Abstract
CALHM1 was recently demonstrated to be a voltage-gated ATP-permeable ion channel and to serve as a bona fide conduit for ATP release from sweet-, umami-, and bitter-sensing type II taste cells. Calhm1 is expressed in taste buds exclusively in type II cells and its product has structural and functional similarities with connexins and pannexins, two families of channel protein candidates for ATP release by type II cells. Calhm1 knockout in mice leads to loss of perception of sweet, umami, and bitter compounds and to impaired gustatory nerve responses to these tastants. These new studies validate the concept of ATP as the primary neurotransmitter from type II cells to gustatory neurons. Furthermore, they identify voltage-gated ATP release through CALHM1 as an essential molecular mechanism of ATP release in taste buds. We discuss these new findings, as well as unresolved issues in peripheral taste signaling that we hope will stimulate future research.
Keywords: ATP, calcium, connexin, pannexin, sensory, TRPM5, voltage-gated
Introduction
Taste of food is vitally important for nutrient detection, toxin avoidance, dietary choices, and for quality of life. Animals have developed taste as one of the five senses along with smell, hearing, sight, and touch. In mammals, taste buds are the taste detectors embedded in the tongue epithelium, which sense chemicals contained in foods and drinks and transmit their taste information to afferent gustatory nerves, and finally to the brain. The morphology of taste buds is specialized for their function. Each taste bud comprises a heterogeneous population of approximately 100 polarized taste cells that originate from local epithelial tissues rather than from neurogenic ectoderm [1]. These elongated cells have distinct apical and basolateral membranes separated by tight junctions. The apical microvilli extend into the oral cavity where they bind to tastants via receptor proteins. The basolateral membrane is involved in the processing and transmission of taste information to the gustatory sensory neurons and possibly to adjacent taste cells.
Sweetness, bitterness, saltiness, sourness, and umami (from Japanese for the “savory” or “meaty” taste of monosodium L-glutamate) are the five basic taste qualities/ modalities recognized by the scientific community. Sweet, umami, and mild salty tastes are generally considered to be attractive, whereas bitter, sour, and strong salty tastes are aversive. Each of the five basic tastes is sensed by dedicated taste cells in the taste buds [2–4], except that concentrated salts (i.e. strong salty taste) recruit both bitter- and sour-sensing cells [5]. Differentiated taste bud cells can be classified into three morphological categories: types I, II, and III defined by their ultrastructural features. These cell types can also be distinguished by their gene expression profiles. Type III cells sense sour taste and only this type of taste bud cell forms conventional synapses with intragemmal nerve processes. Accordingly, type III cells can be identified by their expression of neurotransmitter biosynthesis enzymes and proteins associated with synaptic transmission. Type II cells are divided into three functionally distinct taste cells: sweet-, umami-, and bitter-sensing cells. The type of taste that is sensed by a type II cell is determined by its unique expression of G protein-coupled taste receptor genes (TAS1R and TAS2R gene families). Nevertheless, all three varieties of type II cells share a common intracellular signal transduction pathway and they can all therefore be identified by expression of genes and proteins in the signaling cascade, which is discussed in detail below. Type I cells are generally believed to have a glial-like support function in taste buds. They can be identified by the expression of a glial glutamate/aspartate transporter and nucleoside triphosphate diphosphohydrolase 2. A subset of type I cells was demonstrated to mediate amiloride-sensitive salty taste which involves functional epithelial sodium channels [2], indicating that type I cells can also function as taste receptor cells. Despite their lack of conventional synaptic structures [6], types I and II cells transmit taste information to the nervous system. How do these taste cells accomplish this?
The importance of ATP in extracellular signaling has been well established [7–10]. A role of ATP as the primary neurotransmitter signaling from taste cells to gustatory neurons was suggested by two key findings. First, taste stimuli were found to evoke ATP release from gustatory papillary epithelia [11], and second, double genetic knockout (KO) of ionotropic ATP receptors P2X2 and P2X3, both expressed in the intragemmal gustatory nerve fibers [12, 13], was discovered to eliminate taste nerve responses in mice. The interpretation from these studies was that loss of taste perception was due to the absence of ATP receptors on the afferent nerve terminals in the double KO mice. However, it has subsequently been suggested that taste-evoked ATP release from taste buds itself may be deficient in the double KO animals [14]. Furthermore, while type II cells clearly release ATP upon stimulation, types I or III cells have not been shown to liberate ATP, whereas responses to all five basic taste modalities were eliminated in the P2X2/P2X3 KO mice [15, 16]. Thus, although peripheral taste signal transmission is largely dependent on extracellular ATP, the taste-deficient phenotype of P2X2/P2X3 double KO mice may not simply be attributed to the loss of these ATP receptors on the afferent nerves. Consequently, there has been considerable confusion and there remain critical questions regarding the roles of ATP as the primary neurotransmitter in taste buds.
