Abstract
Interaction of cells with the extracellular matrix (ECM) plays an important role in the regulation of cell behavior. Formation of adhesive contacts leads to transduction of signals into the cell and results in altered gene expression and modulation of the cellular phenotype. Specific adhesive interactions of the fibronectin and vitronectin receptors with their ligands in the matrix modulates expression of ECM-degrading metalloproteases. These proteases are involved in the acquisition of the invasive phenotype by a number of cell types. The activity of matrix metalloproteases (MMPs) is reduced by endogenous inhibitors referred to as tissue inhibitors of metalloproteases (TIMPs). Alterations in the balance between the activity of MMPs and TIMPs alters cellular invasion through effects on matrix degradation. In this study we demonstrate that inhibition of endogenous gelatinase A activity in A2058 human melanoma cells results in enhanced cellular adhesion. To further explore this phenomenon, we have used retroviral infection vectors to control the amount of the MMP inhibitor TIMP-2 in human melanoma A2058 cells. Altering the production of TIMP-2 modulates not only proteolysis of the extracellular matrix, but also the adhesive and spreading properties of the cells and results in altered cell morphology. These effects of TIMP-2 appear to be mediated by inhibition of gelatinase A activity. We conclude that gelatinase A, in addition to contributing to proteolysis of ECM components, also functions to proteolyse cell surface components that mediate attachment of A2058 cells to the ECM. Thus, gelatinase A may function to modulate cell attachment and facilitate cell migration and invasion.
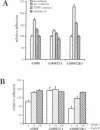
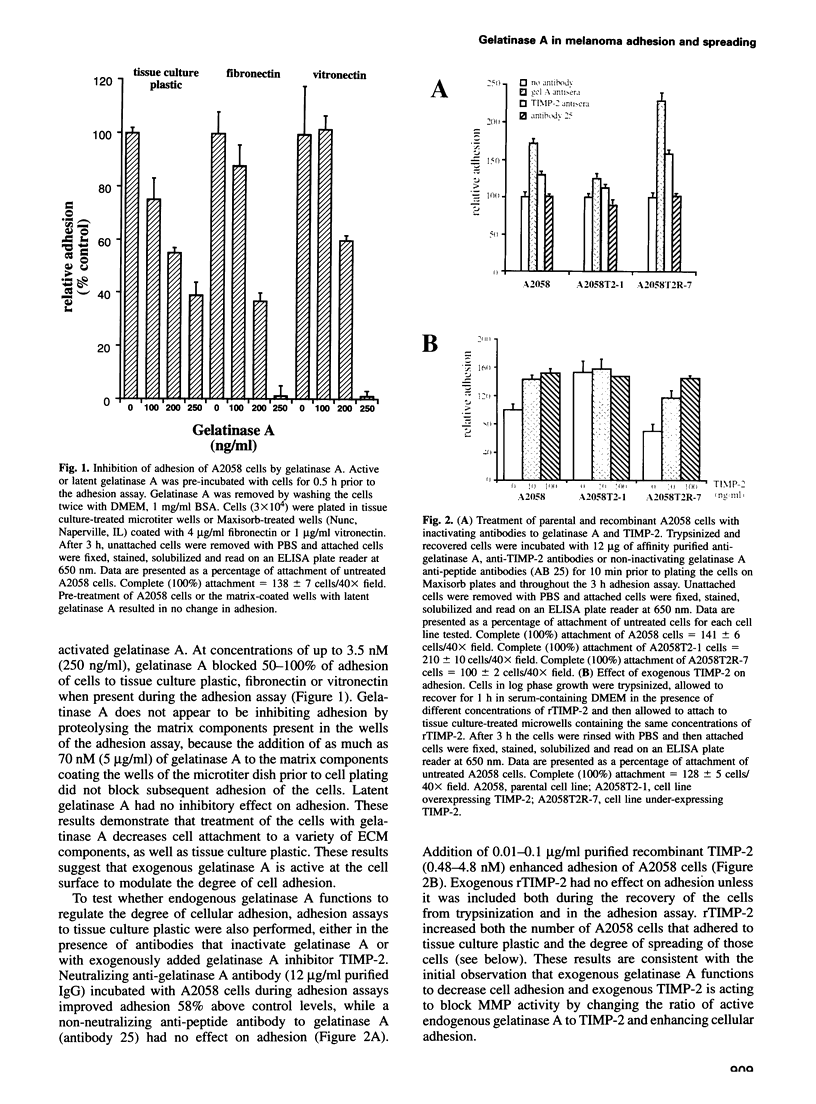
Full text
PDF

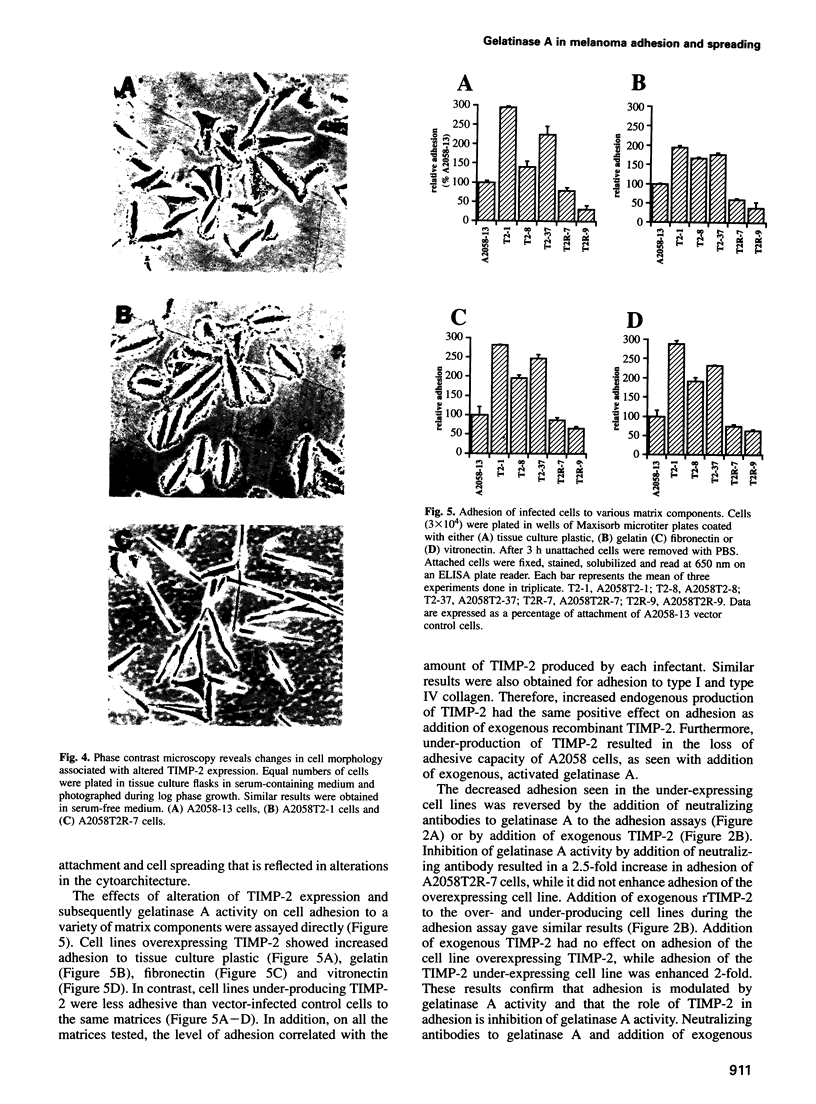


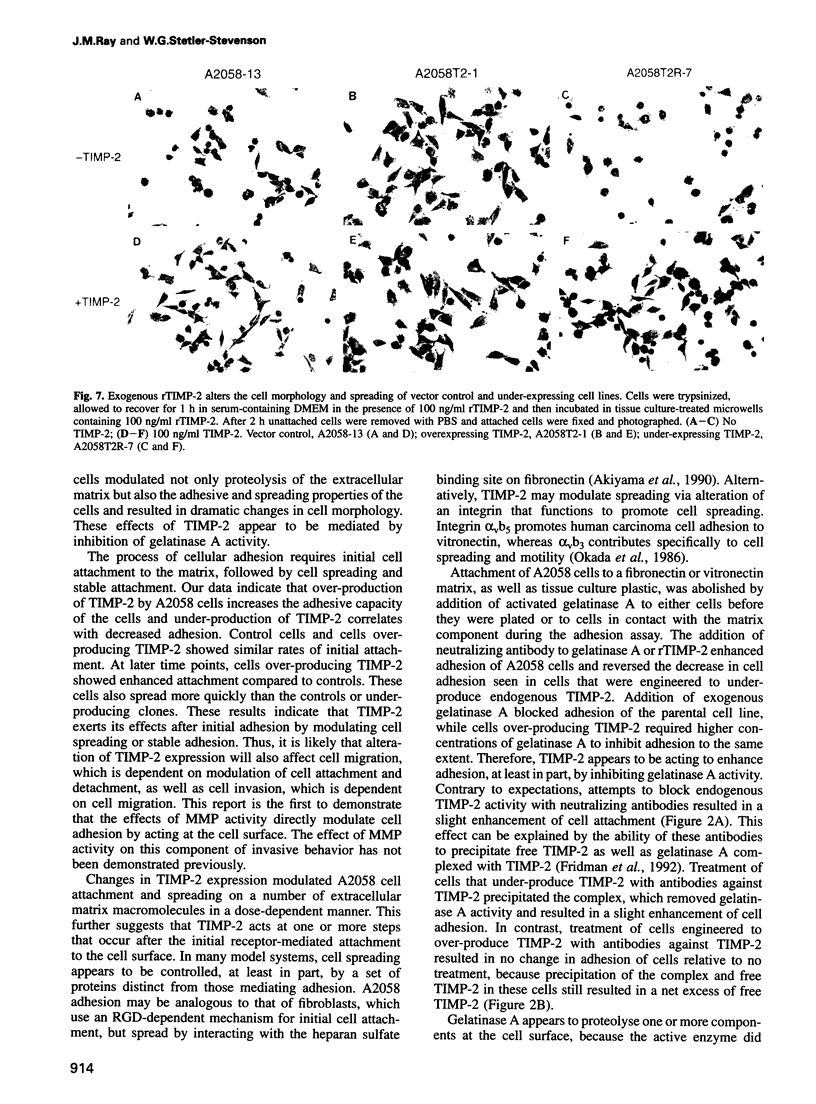
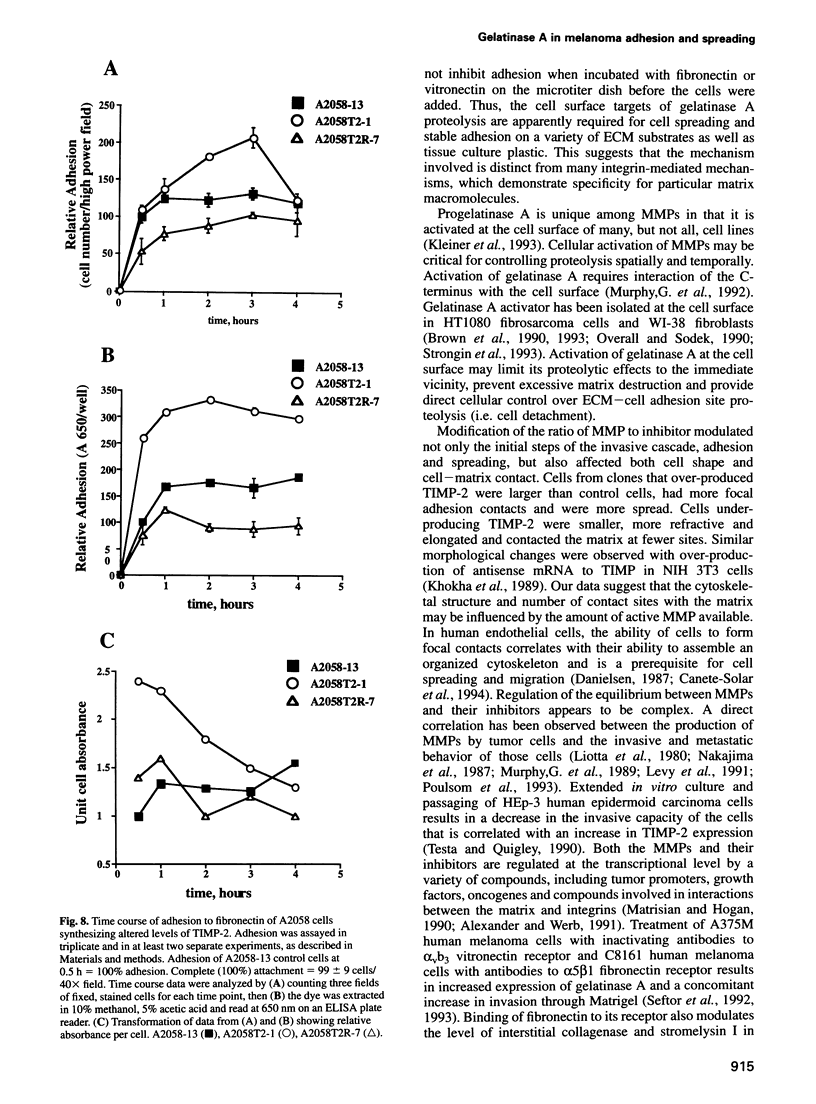


