DOPO sample took more than 60 min for the same weight loss as the DOPO-immobilized SiO2 nanoparticles were incorporated into the PP matrix. Due to the physical barrier effect of the inorganic fillers, the isothermal stability of PP/SiO2 and PP/SiO2–WD70 nanocomposites were a little higher than that of pure PP [38]. Because of the low thermal stability of DOPO, the thermal stability of the PP/DOPO composite deteriorated. The above results indicated that DOPO-immobilized SiO2 nanoparticles could improve the thermal oxidative stability of PP.
Fig. 7.
Isothermal TGA of PP/SiO2 nanocomposites at 200 °C in air atmosphere
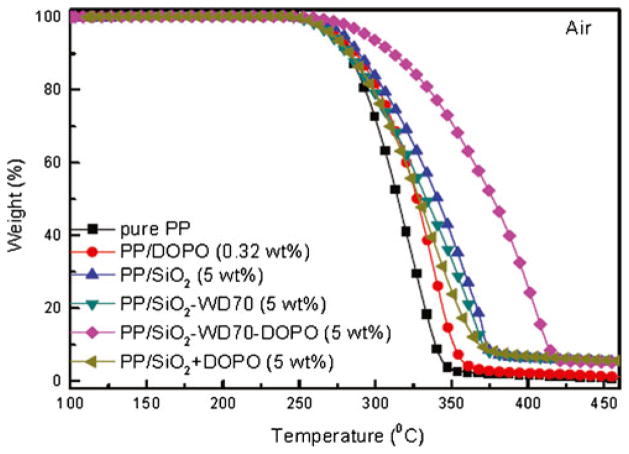
The improvement of the thermal oxidative stability was further confirmed by dynamic oxidation experiments (Fig. 8 and Table 2). It is known that C=C could induce the thermal oxidative degradation of PP [39]. Thus, the thermal oxidative stability of PP/SiO2–WD70 (5 wt%) nanocomposite was a little lower than that of PP/SiO2 (5 wt%) nanocomposite. As shown in Table 2, DOPO could improve the thermal stability, but it could not significantly improve the thermal stability even with the addition of 5 wt% [40, 41]. It was speculated that DOPO could induce cross-linking reaction and carbonization and form thermally-stable char, and therefore reduced the releasing rate of degradation volatiles. Because of the low thermal stability of DOPO, T1 % of PP/DOPO was even a little lower than that of pure PP. The decrease of the initial thermal degradation temperature was also detected for PP/(SiO2+DOPO; Table 2). For PP/(SiO2 +DOPO) nanocomposite, which was simply compounded with 0.32 wt% DOPO and 4.68 wt% SiO2, the thermal oxidative stability was slightly improved but was even lower than that of the PP/SiO2 (5 wt%) nanocomposite.
Fig. 8.
TGA curves of PP/SiO2 nanocomposites in air atmosphere
Table 2.
Typical thermal oxidative degradation data of PP/SiO2 nanocomposites
| Sample | PP 0 % | PP/DOPO 0.32 wt% | PP/DOPO 5 wt% | PP/SiO2 5 wt% | PP/SiO2 10 wt% | PP/SiO2–WD70 5 wt% | PP/SiO2–WD70–DOPO 5 wt% | PP/(SiO2+DOPO) 5 wt% |
|---|---|---|---|---|---|---|---|---|
| T1% | 260 | 260 | 257 | 261 | 263 | 258 | 272 | 256 |
| T50% | 315 | 327 | 330 | 341 | 365 | 334 | 377 | 329 |
| Tmax | 318 | 340 | 354 | 371 | 386 | 340 | 408 | 369 |
T1% is the temperature at which 1 wt% weight loss occurs and Tmax is the temperature at peak of the derivative TGA curve
When SiO2–WD70–DOPO was incorporated into the matrix, the temperature for maximum weight loss (Tmax), i.e., the temperature at peak of the derivative TGA curve increased 90 °C as compared to that of pure PP. ΔT50 % (T50 % of a sample minus T50 % of pure PP) was 62 °C for PP/SiO2–WD70–DOPO (5 wt%) nanocomposite, while that was only 26 °C for PP/SiO2 (5 wt%) nanocomposite. The thermal oxidative stability of PP/SiO2–WD70–DOPO (5 wt%) nanocomposite was even higher than that of PP/SiO2 nanocomposite containing 10 wt% of the pristine SiO2 and this behavior maintained throughout the entire degradation test. The improvement was much higher than the sum of the increment of PP/DOPO and PP/SiO2 or the PP/(SiO2+ DOPO) nanocomposites. It was suggested there was synergistic effect for the DOPO-immobilized SiO2 nanoparticles. It is known that SiO2 can improve stability due to physical effect [42, 43]. This could be explained by the fact that DOPO-immobilized SiO2 nanoparticles could induce carbonization and form thermally-stable char, and therefore to reduce releasing rate of degradation volatiles [20, 44].
