Abstract
Background and Aim. Malignant hilar strictures are a clinical challenge because of the current therapeutic approach and the poor prognosis. In recent years, self-expandable metallic stents have proven more effective than plastic stents for palliation of malignant hilar strictures, with the bilateral stent-in-stent technique registering a high success rate. We report our experience with Y-shaped endoscopic self-expandable metallic stents placement for treatment of advanced malignant hilar strictures. Methods. From April 2009 to August 2012, we prospectively collected data on patients treated with Y-shaped SEMS placement for advanced malignant hilar carcinoma. Data on technical success, clinical success, and complications were collected. Results. Twenty patients (9 males) were treated (mean age 64.2 ± 15.3 years). The grade of malignant hilar strictures according to the Bismuth classification was II in 5 patients (25%), IIIa in 1 (5%), and IV in 14 (70%). The mean bilirubin level was 14.7 ± 4.9 mg/dL. Technical success was achieved in all patients, with a significant reduction in bilirubin levels (2.9 ± 1.7 mg/dL). One patient experienced cholangitis as early complication, while in 2 patients stent ingrowth was observed. No stents migration was recorded. There was no procedure-related mortality. At the end of the follow-up (7.1 ± 3.1 months), 13 of the 20 patients (65%) had died. Conclusions. Our experience confirms endoscopic bilateral self-expandable metallic stents placement with stent-in-stent technique (Y-shaped configuration) as a feasible, effective, and safe procedure for palliation of unresectable malignant hilar strictures.
1. Introduction
Malignant hilar strictures (MHS), type ≥2 according to the Bismuth-Corlette classification of cancers of the human biliary tract [1], are a clinical challenge because of the current therapeutic approach (surgical or nonsurgical) [2] and the poor prognosis associated with this form of cancer. Cholangiocarcinoma is the leading cause of all MHS which require, in inoperable patients, a conservative approach only with stent placement through percutaneous transhepatic cholangiography or endoscopic retrograde cholangiopancreatography (ERCP) [3]. Despite the fact that multiple plastic stenting is a feasible and valid treatment, self-expandable metallic stents (SEMS) have proven to be more effective than plastic stents for hilar tumor palliation [4], with bilateral stent-in-stent placement registering a high success rate [5]. In the current study, we report our experience on feasibility, efficacy, and safety of Y-shaped endoscopic SEMS placement in advanced malignant hilar carcinoma.
2. Methods
From April 2009 to August 2012, we prospectively collected data on patients treated with Y-shaped SEMS placement for advanced malignant hilar carcinoma. All patients were diagnosed as having MHS by computer-assisted tomography or magnetic resonance imaging. Histologic and cytologic confirmation of malignancy was made for all the patients. Indication for stent placement was an increase in bilirubin levels with evidence of intrahepatic bile ducts dilatation. Patients were treated within 48 hours from the admittance. All patients were not treated previously. Data were collected in an electronic database and subsequently exported to the statistical software for final analysis.
All patients were hospitalized and complete full blood count, chemistry, and coagulation parameters were obtained. Stent placement was discussed with the surgeon and both tumor characteristics/location and overall clinical condition were taken into account. An informed consent was always obtained before the procedure. All endoscopic procedures were performed with a therapeutic channel video duodenoscope with the patient under deep sedation with propofol. SEMS (Niti-S Biliary Y stent, Taewoong, Seoul, Republic of Korea) were placed during ERCP and according to manufacturer's instructions, by an experienced endoscopist. The length and the size of SEMS were the same in all treated patients. After common bile duct cannulation, cholangiography, and subsequent sphincterotomy, a guidewire was placed, under fluoroscopic guidance, across the left hepatic duct. Evaluation of the severity of the stricture was made with contrast injection. The first uncovered SEMS, with a wide-open central mesh were placed across the hilar stricture. If needed, balloon dilation was performed before stent placement. The guidewire was then withdrawn slowly and, with the aid of a 5.5 French catheter, inserted under fluoroscopic guidance into the wide-open central mesh of the first stent, identified by radiopaque markers. The second uncovered SEMS were placed through the central crossed mesh of the primary stent (Y-shaped configuration) to drain the right hepatic duct (Figure 1).
Figure 1.
