Abstract
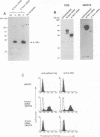
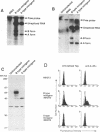
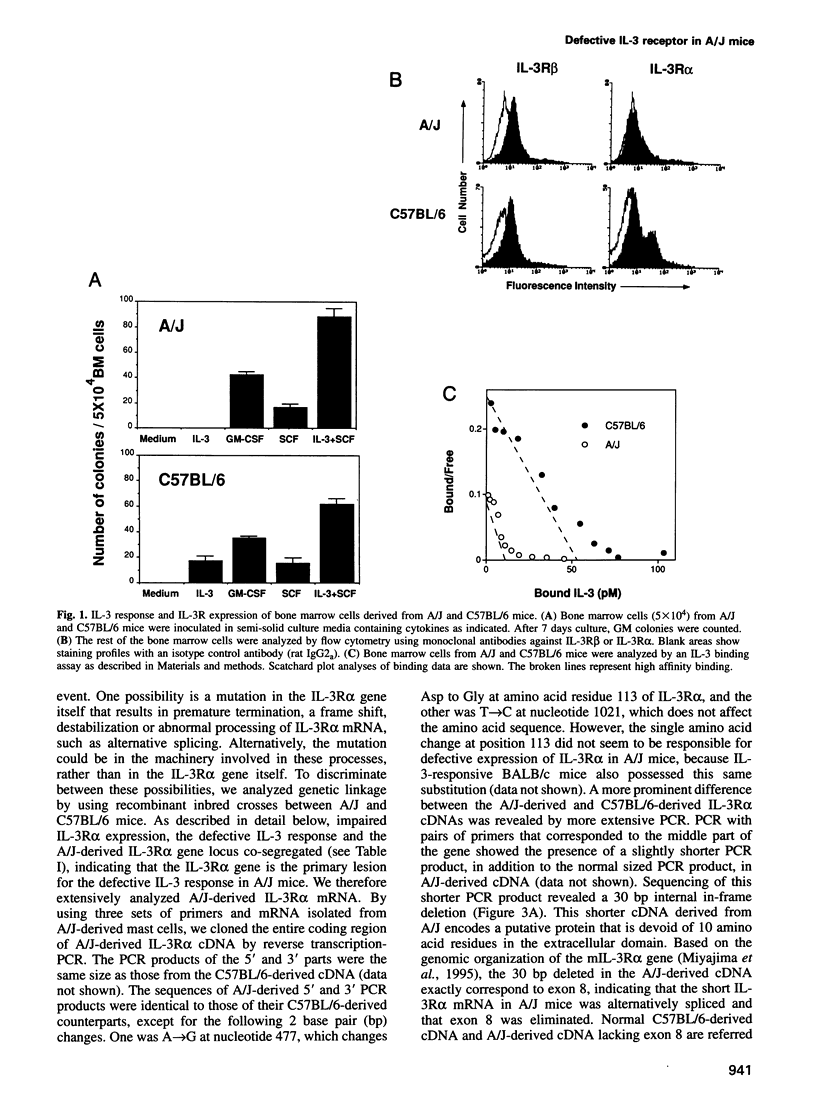
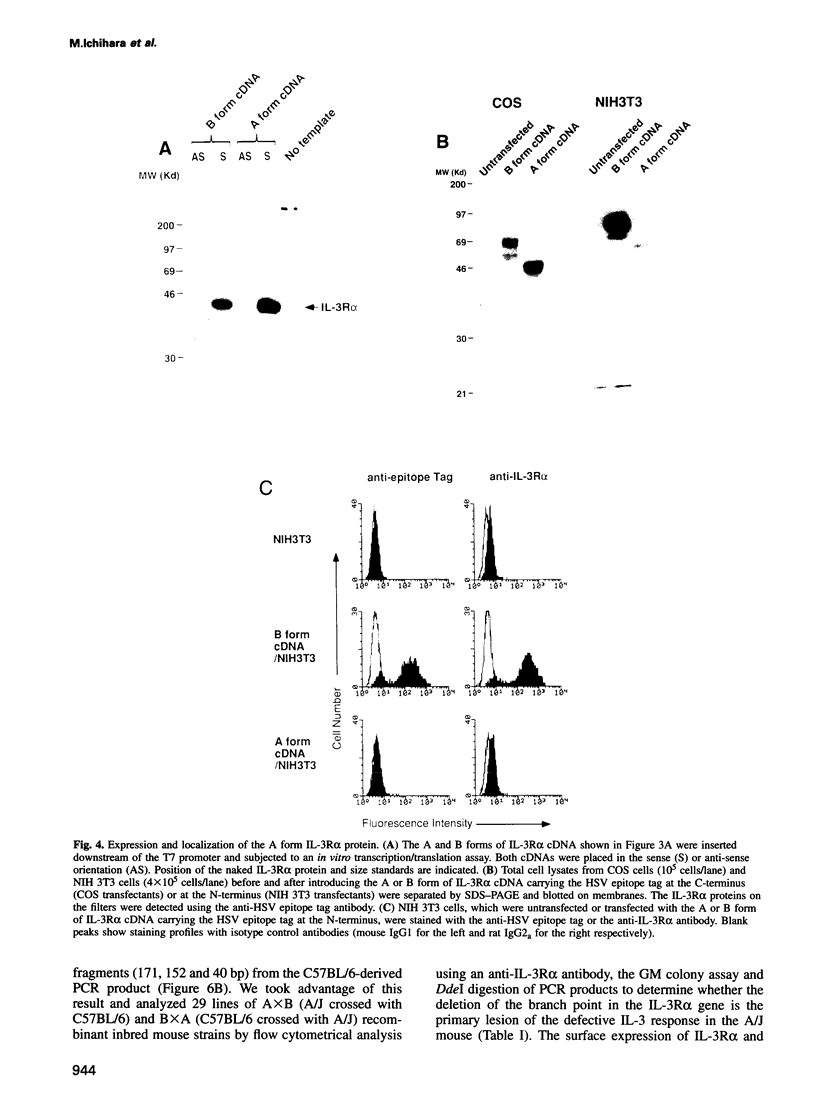
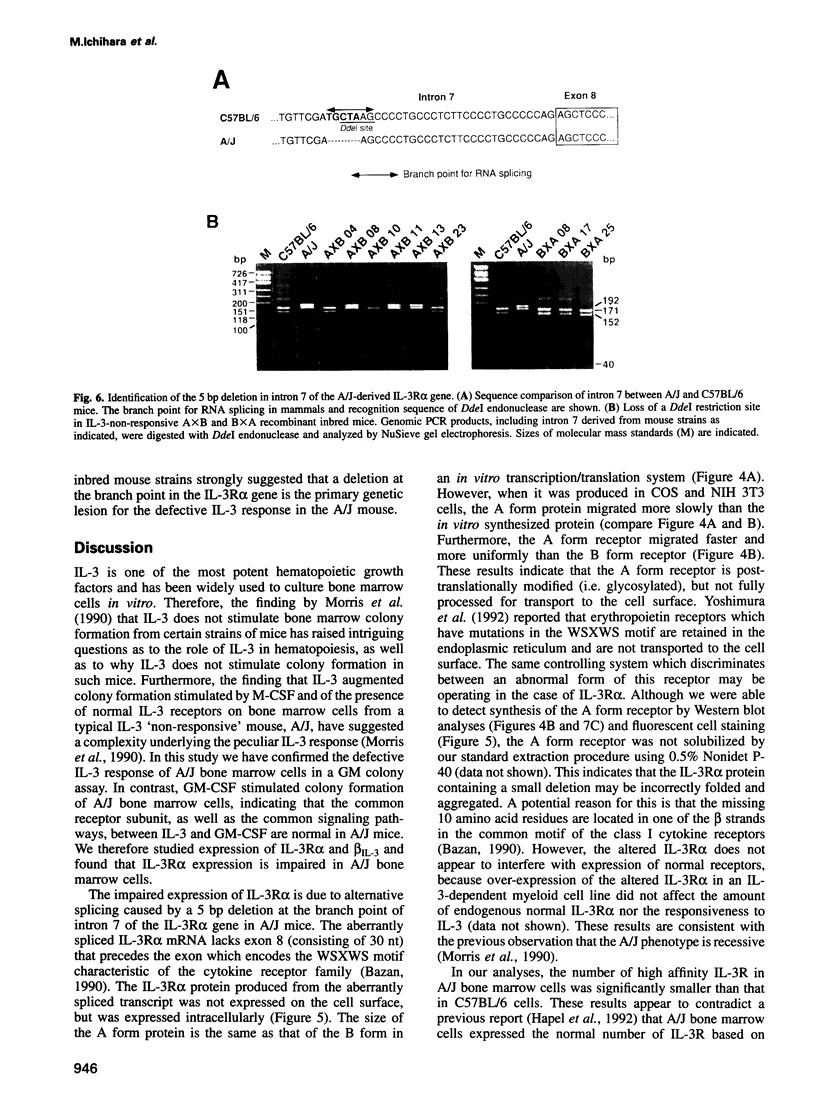
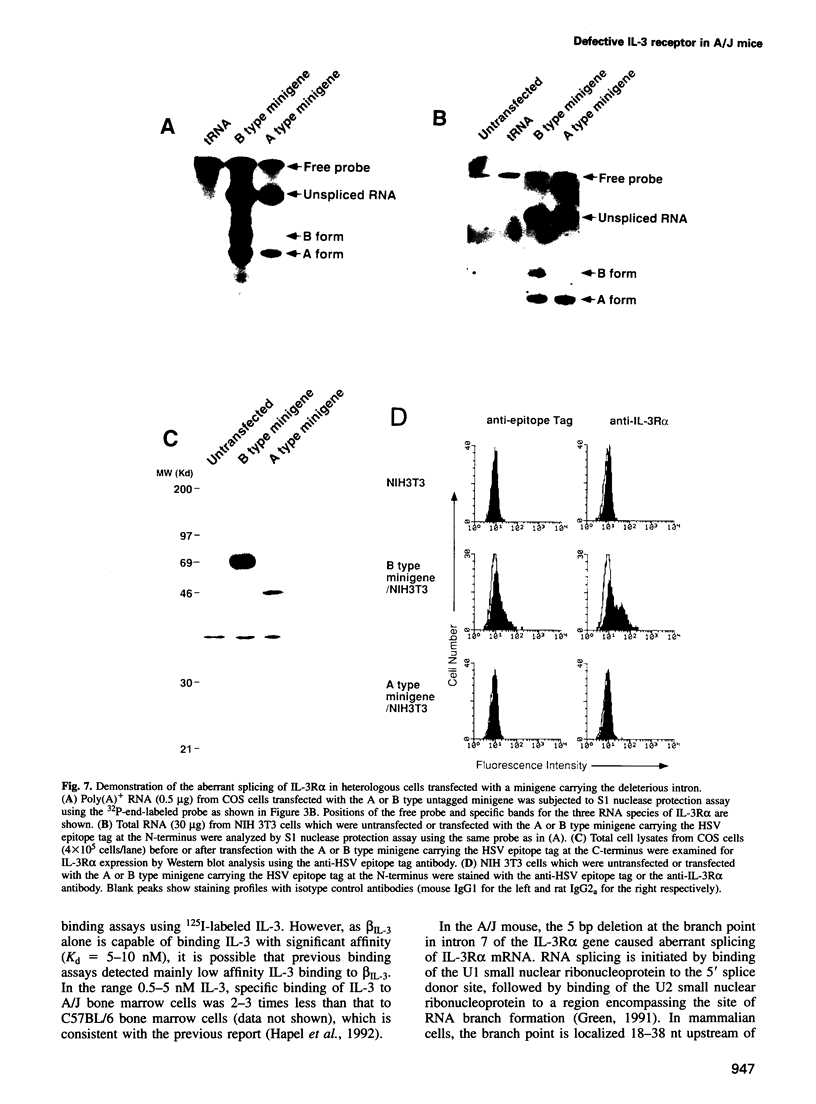
Interleukin-3 (IL-3) alone does not support hematopoietic colony formation of bone marrow cells from the A/J mouse. To elucidate the molecular lesion in A/J mice, we examined expression of the alpha and beta subunits of the IL-3 receptor (IL-3R). While IL-3R beta was normally expressed, IL-3R alpha was not detectable on the surface of A/J-derived cells by antibody staining. Genetic linkage analysis using recombinant inbred mouse strains between A/J and IL-3-responsive C57BL/6 indicated that the IL-3R alpha gene locus was responsible for the impaired IL-3 response in A/J mice. Molecular cloning and characterization of A/J-derived IL-3R alpha cDNA revealed that it lacked the sequence corresponding to exon 8, which encodes 10 amino acid residues in the extracellular domain. The aberrant splicing was due to a 5 base pair deletion at the branch point in intron 7 and was reproduced in heterologous cells by transfecting with an IL-3R alpha minigene carrying the deleterious intron. The A/J-specific abnormal form of IL-3R alpha was localized inside the cells, but not on the cell surface, providing the molecular basis for the impaired IL-3 response in the A/J mouse.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M., Moschonas N., Flavell R. A. Beta + thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981 Dec;27(2 Pt 1):289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colotta F., Bussolino F., Polentarutti N., Guglielmetti A., Sironi M., Bocchietto E., De Rossi M., Mantovani A. Differential expression of the common beta and specific alpha chains of the receptors for GM-CSF, IL-3, and IL-5 in endothelial cells. Exp Cell Res. 1993 Jun;206(2):311–317. doi: 10.1006/excr.1993.1151. [DOI] [PubMed] [Google Scholar]