Kinetics analysis
TGA results have indicated that the DOPO-immobilized SiO2 nanoparticles could significantly improve the thermal stability of PP. The kinetic analysis can provide additional information to the thermal oxidative degradation of the nanocomposites. The kinetics parameters obtained from a kinetic analysis include activation energy, frequency factor, the rate of decomposition, and in some cases, the order of the decomposition reaction [45, 46]. The objective of kinetics is often to provide a mathematical relationship in time, temperature, and conversion.
In this study, the multiple heating rate kinetics method is used to estimate the apparent activation energy by applying the Flynn–Wall–Ozawa method [45–49] specifically derived for heterogeneous chemical reaction under linear heating rates (2.5, 5, 7.5, and 10 °C/min). The derivation of the Flynn–Wall–Ozawa method from the first principle was presented in 1965 [47]. A simplified method is expressed by the equation:
where f(x) denotes the conversional functional relationship, A the pre-exponential factor, Eα the apparent activation energy, α the given extent of conversion, R the gas constant, β the heating rate, and T the absolute temperature. Assuming that the reaction rate is only a function of temperature, the above equation can be further simplified to model free expression as:
Activation energy can be calculated from the slope of the isoconversional plots of log (β) versus 1/T for a fixed degree of conversion.
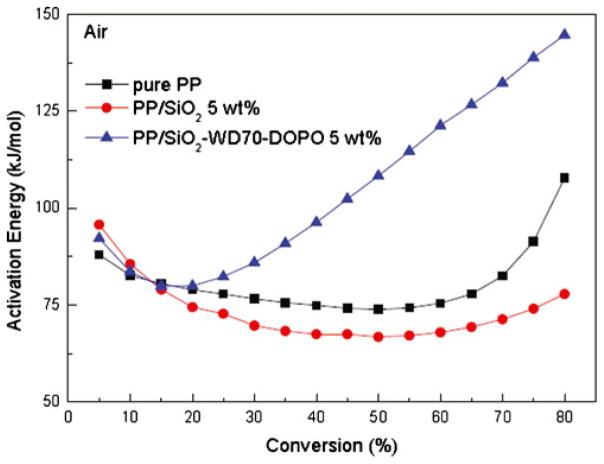
As shown in Fig. 9, the apparent activation energies were plotted as a function of α. There was little difference of the initial activation energies (when α is below 20 %) for all the three samples. It is because that at low temperature, PP undergoes the radical peroxidation chain and the additional functional SiO2 nanoparticles could not influence the process [38, 43, 50]. As the degradation proceeds, Eα of PP/SiO2 (5 wt%) nanocomposite decreased slowly and then increased tardily. It had the similar trend as that of pure PP, but showed a little lower value. It was reported that the silanol groups on the surface of SiO2 could catalyze the thermal degradation [38, 42, 43], thus the apparent activation energies were lower than that of pure PP. The degradation products could be adsorbed on the surface of the SiO2 nanoparticles [1, 42], so the apparent weight loss was slow. For the PP/SiO2–WD70–DOPO (5 wt%) nanocomposite, Eα increased linearly as the degradation proceeded, which was very different from that of PP and PP/SiO2 nanocomposite (5 wt%). The high activation energies of PP/SiO2–WD70–DOPO (5 wt%) nanocomposite might be owing to the synergistic effect of SiO2 and phosphorus on catalyzing the formation of carbonaceous char [20, 44]. Thus, the releasing rate of degradation volatiles was significantly reduced and the activation energies were improved.