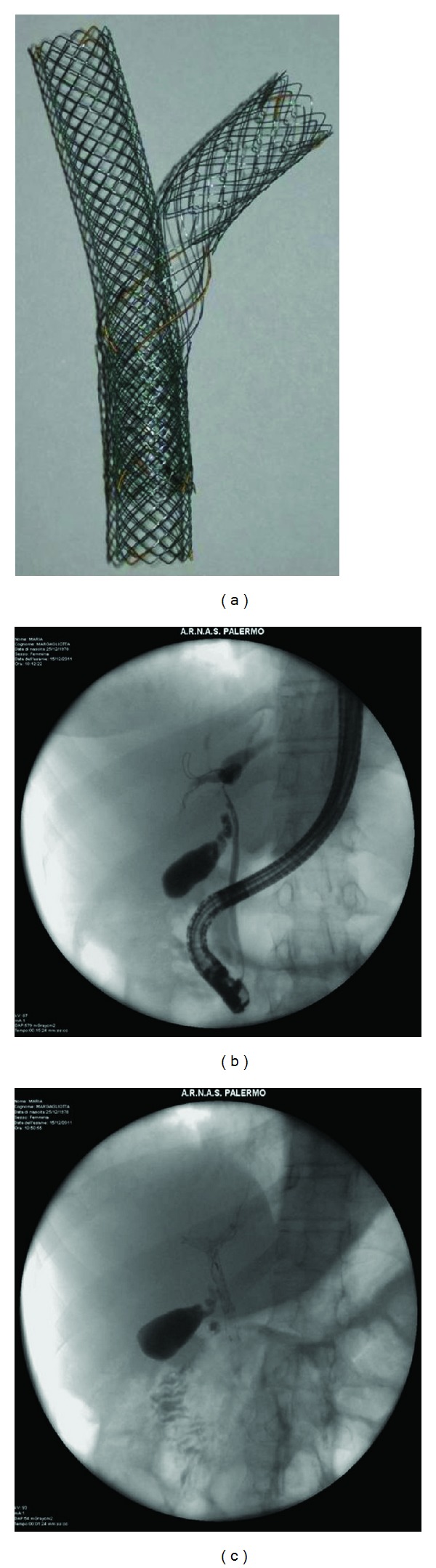
(a) Niti-S stent assembled (Y-shaped configuration). (b-c) Images from a patient with hilar cholangiocarcinoma before (b) and after (c) stent placement.
All patients, data on technical success, clinical success, and complications were collected. All patients were followed up in the outpatient clinic or by phone until death.
Technical success was defined as successful bilateral SEMS placement across the stricture, confirmed by radiological markers at fluoroscopy, with outflow of contrast medium and/or bile through the stents. Clinical success was defined as reduction of bilirubin levels of at least 75% of the pretreatment value within the first month. Complications were defined as any event related to SEMS placement (included occlusion). Complications were defined as early if complications occurred within 30 days and late if occurring after 30 days. We also considered any bleeding due to endoscopic sphincterotomy, according to the Cotton criteria [6], as a complication. Stent occlusion was defined as recurrence of jaundice or significant increase in bilirubin level with concomitant US or CT evidence of intrahepatic bile duct dilatation that required an endoscopic, percutaneous, or surgical procedure. Stent patency was defined as the period of time between insertion and stent occlusion. Each death was investigated as to whether it was related to the SEMS placement or to the natural history of the underlying malignancy.
Data were analyzed using the software package SPSS 15 (SPSS Inc., Chicago, IL, USA). Continuous variables were summarized as mean (±standard deviation [SD]) or median (range) according to their distribution. Categorical variables were summarized as frequency and percentage.
3. Results
We enrolled 20 patients, 9 males (45%) and 11 female (55%), with a mean age of 64.2 ± 15.3 years. The causes of hilar biliary obstruction were cholangiocarcinoma in 10 patients (50%), metastatic colon cancer in 5 (25%), metastatic pancreatic cancer in 3 (15%), and hepatocarcinoma in 2 (10%). The types of MHS according to the Bismuth classification were II in 5 patients (25%), IIIa in 1 patient (5%), and IV in 14 patients (70%). The mean bilirubin level was 14.7 ± 4.9 mg/dL. The diagnosis of malignancy was confirmed with tissue samples (brushing or forceps biopsy). Technical success was achieved in all patients. Eleven of the 20 patients (55%) were treated with balloon dilation before stent placement. After the procedures, a significant reduction in bilirubin levels was observed in all treated patients, with a mean bilirubin level at discharge of 2.6 ± 1.5 mg/dL. No patients received chemotherapy after SEMS placement. The median stent patency was 7 months (range 3–13), while mean survival time was 7.1 ± 3.1 months. No differences of SEMS patency were observed among the II, III, and IV type of MHS. At the end of the follow-up, 13 of the 20 patients (65%) had died of tumor progression.