Although it is firmly established that type II cells release ATP, the mechanisms by which this is achieved have been controversial. In this review, we focus on the mechanisms of ATP release from sweet-, umami-, and bitter-sensing type II taste cells. It has been argued that ATP is released from type II cells by a non-traditional non-exocytotic mechanism. Plasma membrane connexin and pannexin ion channels have hitherto been proposed as the primary mechanisms for the ATP release from these cells [15–19]. Our recent study [20] suggests a new model where, rather than connexins and pannexins, calcium homeostasis modulator 1 (CALHM1) [21], a recently identified subunit of a novel plasma membrane ion channel [22], mediates and is indispensable for type II taste cell ATP release. We will attempt to review the current and somewhat conflicting and confusing evidence about the identity of ATP release channels in this type of taste cell, and to reflect on the following questions: which ion channels are actually involved and how are they activated during taste?
ATP release plays important roles in taste signal transmission
Although the importance of extracellular ATP in taste signal transmission in taste buds is well acknowledged, its role as the primary neurotransmitter remains to be fully clarified. CALHM1 has been shown to be a voltage-gated ATP release channel and its genetic elimination abolished both ATP release from taste buds and gustatory nerve responses to taste qualities (sweetness, umami, and bitterness) mediated by type II cells [20]. These observations strongly indicate that ATP released through CALHM1 channels is the principal neurotransmitter linking sweet-, umami-, and bitter-sensing type II cells to the peripheral nervous system. However, as noted above, all taste-evoked neural activity is eliminated in the P2X2/P2X3 double KO mice [11], suggesting that types I and III cells also utilize ATP as their neurotransmitter. However, ATP release has not yet been detected from these cells [15, 16, 18], and CALHM1 is not expressed in them [20, 23].
ATP released from type II cells has been suggested to be involved in lesser-known cell-to-cell communication within taste buds that has been speculated to play a role in shaping signal outputs from taste buds (see [24] for review). However, the physiological relevance of ATP in cell-cell interactions between taste cells remains to be clarified.
How exactly does taste stimulate ATP secretion?
The molecular biology of taste transduction began with the identification of gustducin (now designated as GNAT3), a gene encoding a G protein α subunit expressed in the majority of type II cells [25]. Subsequent studies have identified many genes involved in sweet, umami, and bitter taste reception (i.e. type II cell functions), including taste receptors (TAS1Rs and TAS2Rs), G proteins, phospholipase C-β2 (PLCB2), inositol trisphosphate receptor type 3 (IP3R3), transient receptor potential M5 (TRPM5), and CALHM1. Following binding of taste substances to G protein-coupled taste receptors located at the apical microvilli, the canonical taste signal transduction in type II cells sequentially involves activation of G proteins and phospholipase C-β2, Ca2+ release from endoplasmic reticulum Ca2+ stores through IP3R3, elevated [Ca2+]i-dependent activation of plasma membrane monovalent cation-selective TRPM5 channels, consequent membrane depolarization and release of ATP through a non-exocytotic mechanism, presumably an ion channel (Fig. 1). Our recent studies [20] suggest that the ion channel is comprised of CALHM1. There has been some debate about whether TRPM5-mediated depolarization is sufficient to activate ATP release during taste [17, 18, 26]. The apparent threshold voltage of ATP secretion from type II cells is about −10 mV [16], whereas depolarization caused by activation of TRPM5 channels can bring the membrane potential to at best 0 mV. This would likely be insufficient to activate the ATP release mechanism strongly and quickly enough to mediate rapid taste transduction. Thus, a model with TRPM5-mediated depolarization as the sole activation mechanism of ATP release seems unlikely. Rather, one that includes action potential firing as a booster of TRPM5-mediated depolarization seems more suitable. Indeed, type II cells fire action potentials upon taste stimulation [18, 27], and tetrodotoxin (TTX), a voltage-gated Na+ channel inhibitor, abolished taste stimuli-dependent ATP secretion to the same extent as did Calhm1 KO [20]. Therefore, voltage-gated Na+ channel(s) likely play a crucial role in the release of ATP from taste cells by mediating action potential firing (Fig. 1). SCN2A (Nav1.2), SCN3A (Nav1.3), and SCN9A (Nav1.7) are expressed in type II cells [28] and are molecular candidates for the generation of action potentials. However, functional analyses of the Na+ channel subtypes in type II cells have yet to be undertaken. Evaluation of the functional significance of action potentials in type II cells will require knowledge about the extent and duration of depolarization elicited by TRPM5 channels and by action potentials upon taste stimuli. Notably, lacking is knowledge about the quantitative relationships among the concentrations of taste substances, the frequency of action potential firing and the amount of ATP released.
Figure 1.