Fig. 9.
Activation energy for PP and PP/SiO2 nanocomposites
Conclusions
In this paper, DOPO was successfully immobilized onto the surface of SiO2 nanoparticle. It was revealed that the novel DOPO-immobilized SiO2 nanoparticles could not only disperse uniformly in the matrix but also significantly improve the thermal oxidative stability of PP. TGA results suggested that there was 3.2 wt% DOPO immobilized onto the surface of SiO2 nanoparticle. FTIR and UV–vis spectroscopy implied that DOPO was anchored onto SiO2 nanoparticle. MAS-NMR gave direct evidence that 31P peak shifted from 16.23 ppm for DOPO to 35.84 ppm for the immobilized DOPO. The electron microscopes studies indicated that the DOPO-immobilized SiO2 nanoparticles could finely disperse in PP matrix. By incorporating the novel nanoparticles, the PP/SiO2–WD70–DOPO (5 wt%) nanocomposite exhibited 90 °C improvement at the temperature of maximum weight loss as compared to pure PP. The decomposition activation energy was estimated by Flynn–Wall–Ozawa method. Kinetic analysis suggested that DOPO-immobilized SiO2 nanoparticles could increase the activation energy linearly, which was different from the activation energy curves of PP and PP/SiO2 nanocomposite. It was suggested that DOPO-immobilized SiO2 nanoparticles could inhibit the degradation of PP and catalyze the formation of carbonaceous char on the surface. Thus, the releasing rate of degradation volatiles was significantly reduced and the activation energies were improved.
Acknowledgments
The authors thank Dr. Junfeng Xiang and Ms. Aijiao Guan for the helpful discussion about the NMR experiment. This work was financially supported by the National Natural Science Foundation of China (grant no. 21074142 and 51133009) and the National High Technology Research and Development Program of China (863 Program; no. 2009AA033601).
Contributor Information
Quanxiao Dong, Beijing National Laboratory for Molecular Science, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, People’s Republic of China.
Yanfen Ding, Beijing National Laboratory for Molecular Science, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, People’s Republic of China.
Bin Wen, Beijing National Laboratory for Molecular Science, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, People’s Republic of China.
Feng Wang, Beijing National Laboratory for Molecular Science, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, People’s Republic of China.
Huicong Dong, Beijing National Laboratory for Molecular Science, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, People’s Republic of China.
Shimin Zhang, Beijing National Laboratory for Molecular Science, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, People’s Republic of China.
Tongxin Wang, Email: twang@howard.edu, Crest Center for Nanomaterials, College of Engineering, Howard University, Washington, DC 20059, USA.
Mingshu Yang, Email: yms@iccas.ac.cn, Beijing National Laboratory for Molecular Science, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, People’s Republic of China.