4. Adverse Events
One patient (5%) experienced cholangitis as an early complication, which resolved with medical therapy. Among the late complications, 2 patients (10%) were treated with plastic stent placement (10 French) through the metallic stent because of SEMS ingrowths at 3 and 10 months, respectively. The “Y-shape” did not cause trouble to insert the plastic stents but the stents were placed on a wire guide previously inserted in the occluded SEMS. There was no procedure-related mortality.
5. Discussion
Endoscopic insertion of plastic endoprosthesis has become widespread in patients with malignant proximal biliary obstruction, limiting surgical intervention to a minority of selected patients in whom the tumor seems to be resectable [7]. Unfortunately, despite technological improvements and long-term antibiotic prophylaxis [8, 9], blockage of plastic stents cannot be prevented indefinitely.
The diffusion of SEMS has modified the palliative approach in unresectable patients. A recent randomized controlled trial [10] on SEMS versus plastic stent placement showed a higher 6-month patency rate for SEMS (81% versus 20%; P = 0.0012), with no significant difference in terms of overall survival time. In addition, the mean number of reinterventions because of stent failure was lower in the SEMS group (0.63 versus 1.80 times/patient; P = 0.0008). The total cost of the treatment was significantly lower in the SEMS group than in the plastic-stent group (P = 0.022).
Drainage of obstructed ductal systems has been strongly advocated as allowing for a significant reduction in morbidity and mortality because of better bile flow [11]. Though technically demanding, bilateral placement of two parallel metal expandable endoprosthesis has proven to be a safe and feasible procedure, with a high success rate and little need for further biliary reintervention in patients with unresectable hilar cholangiocarcinoma. Cheng et al. [12] reported, in 36 patients with Bismuth type I/II-III-IV lesions, an insertion success rate of 97%, with only 14% of complications at 30 days. Furthermore, mean stent patency was 169 days, with a rate of stent occlusion at the end of the follow-up of 31%. In 2009, Naitoh et al. confirmed the feasibility of endoscopic bilateral SEMS placement (90% insertion rate success), also showing that this approach was more effective than unilateral drainage in terms of cumulative stent patency (488 versus 210 days, P = 0.009), especially in cases of cholangiocarcinoma, with the same risk of early/late complications and cumulative survival time [13].
In order to improve the feasibility of bilateral SEMS placement, and to avoid the parallel placement of two stents, Silverman and Slivka [14] presented a new technique, in 1996, in which a stent (Wallstent, Boston Scientific, Natick, MA, USA) was placed through the fenestration of SEMS, obtained with 7 French Soehendra stent extractor, previously placed in the right intrahepatic bile duct. In 2007, Lee et al. [15] carried out a pilot study on a newly designed Y metal stent using a biliary stent with wide-open central mesh first (Niti-S Biliary Y stent; Taewoong, Seoul, Republic of Korea), followed by a second SEMS placement (Niti-S or Niti-D stent, resp.; Taewoong) in the contralateral hepatic duct through the central open mesh of the other stent. The authors reported technical success in 8 of the 10 patients (80%), with a functional success rate of 100% among patients in whom bilateral stents were successfully placed by endoscopy. The early complication rate was 0%, with an occlusion rate of 25% and a median of stent patency period of 217 days.
Since then, several studies have reported similar experiences in larger number of patients treated with the stent-in-stent technique [16–24] showing technical success rate between 85 and 100% and functional success rate between 95 and 100% (Table 1). As reported by Naitoh et al. [23], stent-in-stent technique is to be preferred instead of side-by-side deployment in terms of complications (P = 0.016) despite the fact that there were no significant intergroup differences in technical and functional success between the two technique.
Table 1.
Studies on bilateral self expandable metallic stent placement in malignant hilar strictures.
| Study | Number of patients | Type of SEMS | Technical success | Functional success | Tumor ingrowth or overgrowth |
Complications (early/late) |
SEMS patency |
|---|---|---|---|---|---|---|---|
| Lee et al. (2007) [15] | 10 | Niti-S | 80% | 100% | 25% | 0%/0% | 217 days |
| Park et al. (2009) [16] | 35 | Bonastent | 94.3% | 100% | 0% | 0%/0% | 150 days |
| Kim et al. (2009) [17] | 34 | Niti-S | 85.3% | 100% | 31% | 10.3%/37.9% | 186 days |
| Chahal and Baron (2010) [18] | 21 | Flexxus | 100% | — | 33.3% | — | 189 days |
| Kogure et al. (2011) [19] | 5 | Niti-S LGD* | 100% | — | 40% | 20%/— | 202 days |
| Kanno et al. (2011) [20] | 20 | Niti-S | 100% | 95% | 30% | 5%/0% | 250 days |
| Hwang et al. (2011) [22] | 30 | Niti-S | 86.7% | 100% | 38.5% | 10%/0% | 176 days |
| Naitoh et al. (2012) [23] | 24 | Niti-S | 100% | 100% | 42% | 4%/8% | 104 days |
| Kim et al. (2013) [24] | 66 | Niti-S | 87.9% | 100% | 34.2% | 12.1%/55.2% | 152 days |
| Current study | 20 | Niti-S | 100% | 100% | 10% | 5%/10% | 210 days |
*Large cell D-type.