- Devos R., Plaetinck G., Van der Heyden J., Cornelis S., Vandekerckhove J., Fiers W., Tavernier J. Molecular basis of a high affinity murine interleukin-5 receptor. EMBO J. 1991 Aug;10(8):2133–2137. doi: 10.1002/j.1460-2075.1991.tb07747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G., Crawford A. D., Sadelain M., Ream B., Rashid A., Bronson R. T., Dickersin G. R., Bachurski C. J., Mark E. L., Whitsett J. A. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994 Apr 29;264(5159):713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- Fukumaki Y., Ghosh P. K., Benz E. J., Jr, Reddy V. B., Lebowitz P., Forget B. G., Weissman S. M. Abnormally spliced messenger RNA in erythroid cells from patients with beta+ thalassemia and monkey cells expressing a cloned beta+-thalassemic gene. Cell. 1982 Mar;28(3):585–593. doi: 10.1016/0092-8674(82)90213-6. [DOI] [PubMed] [Google Scholar]
- Gorman D. M., Itoh N., Kitamura T., Schreurs J., Yonehara S., Yahara I., Arai K., Miyajima A. Cloning and expression of a gene encoding an interleukin 3 receptor-like protein: identification of another member of the cytokine receptor gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5459–5463. doi: 10.1073/pnas.87.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Hapel A. J., Fung M. C., Mak N. K., Morris C., Metcalf D., Nicola N. Bone marrow cells from A/J mice do not proliferate in interleukin-3 but express normal numbers of interleukin-3 receptors. Br J Haematol. 1992 Nov;82(3):488–493. doi: 10.1111/j.1365-2141.1992.tb06457.x. [DOI] [PubMed] [Google Scholar]
- Hara T., Miyajima A. Two distinct functional high affinity receptors for mouse interleukin-3 (IL-3). EMBO J. 1992 May;11(5):1875–1884. doi: 10.1002/j.1460-2075.1992.tb05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horland A. A., McMorrow L., Wolman S. R. Growth of granulopoietic bone marrow cells of RF mice. Exp Hematol. 1980 Sep;8(8):1024–1030. [PubMed] [Google Scholar]
