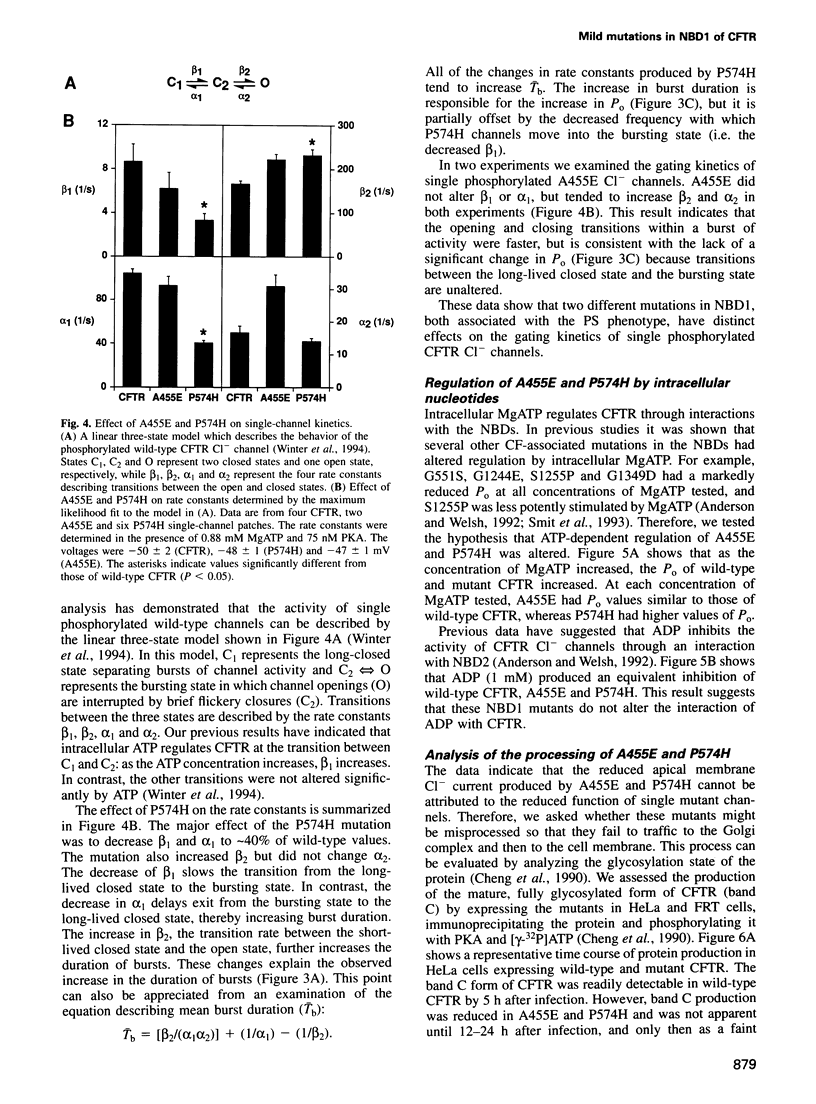
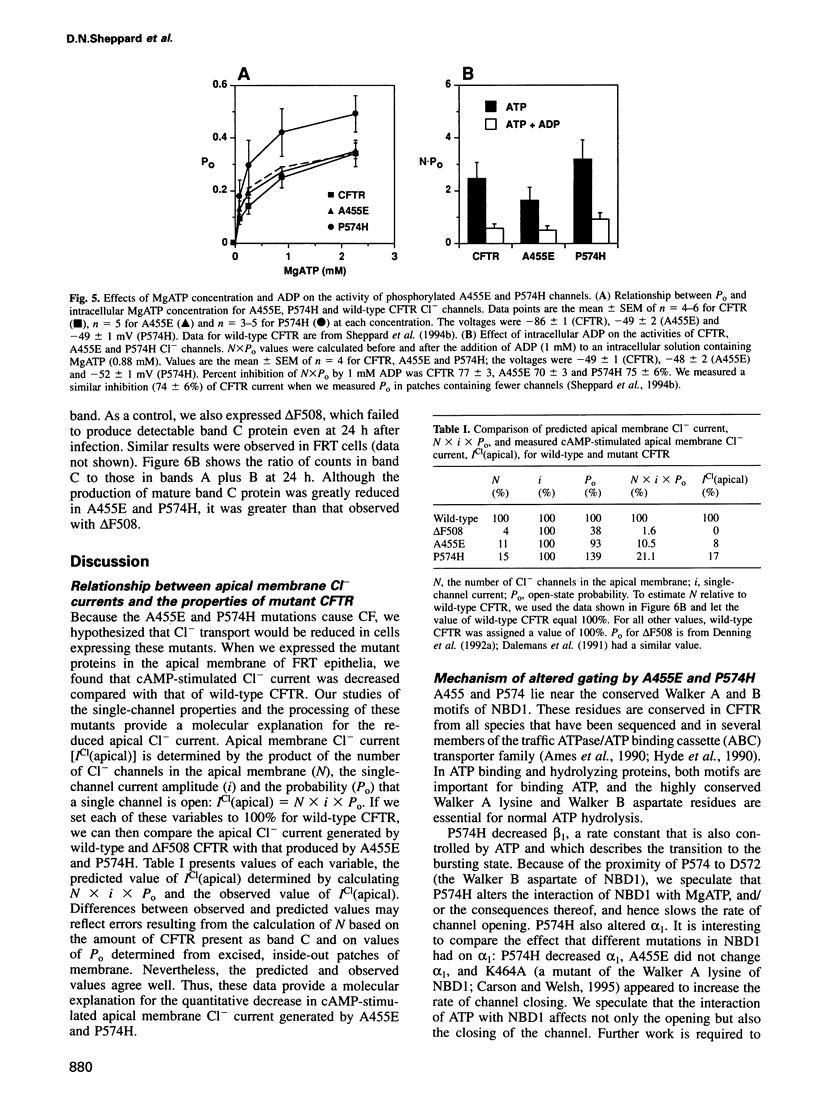
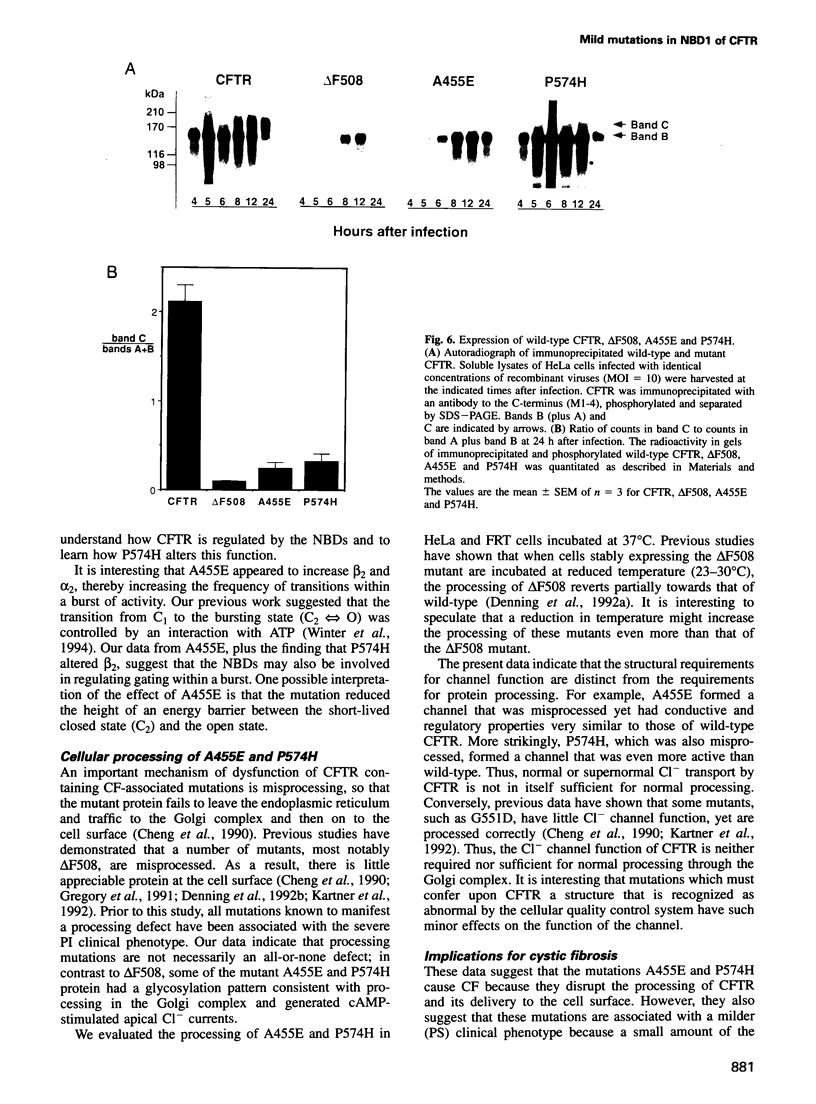
Abstract
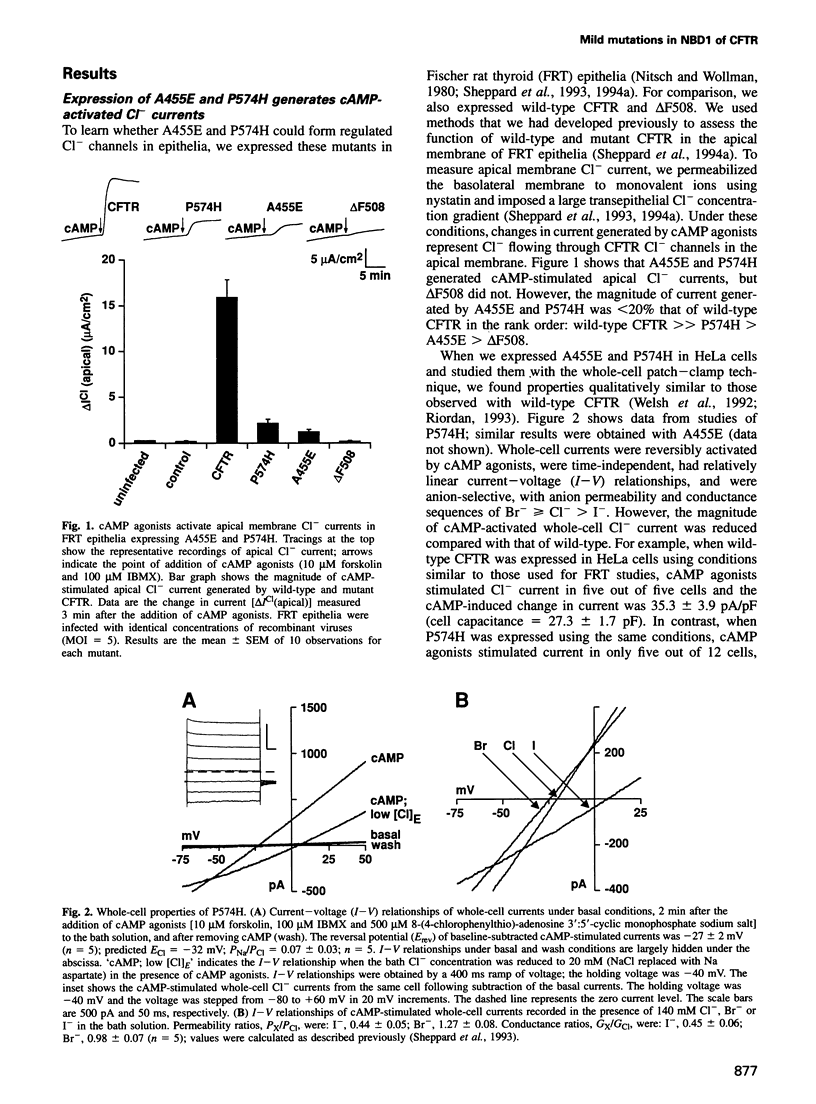
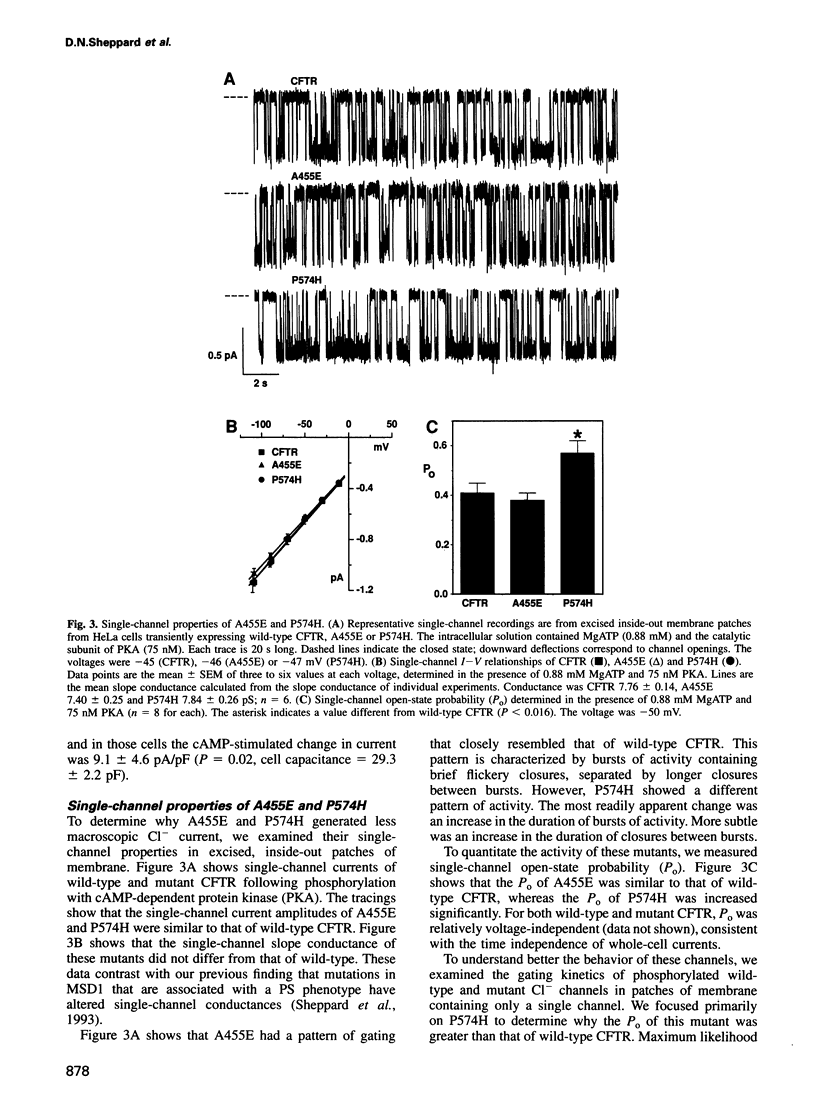
Variability in the severity of cystic fibrosis (CF) is in part due to specific mutations in the CF transmembrane conductance regulator (CFTR) gene. To understand better how mutations in CFTR disrupt Cl- channel function and to learn about the relationship between genotype and phenotype, we studied two CF mutants, A455E and P574H, that are associated with pancreatic sufficiency. A455E and P574H are located close to conserved ATP binding motifs in CFTR. Both mutants generated cAMP-stimulated apical membrane Cl- currents in heterologous epithelial cells, but current magnitudes were reduced compared with wild-type. Patch-clamp analysis revealed that both mutants had normal conductive properties and regulation by phosphorylation and nucleotides. These mutants had normal or increased Cl- channel activity: A455E had an open-state probability (Po) similar to wild-type, and P574H had an increased Po because bursts of activity were prolonged. However, both mutants produced less mature glycosylated protein, although levels were greater than observed with the delta F508 mutant. These changes in channel activity and processing provide a quantitative explanation for the reduced apical Cl- current. These data also dissociate structural requirements for channel function from features that determine processing. Finally, the results suggest that the residual function associated with these two mutants is sufficient to confer a milder clinical