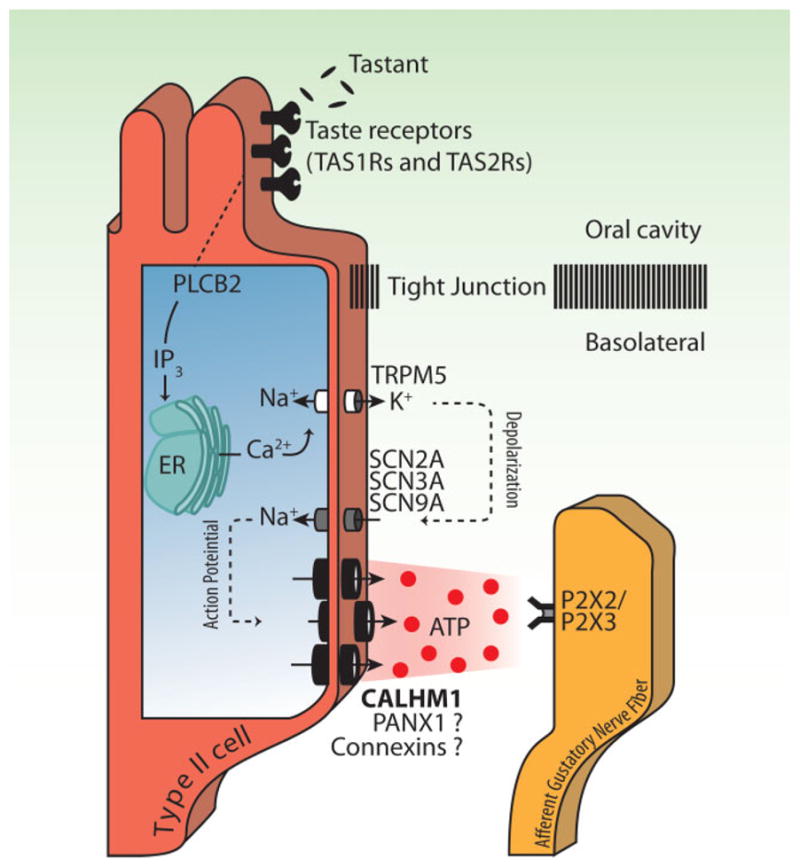
Scheme of taste signal transduction cascade in sweet, bitter, and umami type II taste cells. CALHM1 is an integral component of the ATP release channel, while contributions of pannexin 1 (PANX1) and connexins are still in question, as indicated by the question marks.
Do ion channels mediate non-exocytotic ATP release from type II cells?
To date, type II cells are the only established sites of ATP release from taste buds. Synaptic vesicles of most neuronal cells contain high concentrations of ATP (up to 100 mM) and ATP is co-released from nerve terminals via exocytotic mechanisms with other neurotransmitters [7–10]. Even though type II cells lack synapses, exocytotic ATP release was first suggested as their mechanism of ATP release; the reason being that these cells express vesicular nucleotide transporter (VNUT), which mediates ATP import into synaptic vesicles in neurons [29]. To determine the role of exocytotic fusion of intracellular vesicles with the plasma membrane, Kinnamon and co-workers [30] measured fluctuations of whole-cell membrane electrical capacitance as an indicator of plasma membrane area in isolated types I, II, and III taste bud cells. Membrane depolarization activated plasma membrane voltage-gated Ca2+ channels and elicited an increase in membrane surface area only in type III cells. In contrast, types I and II cells did not show enhanced capacitance, suggesting that exocytotic release of neurotransmitters is not part of their normal repertoire of responses to activation. These findings suggested that depolarization-induced transmitter release by regulated exocytosis is absent in type II cells, in agreement with previous microscopic observations of elaborated presynaptic structures only in type III cells but not in types I or II cells. The implication from these studies was that there must exist non-exocytotic ATP release mechanism(s) in type II cells. There is compelling evidence for non-exocytotic ATP transport mechanisms in plasma membranes of various cell types by, for instance, ATP-binding cassette transporters, voltage-dependent anion channels, connexin gap junction hemichannels (Cxs), and pannexin 1 channels (PANX1), as well as transient cell lysis [31]. Among them, ion channel-mediated mechanisms involving different connexin hemichannel subunits, or the pannexin 1 channel, have been proposed by several groups to constitute the molecular machinery for ATP release in type II cells [15–19]. However, it has remained controversial whether connexins or pannexin(s) are involved. (We do not use the term “hemichannel” to describe pannexin 1 because it has not been shown to form gap junctions [32].)
In the meantime, CALHM1 was found to form an ion channel that is functionally and structurally related to connexin and pannexin channels [33] (Fig. 2), and functional genomic/transcriptome analyses revealed the expression of CALHM genes in primate taste buds, exclusively in type II cells [23]. This knowledge led to the recent discovery of CALHM1 as a bona fide and essential ATP release ion channel in type II taste cells [20], as described below.
Figure 2.
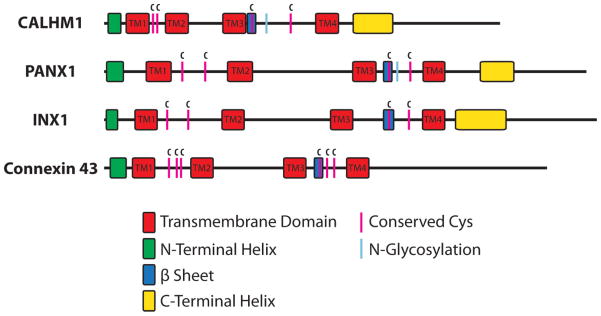
Shared and distinct structural properties of CALHM1, pannexin-1, innexin-1, and connexin43. Schematic depiction of the alignment of the secondary structures. Conserved cysteines and N-linked glycosylation sites are also shown.