An interesting historical control study compared 20 patients with unresectable malignant hilar biliary obstruction who had undergone endoscopic bilateral Y-configured biliary drainage with SEMS placement to 37 patients who had undergone bilateral drainage with plastic stents (control group) [20]. At the end of the study, the technical success rate in the SEMS group was 100% (lack of data from control group). The success rate of biliary decompression was 95% versus 89% (P = 0.65). In the follow-up period, the incidence of stent occlusion was significantly lower in the SEMS group than in the other group (30% versus 62%, P = 0.028), with a mean stent patency of 250 days versus 115 days (P = 0.0061).
A recent large retrospective study by Kim et al. [24], on placement of the newly designed Y-stent with a slimmer open-cell second stent, showed a successful placement and functional success rate of 87% and 97%, respectively.
Although, as reported above, Y-shaped SEMS are effective and safe in MHS treatment, some possible problems should be discussed: (1) insufficient opening of the central portion of the first stent which can be solved with balloon dilation, (2) inaccurate release of the central open mesh at the hilar bifurcation which can be improved by closing and repositioning the stent, and (3) stent occlusion which can be treated with balloon extraction (in case of sludge or stones), with biliary drainage through percutaneous transhepatic cholangiography or with new plastic or metallic stent placement, or.
In our study, we had a technical and clinical success rate of 100% using 2 nitinol metallic stents placed with the stent-in-stent technique to obtain the characteristic Y-shaped configuration. A significant biliary drainage was achieved also in all patients with Bismuth IV. The rate of observed complications was low, and this data is in agreement with data from a recent review by Kogure et al. [21] in which no differences in complications were reported between the different types of SEMS.
In conclusion, endoscopic Y-shaped bilateral stent-in-stent SEMS placement is safe and effective for the palliation of unresectable MHS. This is because the technique more closely resembles the physiological bilateral drainage state than does unilateral drainage. These results should be confirmed by larger prospective series and randomized controlled trials so this technique might gain consensus and become a standard of care.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
R. Di Mitri and F. Mocciaro are responsible for conception and drafting the paper; F. Mocciaro performed the statistical analysis.
References
- 1.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. The New England Journal of Medicine. 1999;341(18):1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 2.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Annals of Surgery. 1992;215(1):31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumonceau J-M, Tringali A, Blero D, et al. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44(3):277–298. doi: 10.1055/s-0031-1291633. [DOI] [PubMed] [Google Scholar]
- 4.Perdue DG, Freeman ML, DiSario JA, et al. Plastic versus self-expanding metallic stents for malignant hilar biliary obstruction: a prospective multicenter observational cohort study. Journal of Clinical Gastroenterology. 2008;42(9):1040–1046. doi: 10.1097/MCG.0b013e31815853e0. [DOI] [PubMed] [Google Scholar]
- 5.Park DH, Lee SS, Moon JH, et al. Newly designed stent for endoscopic bilateral stent-in-stent placement of metallic stents in patients with malignant hilar biliary strictures: multicenter prospective feasibility study (with videos) Gastrointestinal Endoscopy. 2009;69(7):1357–1360. doi: 10.1016/j.gie.2008.12.250. [DOI] [PubMed] [Google Scholar]
- 6.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointestinal Endoscopy. 1991;37(3):383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 7.Polydorou AA, Cairns SR, Dowsett JF, et al. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32(6):685–689. doi: 10.1136/gut.32.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby ED, Leung JW. Prevention of biliary stent clogging: a clinical review. The American Journal of Gastroenterology. 1996;91(7):1301–1308. [PubMed] [Google Scholar]