phenotype and infer approaches to developing treatments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Mimura C. S., Shyamala V. Bacterial periplasmic permeases belong to a family of transport proteins operating from Escherichia coli to human: Traffic ATPases. FEMS Microbiol Rev. 1990 Aug;6(4):429–446. doi: 10.1111/j.1574-6968.1990.tb04110.x. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Berger H. A., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991 Nov 15;67(4):775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 1992 Sep 18;257(5077):1701–1704. doi: 10.1126/science.1382316. [DOI] [PubMed] [Google Scholar]
- Carson M. R., Travis S. M., Welsh M. J. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J Biol Chem. 1995 Jan 27;270(4):1711–1717. doi: 10.1074/jbc.270.4.1711. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Collins F. S. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992 May 8;256(5058):774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., Crystal R. G., Pavirani A., Lecocq J. P., Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991 Dec 19;354(6354):526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992 Aug 27;358(6389):761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Denning G. M., Ostedgaard L. S., Welsh M. J. Abnormal localization of cystic fibrosis transmembrane conductance regulator in primary cultures of cystic fibrosis airway epithelia. J Cell Biol. 1992 Aug;118(3):551–559. doi: 10.1083/jcb.118.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Fuerst T. R., Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5' sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan K. H., Heijerman H. G., Bakker W. Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med. 1994 Mar 24;330(12):865–867. [PubMed] [Google Scholar]