- Ichihara M., Hotta T., Asano H., Inoue C., Murate T., Kobayashi M., Saito H. Effects of stem cell factor (SCF) on human marrow neutrophil, neutrophil/macrophage mixed, macrophage and eosinophil progenitor cell growth. Int J Hematol. 1994 Feb;59(2):81–89. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Fernandes G., Moore M. A., Williams N., Good R. A. Abnormalities in clonable B lymphocytes and myeloid progenitors in autoimmune NZB mice. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3464–3468. doi: 10.1073/pnas.76.7.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Hayashida K., Sakamaki K., Yokota T., Arai K., Miyajima A. Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5082–5086. doi: 10.1073/pnas.88.12.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Miyajima A. Functional reconstitution of the human interleukin-3 receptor. Blood. 1992 Jul 1;80(1):84–90. [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Korpelainen E. I., Gamble J. R., Smith W. B., Goodall G. J., Qiyu S., Woodcock J. M., Dottore M., Vadas M. A., Lopez A. F. The receptor for interleukin 3 is selectively induced in human endothelial cells by tumor necrosis factor alpha and potentiates interleukin 8 secretion and neutrophil transmigration. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11137–11141. doi: 10.1073/pnas.90.23.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke G. J., Stanley E., Grail D., Hodgson G., Sinickas V., Gall J. A., Sinclair R. A., Dunn A. R. Mice lacking both macrophage- and granulocyte-macrophage colony-stimulating factor have macrophages and coexistent osteopetrosis and severe lung disease. Blood. 1994 Jul 1;84(1):27–35. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Miyajima A., Mui A. L., Ogorochi T., Sakamaki K. Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood. 1993 Oct 1;82(7):1960–1974. [PubMed] [Google Scholar]