Candidate ion channels for ATP release from type II cells
Pannexin 1
Shortly after ATP was suggested to be the primary neurotransmitter released from taste cells, Stephen Roper’s group identified pannexin 1 in taste buds and proposed that it is the ATP release channel in type II cells [15]. By immunohistochemistry, in situ hybridization and real-time quantitative RT-PCR, they found expression of pannexin 1 protein and mRNA to be enriched in taste buds compared with the surrounding non-gustatory lingual epithelium. By RT-PCR of single taste cells, pannexin 1 was shown to be preferentially expressed in type II cells (expressed in 100% of type II cells), although it was also detected in about half of type I (50%) and III (60%) cells. Using an in vitro assay for ATP release from freshly isolated taste cells that used ATP biosensor cells (cells stably expressing P2X2 and P2X3 receptors that respond to elevations of extracellular [ATP] by increased [Ca2+]i) positioned in close proximity to taste cells, they confirmed that ATP release occurs only from type II cells. With this assay, they found that ATP release evoked by a taste mixture containing sweet and bitter (and umami in some experiments) compounds was blocked by 5 μM carbenoxolone, a concentration at which it is purported to be a selective blocker of pannexin 1 channels [34–36]. The inhibitory effect of carbenoxolone on taste-evoked ATP release from type II cells was later confirmed by Yuzo Ninomiya and colleagues [18]. Roper’s group also tested probenecid, another, albeit non-selective, pannexin 1 channel blocker [37], in acute lingual slices and observed a partial inhibition of taste-evoked ATP secretion [38]. These studies led to a general recognition in the field that pannexin 1 was the best candidate for the ATP release mechanism in taste cells [39]. Of note, however, a recent study demonstrated that genetic ablation of Panx1 was without effects on voltage-gated ion currents or taste-induced ATP release from type II cells ex vivo [19]. Whether taste perception is altered in the KO animals is as yet unknown. Nevertheless, the role of this channel in type II cell ATP release is now less certain.
Connexins
Evidence that connexins are involved in type II cell ATP release has come mainly from the team of Stanislav Kolesnikov. They were the first to report that type II cells are the only source of membrane depolarization-evoked ATP release from taste buds [16]. They assayed ATP release from freshly isolated taste cells using a cellular ATP biosensor (cells endogenously expressing P2Y receptors) and found that ATP release from type II cells was [Ca2+]i independent but voltage dependent. They established that the presence of nonselective voltage-gated ion currents with slow activation and deactivation kinetics provides an electrophysiological fingerprint of type II cells and, furthermore, that the amount of ATP released is correlated with the magnitude of the slow currents. mRNAs for several connexins (Cx26, Cx30.3, Cx31.1, Cx33, Cx43) were detected by single cell RT-PCR on isolated type II cells, although it has not been established whether these connexin genes are expressed selectively in type II cells or even in taste buds [16]. That is, they could be expressed equally in taste buds and non-gustatory lingual epithelium [15]. Furthermore, it has been suggested that gap junctions exist between taste cells [40], so expression of connexins does not necessarily imply that they play roles as hemichannels. Still, among known connexin inhibitors tested, some, including octanol and a connexin mimetic peptide, 43GAP26 [41–43], blocked the slow voltage-gated currents, while other connexin inhibitors including La3+, NPPB, and niflumic acid, and 10 μM carbenoxolone, a putative pannexin-selective blocker at this concentration, did not. Hence, connexin hemichannels were favored over pannexin 1 channels to play the major role in type II cell ATP release. This notion was later supported by theoretical modeling that predicted a role for ATP-permeable channels with slow deactivation kinetics in ATP release from type II cells [44]. Slow deactivation is a characteristic of connexin hemichannels, whereas pannexin 1 channels deactivate rapidly. Consistent with the mathematical simulations, bell-like and Langmuir isotherm-like potential dependencies were revealed to be characteristic of ATP release observed during prolonged (2 s) and short (100 ms) depolarizations of taste cells, respectively. Thus, connexin hemichannels are possible candidates for the ATP release channel in type II cells.
Are connexin and pannexin channels really involved in ATP release from type II taste cells?