References
- 1.Zou H, Wu SS, Shen J. Polymer/silica nanocomposites: preparation, characterization, properties, and applications. Chem Rev. 2008;108:3893–3957. doi: 10.1021/cr068035q. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez C, Julian B, Belleville P, Popall M. Applications of hybrid organic–inorganic nanocomposites. J Mater Chem. 2005;15:3559–3592. [Google Scholar]
- 3.Slowing II, Vivero-Escoto JL, Trewyn BG, Lin VSY. Mesoporous silica nanoparticles: structural design and applications. J Mater Chem. 2010;20:7924–7937. [Google Scholar]
- 4.Pajonk GM. Some applications of silica aerogels. Colloid Polym Sci. 2003;281:637–651. [Google Scholar]
- 5.Ciprari D, Jacob K, Tannenbaum R. Characterization of polymer nanocomposite interphase and its impact on mechanical properties. Macromolecules. 2006;39:6565–6573. [Google Scholar]
- 6.Rong MZ, Zhang MQ, Zheng YX, Zeng HM, Walter R, Friedrich K. Structure-property relationships of irradiation grafted nano-inorganic particle filled polypropylene composites. Polymer. 2001;42:167–183. [Google Scholar]
- 7.Chinthamanipeta PS, Kobukata S, Nakata H, Shipp DA. Synthesis of poly(methyl methacrylate)-silica nanocomposites using methacrylate-functionalized silica nanoparticles and RAFT polymerization. Polymer. 2008;49:5636–5642. [Google Scholar]
- 8.Yao XY, Tian XY, Xie DH, Zhang X, Zheng K, Xu J, Zhang GZ, Cui P. Interface structure of poly(ethylene terephthalate)/silica nanocomposites. Polymer. 2009;50:1251–1256. [Google Scholar]
- 9.Asuka K, Liu BP, Terano M, Nitta KH. Homogeneously dispersed poly(propylene)/SiO2 nanocomposites with unprecedented transparency. Macromol Rapid Commun. 2006;27:910–913. [Google Scholar]
- 10.Bula K, Jesionowski T, Krysztafkiewicz A, Janik J. The effect of filler surface modification and processing conditions on distribution behaviour of silica nanofillers in polyesters. Colloid Polym Sci. 2007;285:1267–1273. [Google Scholar]
- 11.Shukoor MI, Natalio F, Therese HA, Tahir MN, Ksenofontov V, Panthofer M, Eberhardt M, Theato P, Schroder HC, Muller WEG, Tremel W. Fabrication of a silica coating on magnetic gamma-Fe2O3 nanoparticles by an immobilized enzyme. Chem Mater. 2008;20:3567–3573. [Google Scholar]
- 12.Qhobosheane M, Santra S, Zhang P, Tan WH. Biochemically functionalized silica nanoparticles. Analyst. 2001;126:1274–1278. doi: 10.1039/b101489g. [DOI] [PubMed] [Google Scholar]
- 13.Jesionowski T, Krysztafkiewicz A. Influence of silane coupling agents on surface properties of precipitated silicas. Appl Surf Sci. 2001;172:18–32. [Google Scholar]
- 14.Jal PK, Patel S, Mishra B. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta. 2004;62:1005–1028. doi: 10.1016/j.talanta.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Mahmoud ME, Soliman EM. Study of the selective extraction of iron (III) by silica-immobilized 5-formyl-3-arylazo-salicylic acid derivatives. Talanta. 1997;44:1063–1071. doi: 10.1016/s0039-9140(96)02194-7. [DOI] [PubMed] [Google Scholar]
- 16.Rioux RM, Song H, Hoefelmeyer JD, Yang P, Somorjai GA. High-surface-area catalyst design: synthesis, characterization, and reaction studies of platinum nanoparticles in mesoporous SBA-15 silica. J Phys Chem B. 2005;109:2192–2202. doi: 10.1021/jp048867x. [DOI] [PubMed] [Google Scholar]
- 17.Phan NTS, Brown DH, Styring P. A facile method for catalyst immobilisation on silica: nickel-catalysed Kumada reactions in mini-continuous flow and batch reactors. Green Chem. 2004;6:526–532. [Google Scholar]