- Miyajima A., Otsu K., Schreurs J., Bond M. W., Abrams J. S., Arai K. Expression of murine and human granulocyte-macrophage colony-stimulating factors in S. cerevisiae: mutagenesis of the potential glycosylation sites. EMBO J. 1986 Jun;5(6):1193–1197. doi: 10.1002/j.1460-2075.1986.tb04346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Schreurs J., Otsu K., Kondo A., Arai K., Maeda S. Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level expression and secretion of biologically active mouse interleukin-3. Gene. 1987;58(2-3):273–281. doi: 10.1016/0378-1119(87)90382-9. [DOI] [PubMed] [Google Scholar]
- Morris C. F., Salisbury J., Kobayashi M., Townsend P. V., Hapel A. J. Interleukin 3 alone does not support the proliferation of bone marrow cells from A/J mice: a novel system for studying the synergistic activities of IL-3. Br J Haematol. 1990 Feb;74(2):131–137. doi: 10.1111/j.1365-2141.1990.tb02555.x. [DOI] [PubMed] [Google Scholar]
- Murata Y., Takaki S., Migita M., Kikuchi Y., Tominaga A., Takatsu K. Molecular cloning and expression of the human interleukin 5 receptor. J Exp Med. 1992 Feb 1;175(2):341–351. doi: 10.1084/jem.175.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogorochi T., Hara T., Wang H. M., Maruyama K., Miyajima A. Monoclonal antibodies specific for low-affinity interleukin-3 (IL-3) binding protein AIC2A: evidence that AIC2A is a component of a high-affinity IL-3 receptor. Blood. 1992 Feb 15;79(4):895–903. [PubMed] [Google Scholar]
- Park L. S., Martin U., Sorensen R., Luhr S., Morrissey P. J., Cosman D., Larsen A. Cloning of the low-affinity murine granulocyte-macrophage colony-stimulating factor receptor and reconstitution of a high-affinity receptor complex. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4295–4299. doi: 10.1073/pnas.89.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Jouet M., Kenwrick S. Aberrant splicing of neural cell adhesion molecule L1 mRNA in a family with X-linked hydrocephalus. Nat Genet. 1992 Oct;2(2):107–112. doi: 10.1038/ng1092-107. [DOI] [PubMed] [Google Scholar]