The conflicting pharmacological data obtained so far for connexins and pannexin 1 (see Table 1) prevent us from drawing firm conclusions. The reasons for the discrepancies among studies might be related to altered efficacies of the compounds under different experimental conditions and to unknown or unappreciated actions of these drugs. For example, although carbenoxolone is indeed a blocker of connexins and pannexins and has been used to discriminate these channels by taking advantage of the difference in their dose dependency, carbenoxolone is not specific to these channels. It also inhibits volume-regulated anion [45] and voltage-gated calcium [46] channels. Moreover, carbenoxolone was shown to broadly affect several neuronal membrane conductances and synaptic transmission, independent of its effects on connexins and pannexins [47]. Probenecid, which has been used to dissect pannexin 1 function, also has other actions. It is a well-known FDA-approved inhibitor of multidrug resistance protein 1 and other organic anion transporters [48, 49] and is used clinically as a medicine for gout [50]. Probenecid has recently been described as an agonist of TRPV2 channels [51] and, importantly, as an antagonist of a subset of bitter taste receptors including TAS2R16, TAS2R38, and TAS2R43 [52]. Thus, the observed partial inhibition by probenecid of ATP release induced by a sweet/umami/bitter taste mixture [38] could possibly be attributed to its inhibitory action on bitter taste receptors. Effects of inhibitors with multiple targets can only be interpreted rigorously when assessed in isolation. They should be used with great caution to infer a protein’s role in complex biological systems involving several components, such as taste-evoked ATP release from taste cells. In the absence of reliably specific blockers, genetic ablation of a specific gene is the standard for determining its role(s) in a biological system (Table 2). Gene KO effects on behavioral and neural responses can be rigorously interpreted to determine the requirement of the targeted gene in taste. Notably, ATP release from taste cells ex vivo from Panx1 KO mice was normal [19], although the relevance of these observations for ATP release in vivo remains to be established. Evaluating the effects of connexin and pannexin 1 gene KOs in vivo will provide the best insights into which genes have physiological significance in taste perception.
Table 1.
Pharmacological effects of inhibitors of CALHM1, pannexin-1, and connexins on voltage-gated currents and ATP release in type II taste bud cells
| Channel | Inhibitorsa | Voltage-gated currents | ATP release |
|---|---|---|---|
| Pannexin-1 | 10Px1 | No [44] | ND |
| Carbenoxolone (<10 μM) | No [16] | Yes [15, 18] | |
| Probenecid | No [20] | Yes [38] | |
|
| |||
| Connexins | Octanol | Yes [16] | ND |
| Heptanol | No [20] | ND | |
| 43GAP26 | Yes [16] | No [18] | |
| 32GAP27 | No [16] | No [18] | |
| Gd3+ | Yes [20]/No [16] | ND | |
| La3+, NPPB, Niflumic acid | No [16] | ND | |
|
| |||
| CALHM1 | Gd3+ | Yes [20]/No [16] | ND |
| Ruthenium Red | ND | ND | |
Listed are inhibitors so far tested that can reportedly discriminate contributions of connexins, pannexin 1, and CALHM1.
Table 2.
Effects of gene knockout of CALHM1, pannexin-1, and connexins on voltage-gated currents and ATP release in type II cells and the ability of animals to taste
| Channel | KO mouse modelsa | Voltage-gated currents | Taste-evoked ATP release | Taste perception |
|---|---|---|---|---|
| Pannexin-1 | Panx1−/− [58, 59] | No effect [19] | No effect [19] | ND |
|
| ||||
| Connexins | Cx26loxP/loxP [60] | |||
| Cx30−/− [61–63] | ND | ND | ND | |
| Cx31−/− [64] | ||||
| Cx43loxP/loxP [65] | ||||
|
| ||||
| CALHM1 | Calhm1−/− [22, 66] | Strongly reduced [20] | Eliminated [20] | Eliminated [20] |
loxP/loxP and −/− mean loxP-floxed and conventional KO mouse models, respectively.
KO mouse models only of genes identified in taste buds are listed. ND, not determined.
Calcium homeostasis modulator 1: A bona fide ATP release channel of taste cells
CALHM1 expression was identified specifically in type II cells of primate taste buds [23], suggesting that CALHM1 may have physiological functions in the processing or transmission of taste information during perception of sweetness, umami, and bitterness. CALHM1 was originally identified as a susceptibility factor for late-onset Alzheimer’s disease that influences age of disease onset [21, 53], possibly by influencing amyloid beta peptide levels [21]. CALHM1 encodes a 346-amino-acid membrane protein consisting of four transmembrane domains, cytoplasmic N- and C-termini, two extracellular loops and one cytoplasmic loop and forms a homo-hexameric plasma membrane voltage-gated ion channel [22, 33]. CALHM1 channels are gated by voltage, activating and deactivating slowly in response to depolarization and hyperpolarization, respectively, in the presence of physiological levels of [Ca2+]o [22]. CALHM1 can also be activated by reductions of the concentrations of extracellular divalent cations, particularly Ca2+, by a mechanism that is allosterically coupled to voltage regulation. CALHM1 currents are inhibited by non-specific ion channel blockers including ruthenium red, Gd3+ and Zn2+ [22]. No specific CALHM1 blockers are known yet. The ion selectivity of CALHM1 channels is poor, as monovalent and divalent cations and anions can pass through the channel (relative permeabilities PCa:PNa:PK:PCl = 11:1:1.17:0.56) [22]. The ion conducting pore of CALHM1 channels is unusually wide with the diameter estimated to be about 14 Å [33], similar to that of connexins. Furthermore, connexins are also hexameric ion channels, each subunit having four transmembrane domains and cytoplasmic N- and C-termini. Indeed, it was shown that CALHM1 shares functional and quaternary and secondary structural similarities with connexins and evolutionarily distinct invertebrate gap junction-forming innexins and their vertebrate pannexin homologs (see [33] for details) (Fig. 2). Thus, CALHMs, connexins, and pannexins and innexins are evolutionarily distinct, structurally related protein families with shared and distinct functional properties (Table 1 and Table 3).