- Gregory R. J., Cheng S. H., Rich D. P., Marshall J., Paul S., Hehir K., Ostedgaard L., Klinger K. W., Welsh M. J., Smith A. E. Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature. 1990 Sep 27;347(6291):382–386. doi: 10.1038/347382a0. [DOI] [PubMed] [Google Scholar]
- Gregory R. J., Rich D. P., Cheng S. H., Souza D. W., Paul S., Manavalan P., Anderson M. P., Welsh M. J., Smith A. E. Maturation and function of cystic fibrosis transmembrane conductance regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2. Mol Cell Biol. 1991 Aug;11(8):3886–3893. doi: 10.1128/mcb.11.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Kartner N., Augustinas O., Jensen T. J., Naismith A. L., Riordan J. R. Mislocalization of delta F508 CFTR in cystic fibrosis sweat gland. Nat Genet. 1992 Aug;1(5):321–327. doi: 10.1038/ng0892-321. [DOI] [PubMed] [Google Scholar]
- Kerem B. S., Zielenski J., Markiewicz D., Bozon D., Gazit E., Yahav J., Kennedy D., Riordan J. R., Collins F. S., Rommens J. M. Identification of mutations in regions corresponding to the two putative nucleotide (ATP)-binding folds of the cystic fibrosis gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8447–8451. doi: 10.1073/pnas.87.21.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristidis P., Bozon D., Corey M., Markiewicz D., Rommens J., Tsui L. C., Durie P. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am J Hum Genet. 1992 Jun;50(6):1178–1184. [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs G. L., Chang X. B., Bear C., Kartner N., Mohamed A., Riordan J. R., Grinstein S. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem. 1993 Oct 15;268(29):21592–21598. [PubMed] [Google Scholar]