- 18.Behr M, Rosentritt M, Hagenbuch K, Faltermeier A, Handel G. Flexural strength of experimentally filled resins made of electron beam irradiated silica fillers. J Mech Behav Biomed Mater. 2009;2:61–64. doi: 10.1016/j.jmbbm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Alongi J, Ciobanu M, Malucelli G. Novel flame retardant finishing systems for cotton fabrics based on phosphorus-containing compounds and silica derived from sol-gel processes. Carbohydr Polym. 2011;85:599–608. [Google Scholar]
- 20.Brancatelli G, Colleoni C, Massafra MR, Rosace G. Effect of hybrid phosphorus-doped silica thin films produced by sol-gel method on the thermal behavior of cotton fabrics. Polym Degrad Stab. 2011;96:483–490. [Google Scholar]
- 21.Gao XW, Meng XF, Wang HT, Wen B, Ding YF, Zhang SM, Yang MS. Antioxidant behaviour of a nanosilica-immobilized antioxidant in polypropylene. Polym Degrad Stab. 2008;93:1467–1471. [Google Scholar]
- 22.Gao XW, Hu GJ, Qian ZZ, Ding YF, Zhang SM, Wang DJ, Yang MS. Immobilization of antioxidant on nanosilica and the antioxidative behavior in low density polyethylene. Polymer. 2007;48:7309–7315. [Google Scholar]
- 23.Chen J, Yang MS, Zhang SM. Immobilization of antioxidant on nanosilica and the aging resistance behavior in polypropylene. Compos Part A-Appl S. 2011;42:471–477. [Google Scholar]
- 24.Levchik SV, Weil ED. A review of recent progress in phosphorus-based flame retardants. J Fire Sci. 2006;24:345–364. [Google Scholar]
- 25.Liu YL, Wu CS, Hsu KY, Chang TC. Flame-retardant epoxy resins from o-cresol novolac epoxy cured with a phosphorus-containing aralkyl novolac. J Polym Sci Part A Polym Chem. 2002;40:2329–2339. [Google Scholar]
- 26.Semenzin D, Etemad Moghadam G, Albouy D, Diallo O, Koenig M. Dual radical/polar Pudovik reaction: application field of new activation methods. J Org Chem. 1997;62:2414–2422. doi: 10.1021/jo9622441. [DOI] [PubMed] [Google Scholar]
- 27.Perret B, Schartel B, Stoess K, Ciesielski M, Diederichs J, Doering M, Kraemer J, Altstaedt V. Novel DOPO-based flame retardants in high-performance carbon fibre epoxy composites for aviation. Eur Polym J. 2011;47:1081–1089. [Google Scholar]
- 28.Wang X, Hu Y, Song L, Yang H, Xing W, Lu H. Synthesis and characterization of a DOPO-substitued organophosphorus oligomer and its application in flame retardant epoxy resins. Prog Org Coat. 2011;71:72–82. [Google Scholar]
- 29.Zhang W, Li X, Guo X, Yang R. Mechanical and thermal properties and flame retardancy of phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS)/polycarbonate composites. Polym Degrad Stab. 2010;95:2541–2546. [Google Scholar]
- 30.Zang L, Wagner S, Ciesielski M, Mueller P, Doering M. Novel star-shaped and hyperbranched phosphorus-containing flame retardants in epoxy resins. Polym Adv Technol. 2011;22:1182–1191. [Google Scholar]
- 31.Ma BY, Lii JH, Schaefer HF, Allinger NL. Systematic comparison of experimental, quantum mechanical, and molecular mechanical bond lengths for organic molecules. J Phys Chem. 1996;100:8763–8769. [Google Scholar]
- 32.Bauer F, Ernst H, Decker U, Findeisen M, Glasel HJ, Langguth H, Hartmann E, Mehnert R, Peuker C. Preparation of scratch and abrasion resistant polymeric nanocomposites by monomer grafting onto nanoparticles, 1-FTIR and multi-nuclear NMR spectroscopy to the characterization of methacryl grafting. Macromol Chem Phys. 2000;201:2654–2659. [Google Scholar]