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Larjava H., Yamada K. M. Differences in the biosynthesis and localization of the fibronectin receptor in normal and transformed cultured human cells. Cancer Res. 1990 Mar 1;50(5):1601–1607. [PubMed] [Google Scholar]
- Albini A., Melchiori A., Santi L., Liotta L. A., Brown P. D., Stetler-Stevenson W. G. Tumor cell invasion inhibited by TIMP-2. J Natl Cancer Inst. 1991 Jun 5;83(11):775–779. doi: 10.1093/jnci/83.11.775. [DOI] [PubMed] [Google Scholar]
- Alexander C. M., Werb Z. Targeted disruption of the tissue inhibitor of metalloproteinases gene increases the invasive behavior of primitive mesenchymal cells derived from embryonic stem cells in vitro. J Cell Biol. 1992 Aug;118(3):727–739. doi: 10.1083/jcb.118.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez O. A., Carmichael D. F., DeClerck Y. A. Inhibition of collagenolytic activity and metastasis of tumor cells by a recombinant human tissue inhibitor of metalloproteinases. J Natl Cancer Inst. 1990 Apr 4;82(7):589–595. doi: 10.1093/jnci/82.7.589. [DOI] [PubMed] [Google Scholar]
- Behrens J., Mareel M. M., Van Roy F. M., Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989 Jun;108(6):2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Kleiner D. E., Unsworth E. J., Stetler-Stevenson W. G. Cellular activation of the 72 kDa type IV procollagenase/TIMP-2 complex. Kidney Int. 1993 Jan;43(1):163–170. doi: 10.1038/ki.1993.27. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Levy A. T., Margulies I. M., Liotta L. A., Stetler-Stevenson W. G. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990 Oct 1;50(19):6184–6191. [PubMed] [Google Scholar]
- Canete-Soler R., Litzky L., Lubensky I., Muschel R. J. Localization of the 92 kd gelatinase mRNA in squamous cell and adenocarcinomas of the lung using in situ hybridization. Am J Pathol. 1994 Mar;144(3):518–527. [PMC free article] [PubMed] [Google Scholar]