- Nitsch L., Wollman S. H. Suspension culture of separated follicles consisting of differentiated thyroid epithelial cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):472–476. doi: 10.1073/pnas.77.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990 Sep 27;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Riordan J. R. The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol. 1993;55:609–630. doi: 10.1146/annurev.ph.55.030193.003141. [DOI] [PubMed] [Google Scholar]
- Rozen R., De Braekeleer M., Daigneault J., Ferreira-Rajabi L., Gerdes M., Lamoureux L., Aubin G., Simard F., Fujiwara T. M., Morgan K. Cystic fibrosis mutations in French Canadians: three CFTR mutations are relatively frequent in a Quebec population with an elevated incidence of cystic fibrosis. Am J Med Genet. 1992 Feb 1;42(3):360–364. doi: 10.1002/ajmg.1320420322. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Carson M. R., Ostedgaard L. S., Denning G. M., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator in a model epithelium. Am J Physiol. 1994 Apr;266(4 Pt 1):L405–L413. doi: 10.1152/ajplung.1994.266.4.L405. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Ostedgaard L. S., Rich D. P., Welsh M. J. The amino-terminal portion of CFTR forms a regulated Cl- channel. Cell. 1994 Mar 25;76(6):1091–1098. doi: 10.1016/0092-8674(94)90385-9. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Rich D. P., Ostedgaard L. S., Gregory R. J., Smith A. E., Welsh M. J. Mutations in CFTR associated with mild-disease-form Cl- channels with altered pore properties. Nature. 1993 Mar 11;362(6416):160–164. doi: 10.1038/362160a0. [DOI] [PubMed] [Google Scholar]