- 33.Derouet D, Forgeard S, Brosse JC, Emery J, Buzare JY. Application of solid-state NMR (C-13 and Si-29 CP/MAS NMR) spectroscopy to the characterization of alkenyltrialkoxysilane and trialkoxysilyl-terminated polyisoprene grafting onto silica micro-particles. J Polym Sci Part A Polym Chem. 1998;36:437–453. [Google Scholar]
- 34.Zhong HF, Wu D, Wei P, Jiang PK, Li Q, Hao JW. Synthesis, characteristic of a novel additive-type flame retardant containing silicon and its application in PC/ABS alloy. J Mater Sci. 2007;42:10106–10112. [Google Scholar]
- 35.Liu CH, Pan CY. Grafting polystyrene onto silica nanoparticles via RAFT polymerization. Polymer. 2007;48:3679–3685. [Google Scholar]
- 36.Jana SC, Jain S. Dispersion of nanofillers in high performance polymers using reactive solvents as processing aids. Polymer. 2001;42:6897–6905. [Google Scholar]
- 37.Wang Y, Wu X, Yang W, Zhai Y, Xie B, Yang M. Aggregate of nanoparticles: rheological and mechanical properties. Nanoscal Res Lett. 2011;6:114. doi: 10.1186/1556-276X-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanetti M, Camino G, Reichert P, Mulhaupt R. Thermal behaviour of poly(propylene) layered silicate nanocomposites. Macromol Rapid Commun. 2001;22:176–180. [Google Scholar]
- 39.Hinsken H, Moss S, Pauquet JR, Zweifel H. Degradation of polyolefins during melt processing. Polym Degrad Stab. 1991;34:279–293. [Google Scholar]
- 40.Wang X, Hu Y, Song L, Xing WY, Lu HD, Lv P, Jie GX. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer. 2010;51:2435–2445. [Google Scholar]
- 41.Qian LJ, Zhi JG, Tong B, Shi JB, Yang F, Dong YP. Synthesis and characterization of main-chain liquid crystalline copolyesters containing phosphaphenanthrene side-groups. Polymer. 2009;50:4813–4820. [Google Scholar]
- 42.Palza H, Vergara R, Zapata P. Improving the thermal behavior of poly(propylene) by addition of spherical silica nanoparticles. Macromol Mater Eng. 2010;295:899–905. [Google Scholar]
- 43.Gianelli W, Ferrara G, Camino G, Pellegatti G, Rosenthal J, Trombini RC. Effect of matrix features on polypropylene layered silicate nanocomposites. Polymer. 2005;46:7037–7046. [Google Scholar]
- 44.Liu YL, Wei WL, Hsu KY, Ho WH. Thermal stability of epoxy-silica hybrid materials by thermogravimetric analysis. Thermochim Acta. 2004;412:139–147. [Google Scholar]
- 45.Menczel JD, Prime RB. Thermal analysis of polymers—fundamentals and applications. Wiley; New Jersey: 2009. [Google Scholar]
- 46.Chigwada G, Kandare E, Wang DY, Majoni S, Mlambo D, Wilkie CA, Hossenlopp JM. Therma stabiliy and degradation kinetics of polystyrene/organically-modified mont-morillonite nanocomposites. J Nanosci Nanotech. 2008;8:1927–1936. [PubMed] [Google Scholar]
- 47.Ozawa T. A new method of analyzing thermogravimetric data. B Chem Soc Jpn. 1965;38:1881–1886. [Google Scholar]
- 48.Flynn JH, Wall LA. A quick direct method for determination of activation energy from thermogravimetric data. J Polym Sci B Polym Lett. 1966;4:323–328. [Google Scholar]
- 49.Flynn JH. The isoconversional method for determination of energy of activation at constant heating rates—corrections for the Doyle approximation. J Therm Anal. 1983;27:95–102. [Google Scholar]
- 50.Benson SW, Nangia PS. Some unresolved problems in oxidation and combustion. Acc Chem Res. 1979;12:223–228. [Google Scholar]