- Clark W. H. Tumour progression and the nature of cancer. Br J Cancer. 1991 Oct;64(4):631–644. doi: 10.1038/bjc.1991.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen C. C. Thermal stability of human-fibroblast-collagenase-cleavage products of type-I and type-III collagens. Biochem J. 1987 Nov 1;247(3):725–729. doi: 10.1042/bj2470725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClerck Y. A., Perez N., Shimada H., Boone T. C., Langley K. E., Taylor S. M. Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res. 1992 Feb 1;52(3):701–708. [PubMed] [Google Scholar]
- Fridman R., Fuerst T. R., Bird R. E., Hoyhtya M., Oelkuct M., Kraus S., Komarek D., Liotta L. A., Berman M. L., Stetler-Stevenson W. G. Domain structure of human 72-kDa gelatinase/type IV collagenase. Characterization of proteolytic activity and identification of the tissue inhibitor of metalloproteinase-2 (TIMP-2) binding regions. J Biol Chem. 1992 Aug 5;267(22):15398–15405. [PubMed] [Google Scholar]
- Gehlsen K. R., Argraves W. S., Pierschbacher M. D., Ruoslahti E. Inhibition of in vitro tumor cell invasion by Arg-Gly-Asp-containing synthetic peptides. J Cell Biol. 1988 Mar;106(3):925–930. doi: 10.1083/jcb.106.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Graham C. H., Lala P. K. Mechanisms of placental invasion of the uterus and their control. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):867–874. doi: 10.1139/o92-135. [DOI] [PubMed] [Google Scholar]
- Khokha R., Waterhouse P., Yagel S., Lala P. K., Overall C. M., Norton G., Denhardt D. T. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989 Feb 17;243(4893):947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- Kleiner D. E., Jr, Tuuttila A., Tryggvason K., Stetler-Stevenson W. G. Stability analysis of latent and active 72-kDa type IV collagenase: the role of tissue inhibitor of metalloproteinases-2 (TIMP-2). Biochemistry. 1993 Feb 16;32(6):1583–1592. doi: 10.1021/bi00057a024. [DOI] [PubMed] [Google Scholar]
- Kleiner D. E., Jr, Unsworth E. J., Krutzsch H. C., Stetler-Stevenson W. G. Higher-order complex formation between the 72-kilodalton type IV collagenase and tissue inhibitor of metalloproteinases-2. Biochemistry. 1992 Feb 18;31(6):1665–1672. doi: 10.1021/bi00121a013. [DOI] [PubMed] [Google Scholar]