Table 3.
Shared and distinct functional properties of CALHM1, pannexin-1, and connexins
| Channel | Voltage gatinga | ATP permeability | Carbenoxolone sensitivity | Ca2+ sensitivity | Gap junction formation |
|---|---|---|---|---|---|
| Pannexin-1 | Yes [35, 67, 68] | Yes [67] | Yes [35] | No [35] | No [32] |
| Connexins | Yes [69–71] | Yes [72, 73] | Yes [73, 74] | Yes [69–71] | Yes [75, 76] |
| CALHM1 | Yes [22] | Yes [20] | No [20, 22] | Yes [20, 22] | No [33] |
Regardless of response kinetics.
Connexin hemichannels and pannexin 1 are permeable to ATP molecules (~12 Å in diameter) [47–49] and are involved in physiological cellular ATP release [54–56]. The apparent structural and functional similarities to those channels, the anion permeability and the wide pore of CALHM1 channel led us to develop a hypothesis that ATP can permeate through the CALHM1 ion-conducting pore. When expressed in HeLa and COS-1 cells, and Xenopus laevis oocytes, CALHM1 mediated robust ruthenium red-blockable cellular ATP release into the extracellular medium that was induced by maneuvers that activate CALHM1 channels, such as lowering [Ca2+]o and membrane depolarization [20, 22]. The results demonstrated that CALHM1 is a novel voltage-gated ATP-permeable channel.
The ATP permeability of CALHM1 and type II cell-specific expression of CALHM1 mRNA in primate taste buds [23] suggested a role for CALHM1 as an ATP release channel in peripheral taste transduction in type II cells. Notably, the slow deactivation kinetics of the CALHM1 channel [22] are compatible with biophysical inferences made by the Kolesnikov group about ATP release channels in mouse type II cells, as described above [44]. We recorded slowly activating non-selective voltage-gated currents that were reminiscent of CALHM1 currents in type II cells but not in type I or III cells. The CALHM1-like slow currents in type II cells were inhibited by Gd3+ and, importantly, by genetic elimination of Calhm1. Most notably, Calhm1 KO mice displayed severely depressed behavioral and gustatory nerve responses to sweet, umami, and bitter tastes. Furthermore, taste-evoked ATP release from taste buds observed in wild type mice was absent in the Calhm1 KO mice. As in primates, Calhm1 mRNA expression is confined to type II cells in the mouse tongue, demonstrated by multiple independent approaches, including single cell RT-PCR and double- and single-labeling in situ hybridization using wild-type and type II cell-null mice [57]. The combined physiological, behavioral, biochemical, and genetic evidence strongly implicate an essential role for CALHM1 in sweet, umami, and bitter taste perception by mediating the release of ATP from type II cells. These observations, in turn, strongly indicate that ATP released through CALHM1 channels is the principal neurotransmitter linking sweet-, umami-, and bitter-sensing type II cells to the peripheral nervous system.
CALHM1 is therefore essential for ATP release by type II cells. Nevertheless, it should be noted that there is some inconsistency between the pharmacological profile of the CALHM1 channel [20, 22], notably its insensitivity to carbenoxolone, and previous pharmacological studies of ATP release from taste cells (Table 1). This discrepancy may be explained trivially as a consequence of the lack of specificity of carbenoxolone, or it may suggest that CALHM1 is not the entire story. That is, it is possible that whereas CALHM1 is an essential component of the ATP release channel in type II cells, it may associate with other components that confer specific physiological and pharmacological properties. Molecular identification of such protein complexes is now an important goal in the field.
Conclusions and prospects
It has been a long-standing enigma how taste cells devoid of synapses transmit taste information to the nervous system. The taste blind phenotype of P2X2/P2X3 double-KO mice shed light on the importance of ATP in the chemical communication between taste cells and gustatory neurons [11], but the role of ATP as the primary neurotransmitter was in question [14]. Therefore, two major unresolved issues in the peripheral taste signal transmission were (1) the identity of the primary neurotransmitter(s) signaling from taste cells to gustatory neurons and (2) the mechanism(s) of how the neurotransmitter is released from taste cells. Our recent report [20] has addressed these issues. CALHM1, a voltage-gated large-pore ion channel expressed exclusively in type II cells, mediates non-exocytotic ATP release and is required for sweet, umami, and bitter taste reception, establishing CALHM1 as an essential mediator of non-synaptic neurotransmission in type II cells. The loss of taste perception by KO of an ATP release channel exclusively expressed in type II cells (i.e. Calhm1), in turn, reinforces the concept of ATP as the primary neurotransmitter for, at least, sweet, umami, and bitter signal transmission. However, the nature of protein complexes associated with the ATP release channel, the tuning mechanisms of ATP release and the functional significance of ATP in cell-cell interactions within taste buds are compelling questions for future research.