- 9.Donelli G, Guaglianone E, di Rosa R, Fiocca F, Basoli A. Plastic biliary stent occlusion: factors involved and possible preventive approaches. Clinical Medicine & Research. 2007;5(1):53–60. doi: 10.3121/cmr.2007.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukai T, Yasuda I, Nakashima M, et al. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. Journal of Hepato-Biliary-Pancreatic Sciences. 2013;20(2):214–222. doi: 10.1007/s00534-012-0508-8. [DOI] [PubMed] [Google Scholar]
- 11.Dumas R, Demuth N, Buckley M, et al. Endoscopic bilateral metal stent placement for malignant hilar stenoses: identification of optimal technique. Gastrointestinal Endoscopy. 2000;51(3):334–338. doi: 10.1016/s0016-5107(00)70364-6. [DOI] [PubMed] [Google Scholar]
- 12.Cheng JLS, Bruno MJ, Bergman JJ, Rauws EA, Tytgat GN, Huibregtse K. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: efficacy of self-expandable metallic Wallstents. Gastrointestinal Endoscopy. 2002;56(1):33–39. doi: 10.1067/mge.2002.125364. [DOI] [PubMed] [Google Scholar]
- 13.Naitoh I, Ohara H, Nakazawa T, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. Journal of Gastroenterology and Hepatology. 2009;24(4):552–557. doi: 10.1111/j.1440-1746.2008.05750.x. [DOI] [PubMed] [Google Scholar]
- 14.Silverman W, Silvka A. New technique for bilateral metal mesh stent insertion to treat hilar cholangiocarcinoma. Gastrointestinal Endoscopy. 1996;43(1):61–63. doi: 10.1016/s0016-5107(96)70263-8. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Kang DH, Kim JY, et al. Endoscopic bilateral metal stent placement for advanced hilar cholangiocarcinoma: a pilot study of a newly designed Y stent. Gastrointestinal Endoscopy. 2007;66(2):364–369. doi: 10.1016/j.gie.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 16.Park DH, Lee SS, Moon JH, et al. Newly designed stent for endoscopic bilateral stent-in-stent placement of metallic stents in patients with malignant hilar biliary strictures: multicenter prospective feasibility study (with videos) Gastrointestinal Endoscopy. 2009;69(7):1357–1360. doi: 10.1016/j.gie.2008.12.250. [DOI] [PubMed] [Google Scholar]
- 17.Kim JY, Kang DH, Kim HW, et al. Usefulness of slimmer and open-cell-design stents for endoscopic bilateral stenting and endoscopic revision in patients with hilar cholangiocarcinoma (with video) Gastrointestinal Endoscopy. 2009;70(6):1109–1115. doi: 10.1016/j.gie.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Chahal P, Baron TH. Expandable metal stents for endoscopic bilateral stent-within-stent placement for malignant hilar biliary obstruction. Gastrointestinal Endoscopy. 2010;71(1):195–199. doi: 10.1016/j.gie.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Kogure H, Isayama H, Nakai Y, et al. Newly designed large cell Niti-S stent for malignant hilar biliary obstruction: a pilot study. Surgical Endoscopy. 2011;25(2):463–467. doi: 10.1007/s00464-010-1194-8. [DOI] [PubMed] [Google Scholar]
- 20.Kanno Y, Ito K, Fujita N, et al. Single-session endoscopic bilateral Y-configured placement of metal stents for hilar malignant biliary obstruction. Digestive Endoscopy. 2011;23(1):91–96. doi: 10.1111/j.1443-1661.2010.01048.x. [DOI] [PubMed] [Google Scholar]
- 21.Kogure H, Isayama H, Kawakubo K, et al. Endoscopic bilateral metallic stenting for malignant hilar obstruction using newly designed stents. Journal of Hepato-Biliary-Pancreatic Sciences. 2011;18(5):653–657. doi: 10.1007/s00534-011-0407-4. [DOI] [PubMed] [Google Scholar]
- 22.Hwang JC, Kim JH, Lim SG, Kim SS, Yoo BM, Cho SW. Y-shaped endoscopic bilateral metal stent placement for malignant hilar biliary obstruction: prospective long-term study. Scandinavian Journal of Gastroenterology. 2011;46(3):326–332. doi: 10.3109/00365521.2010.536253. [DOI] [PubMed] [Google Scholar]
- 23.Naitoh I, Hayashi K, Nakazawa T, et al. Side-by-side versus stent-in-stent deployment in bilateral endoscopic metal stenting for malignant hilar biliary obstruction. Digestive Diseases and Sciences. 2012;57(12):3279–3285. doi: 10.1007/s10620-012-2270-9. [DOI] [PubMed] [Google Scholar]
- 24.Kim DU, Kang DH, Kim GH, et al. Bilateral biliary drainage for malignant hilar obstruction using the “stent-in-stent” method with a Y-stent: efficacy and complications. European Journal of Gastroenterology & Hepatology. 2013;25(1):99–106. doi: 10.1097/MEG.0b013e3283590a2a. [DOI] [PubMed] [Google Scholar]