- Levy A. T., Cioce V., Sobel M. E., Garbisa S., Grigioni W. F., Liotta L. A., Stetler-Stevenson W. G. Increased expression of the Mr 72,000 type IV collagenase in human colonic adenocarcinoma. Cancer Res. 1991 Jan 1;51(1):439–444. [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Hogan B. L. Growth factor-regulated proteases and extracellular matrix remodeling during mammalian development. Curr Top Dev Biol. 1990;24:219–259. doi: 10.1016/s0070-2153(08)60089-7. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Pepper M. S., Möhle-Steinlein U., Risau W., Wagner E. F., Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990 Aug 10;62(3):435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Moses M. A., Sudhalter J., Langer R. Identification of an inhibitor of neovascularization from cartilage. Science. 1990 Jun 15;248(4961):1408–1410. doi: 10.1126/science.1694043. [DOI] [PubMed] [Google Scholar]
- Murphy A. N., Unsworth E. J., Stetler-Stevenson W. G. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J Cell Physiol. 1993 Nov;157(2):351–358. doi: 10.1002/jcp.1041570219. [DOI] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Hembry R. M. Metalloproteinases and cancer invasion and metastasis. Int J Cancer. 1989 Oct 15;44(4):757–760. doi: 10.1002/ijc.2910440434. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ward R., Gavrilovic J., Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992;1:224–230. [PubMed] [Google Scholar]
- Nakajima M., Welch D. R., Belloni P. N., Nicolson G. L. Degradation of basement membrane type IV collagen and lung subendothelial matrix by rat mammary adenocarcinoma cell clones of differing metastatic potentials. Cancer Res. 1987 Sep 15;47(18):4869–4876. [PubMed] [Google Scholar]