Abbreviations
- ATP
adenosine-5′-triphosphate
- FDA
U.S. Food and Drug Administration
- KO
knockout
- TTX
tetrodotoxin
References
- 1.Stone LM, Finger TE, Tam PP, Tan SS. Taste receptor cells arise from local epithelium, not neurogenic ectoderm. Proc Natl Acad Sci USA. 1995;92:1916–20. doi: 10.1073/pnas.92.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumoto I, Ohmoto M, Abe K. Functional diversification of taste cells in vertebrates. Semin Cell Dev Biol. 2013;24:210–4. doi: 10.1016/j.semcdb.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–44. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, et al. High salt recruits aversive taste pathways. Nature. 2013;494:472–5. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clapp TR, Yang R, Stoick CL, Kinnamon SC, et al. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–21. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Purinergic cotransmission. Brain Res Bull. 1999;50:355–7. doi: 10.1016/s0361-9230(99)00103-3. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27:166–76. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. BioEssays. 2012;34:218–25. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 11.Finger TE, Danilova V, Barrows J, Bartel DL, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–9. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 12.Bo X, Alavi A, Xiang Z, Oglesby I, et al. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 1999;10:1107–11. doi: 10.1097/00001756-199904060-00037. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto I, Emori Y, Nakamura S, Shimizu K, et al. DNA microarray cluster analysis reveals tissue similarity and potential neuron-specific genes expressed in cranial sensory ganglia. J Neurosci Res. 2003;74:818–28. doi: 10.1002/jnr.10814. [DOI] [PubMed] [Google Scholar]
- 14.Huang YA, Stone LM, Pereira E, Yang R, et al. Knocking out P2X receptors reduces transmitter secretion in taste buds. J Neurosci. 2011;31:13654–61. doi: 10.1523/JNEUROSCI.3356-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, et al. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104:6436–41. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, et al. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–67. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YA, Roper SD. Intracellular Ca2+ and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol. 2010;588:2343–50. doi: 10.1113/jphysiol.2010.191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murata Y, Yasuo T, Yoshida R, Obata K, et al. Action potential-enhanced ATP release from taste cells through hemichannels. J Neurophysiol. 2010;104:896–901. doi: 10.1152/jn.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romanov RA, Bystrova MF, Rogachevskaya OA, Sadovnikov VB, et al. The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J Cell Sci. 2012;125:5514–23. doi: 10.1242/jcs.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–6. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreses-Werringloer U, Lambert JC, Vingtdeux V, Zhao H, et al. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell. 2008;133:1149–61. doi: 10.1016/j.cell.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Z, Siebert AP, Cheung KH, Lee RJ, et al. Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proc Natl Acad Sci USA. 2012;109:E1963–71. doi: 10.1073/pnas.1204023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyer BD, Hevezi P, Gao N, Lu M, et al. Expression of genes encoding multi-transmembrane proteins in specific primate taste cell populations. PLoS One. 2009;4:e7682. doi: 10.1371/journal.pone.0007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roper SD. Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol. 2013;24:71–9. doi: 10.1016/j.semcdb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–9. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 26.Vandenbeuch A, Kinnamon SC. Why do taste cells generate action potentials? J Biol. 2009;8:42. doi: 10.1186/jbiol138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, et al. Taste responsiveness of fungiform taste cells with action potentials. J Neurophysiol. 2006;96:3088–95. doi: 10.1152/jn.00409.2006. [DOI] [PubMed] [Google Scholar]
- 28.Gao N, Lu M, Echeverri F, Laita B, et al. Voltage-gated sodium channels in taste bud cells. BMC Neurosci. 2009;10:20. doi: 10.1186/1471-2202-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, et al. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun. 2009;388:1–15. doi: 10.1016/j.bbrc.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 30.Vandenbeuch A, Zorec R, Kinnamon SC. Capacitance measurements of regulated exocytosis in mouse taste cells. J Neurosci. 2010;30:14695–701. doi: 10.1523/JNEUROSCI.1570-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signal. 2005;1:311–28. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, et al. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011;5:193–7. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siebert AP, Ma Z, Grevet JD, Demuro A, et al. Structural and functional similarities of calcium homeostasis modulator 1 (CALHM1) ion channel with connexins, pannexins, and innexins. J Biol Chem. 2013;288:6140–53. doi: 10.1074/jbc.M112.409789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda) 2006;21:103–14. doi: 10.1152/physiol.00048.2005. [DOI] [PubMed] [Google Scholar]
- 35.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–43. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 36.Li A, Leung CT, Peterson-Yantorno K, Stamer WD, et al. Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol. 2012;227:172–82. doi: 10.1002/jcp.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–7. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–96. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshii K. Gap junctions among taste bud cells in mouse fungiform papillae. Chem Senses. 2005;30:i35–6. doi: 10.1093/chemse/bjh100. [DOI] [PubMed] [Google Scholar]