- Smit L. S., Wilkinson D. J., Mansoura M. K., Collins F. S., Dawson D. C. Functional roles of the nucleotide-binding folds in the activation of the cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9963–9967. doi: 10.1073/pnas.90.21.9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani J. A., Rommens J. M., Hou Y. X., Chang X. B., Tsui L. C., Riordan J. R., Hanrahan J. W. Multi-ion pore behaviour in the CFTR chloride channel. Nature. 1993 Nov 4;366(6450):79–82. doi: 10.1038/366079a0. [DOI] [PubMed] [Google Scholar]
- Veeze H. J., Halley D. J., Bijman J., de Jongste J. C., de Jonge H. R., Sinaasappel M. Determinants of mild clinical symptoms in cystic fibrosis patients. Residual chloride secretion measured in rectal biopsies in relation to the genotype. J Clin Invest. 1994 Feb;93(2):461–466. doi: 10.1172/JCI116993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Anderson M. P., Rich D. P., Berger H. A., Denning G. M., Ostedgaard L. S., Sheppard D. N., Cheng S. H., Gregory R. J., Smith A. E. Cystic fibrosis transmembrane conductance regulator: a chloride channel with novel regulation. Neuron. 1992 May;8(5):821–829. doi: 10.1016/0896-6273(92)90196-k. [DOI] [PubMed] [Google Scholar]
- Winter M. C., Sheppard D. N., Carson M. R., Welsh M. J. Effect of ATP concentration on CFTR Cl- channels: a kinetic analysis of channel regulation. Biophys J. 1994 May;66(5):1398–1403. doi: 10.1016/S0006-3495(94)80930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]