- Ohnishi T., Arita N., Hayakawa T., Izumoto S., Taki T., Yamamoto H. Motility factor produced by malignant glioma cells: role in tumor invasion. J Neurosurg. 1990 Dec;73(6):881–888. doi: 10.3171/jns.1990.73.6.0881. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Overall C. M., Sodek J. Concanavalin A produces a matrix-degradative phenotype in human fibroblasts. Induction and endogenous activation of collagenase, 72-kDa gelatinase, and Pump-1 is accompanied by the suppression of the tissue inhibitor of matrix metalloproteinases. J Biol Chem. 1990 Dec 5;265(34):21141–21151. [PubMed] [Google Scholar]
- Pepper M. S., Belin D., Montesano R., Orci L., Vassalli J. D. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990 Aug;111(2):743–755. doi: 10.1083/jcb.111.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsom R., Hanby A. M., Pignatelli M., Jeffery R. E., Longcroft J. M., Rogers L., Stamp G. W. Expression of gelatinase A and TIMP-2 mRNAs in desmoplastic fibroblasts in both mammary carcinomas and basal cell carcinomas of the skin. J Clin Pathol. 1993 May;46(5):429–436. doi: 10.1136/jcp.46.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaper H. W., Grant D. S., Stetler-Stevenson W. G., Fridman R., D'Orazi G., Murphy A. N., Bird R. E., Hoythya M., Fuerst T. R., French D. L. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J Cell Physiol. 1993 Aug;156(2):235–246. doi: 10.1002/jcp.1041560204. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Silberman S., Persky B., Bajkowski A. S., Carmichael D. F. Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res. 1988 Oct 1;48(19):5539–5545. [PubMed] [Google Scholar]
- Seftor R. E., Seftor E. A., Gehlsen K. R., Stetler-Stevenson W. G., Brown P. D., Ruoslahti E., Hendrix M. J. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor R. E., Seftor E. A., Stetler-Stevenson W. G., Hendrix M. J. The 72 kDa type IV collagenase is modulated via differential expression of alpha v beta 3 and alpha 5 beta 1 integrins during human melanoma cell invasion. Cancer Res. 1993 Jul 15;53(14):3411–3415. [PubMed] [Google Scholar]