- 41.Chaytor AT, Evans WH, Griffith TM. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J Physiol. 1997;503:99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desplantez T, Verma V, Leybaert L, Evans WH, et al. Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacol Res. 2012;65:546–52. doi: 10.1016/j.phrs.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Leybaert L, Braet K, Vandamme W, Cabooter L, et al. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes. 2003;10:251–7. doi: 10.1080/cac.10.4-6.251.257. [DOI] [PubMed] [Google Scholar]
- 44.Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol. 2008;132:731–44. doi: 10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benfenati V, Caprini M, Nicchia GP, Rossi A, et al. Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels (Austin) 2009;3:323–36. doi: 10.4161/chan.3.5.9568. [DOI] [PubMed] [Google Scholar]
- 46.Vessey JP, Lalonde MR, Mizan HA, Welch NC, et al. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–6. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- 47.Tovar KR, Maher BJ, Westbrook GL. Direct actions of carbenoxolone on synaptic transmission and neuronal membrane properties. J Neurophysiol. 2009;102:974–8. doi: 10.1152/jn.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakos E, Evers R, Sinko E, Varadi A, et al. Interactions of the human multidrug resistance proteins MRP1 and MRP2 with organic anions. Mol Pharmacol. 2000;57:760–8. doi: 10.1124/mol.57.4.760. [DOI] [PubMed] [Google Scholar]
- 49.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–99. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 50.Stocker SL, Williams KM, McLachlan AJ, Graham GG, et al. Pharmacokinetic and pharmacodynamic interaction between allopurinol and probenecid in healthy subjects. Clin Pharmacokinet. 2008;47:111–8. doi: 10.2165/00003088-200847020-00004. [DOI] [PubMed] [Google Scholar]
- 51.Bang S, Kim KY, Yoo S, Lee SH, et al. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007;425:120–5. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 52.Greene TA, Alarcon S, Thomas A, Berdougo E, et al. Probenecid inhibits the human bitter taste receptor TAS2R16 and suppresses bitter perception of salicin. PLoS One. 2011;6:e20123. doi: 10.1371/journal.pone.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambert JC, Sleegers K, Gonzalez-Perez A, Ingelsson M, et al. The CALHM1 P86L polymorphism is a genetic modifier of age at onset in Alzheimer’s disease: a meta-analysis study. J Alzheimers Dis. 2010;22:247–55. doi: 10.3233/JAD-2010-100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, et al. ATP-mediated glia signaling. J Neurosci. 2000;20:2835–44. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998;18:8794–804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103:7655–9. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, et al. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 2011;14:685–7. doi: 10.1038/nn.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anselmi F, Hernandez VH, Crispino G, Seydel A, et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA. 2008;105:18770–5. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–86. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen-Salmon M, Ott T, Michel V, Hardelin JP, et al. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002;12:1106–11. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boulay AC, del Castillo FJ, Giraudet F, Hamard G, et al. Hearing is normal without connexin30. J Neurosci. 2013;33:430–4. doi: 10.1523/JNEUROSCI.4240-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liebmann M, Stahr A, Guenther M, Witte OW, et al. Astrocytic Cx43 and Cx30 differentially modulate adult neurogenesis in mice. Neurosci Lett. 2013;545:40–5. doi: 10.1016/j.neulet.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 63.Teubner B, Michel V, Pesch J, Lautermann J, et al. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet. 2003;12:13–21. doi: 10.1093/hmg/ddg001. [DOI] [PubMed] [Google Scholar]
- 64.Plum A, Winterhager E, Pesch J, Lautermann J, et al. Connexin31-deficiency in mice causes transient placental dysmorphogenesis but does not impair hearing and skin differentiation. Dev Biol. 2001;231:334–47. doi: 10.1006/dbio.2000.0148. [DOI] [PubMed] [Google Scholar]
- 65.Theis M, de Wit C, Schlaeger TM, Eckardt D, et al. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis. 2001;29:1–13. doi: 10.1002/1526-968x(200101)29:1<1::aid-gene1000>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 66.Wu J, Peng S, Wu R, Hao Y, et al. Generation of Calhm1 knockout mouse and characterization of calhm1 gene expression. Protein Cell. 2012;3:470–80. doi: 10.1007/s13238-012-2932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–8. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 68.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, et al. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA. 2003;100:13644–9. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ebihara L. New roles for connexons. News Physiol Sci. 2003;18:100–3. doi: 10.1152/nips.01431.2002. [DOI] [PubMed] [Google Scholar]
- 70.Srinivas M, Calderon DP, Kronengold J, Verselis VK. Regulation of connexin hemichannels by monovalent cations. J Gen Physiol. 2006;127:67–75. doi: 10.1085/jgp.200509397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol. 2008;132:315–27. doi: 10.1085/jgp.200810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–43. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang J, Kang N, Lovatt D, Torres A, et al. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–11. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–96. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nielsen MS, Nygaard Axelsen L, Sorgen PL, Verma V, et al. Gap junctions. Compr Physiol. 2012;2:1981–2035. doi: 10.1002/cphy.c110051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rozental R, Giaume C, Spray DC. Gap junctions in the nervous system. Brain Res Brain Res Rev. 2000;32:11–5. doi: 10.1016/s0165-0173(99)00095-8. [DOI] [PubMed] [Google Scholar]