- Seiki M., Sato H., Liotta L. A., Schiffmann E. Comparison of autocrine mechanisms promoting motility in two metastatic cell lines: human melanoma and ras-transfected NIH3T3 cells. Int J Cancer. 1991 Nov 11;49(5):717–720. doi: 10.1002/ijc.2910490515. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Brown P. D., Onisto M., Levy A. T., Liotta L. A. Tissue inhibitor of metalloproteinases-2 (TIMP-2) mRNA expression in tumor cell lines and human tumor tissues. J Biol Chem. 1990 Aug 15;265(23):13933–13938. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Wacher M. P., Margulies I. M., Liotta L. A. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem. 1989 Jan 25;264(3):1353–1356. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Liotta L. A., Kleiner D. E., Jr Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993 Dec;7(15):1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- Strongin A. Y., Marmer B. L., Grant G. A., Goldberg G. I. Plasma membrane-dependent activation of the 72-kDa type IV collagenase is prevented by complex formation with TIMP-2. J Biol Chem. 1993 Jul 5;268(19):14033–14039. [PubMed] [Google Scholar]
- Sympson C. J., Talhouk R. S., Alexander C. M., Chin J. R., Clift S. M., Bissell M. J., Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994 May;125(3):681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa J. E., Quigley J. P. The role of urokinase-type plasminogen activator in aggressive tumor cell behavior. Cancer Metastasis Rev. 1990 Dec;9(4):353–367. doi: 10.1007/BF00049524. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi R., Rifkin D. B. Bimodal relationship between invasion of the amniotic membrane and plasminogen activator activity. Int J Cancer. 1990 Jul 15;46(1):56–60. doi: 10.1002/ijc.2910460112. [DOI] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brûle F. A., Engel J., Stetler-Stevenson W. G., Liu F. T., Sobel M. E., Castronovo V. Genes involved in tumor invasion and metastasis are differentially modulated by estradiol and progestin in human breast-cancer cells. Int J Cancer. 1992 Oct 21;52(4):653–657. doi: 10.1002/ijc.2910520426. [DOI] [PubMed] [Google Scholar]