- Sakamaki K., Miyajima I., Kitamura T., Miyajima A. Critical cytoplasmic domains of the common beta subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992 Oct;11(10):3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Caux C., Kitamura T., Watanabe Y., Arai K., Banchereau J., Miyajima A. Expression and factor-dependent modulation of the interleukin-3 receptor subunits on human hematopoietic cells. Blood. 1993 Aug 1;82(3):752–761. [PubMed] [Google Scholar]
- Shultz L. D., Schweitzer P. A., Rajan T. V., Yi T., Ihle J. N., Matthews R. J., Thomas M. L., Beier D. R. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993 Jul 2;73(7):1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Takaki S., Mita S., Kitamura T., Yonehara S., Yamaguchi N., Tominaga A., Miyajima A., Takatsu K. Identification of the second subunit of the murine interleukin-5 receptor: interleukin-3 receptor-like protein, AIC2B is a component of the high affinity interleukin-5 receptor. EMBO J. 1991 Oct;10(10):2833–2838. doi: 10.1002/j.1460-2075.1991.tb07832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier J., Devos R., Cornelis S., Tuypens T., Van der Heyden J., Fiers W., Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991 Sep 20;66(6):1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- Weiss M., Yokoyama C., Shikama Y., Naugle C., Druker B., Sieff C. A. Human granulocyte-macrophage colony-stimulating factor receptor signal transduction requires the proximal cytoplasmic domains of the alpha and beta subunits. Blood. 1993 Dec 1;82(11):3298–3306. [PubMed] [Google Scholar]
- Yoshimura A., Zimmers T., Neumann D., Longmore G., Yoshimura Y., Lodish H. F. Mutations in the Trp-Ser-X-Trp-Ser motif of the erythropoietin receptor abolish processing, ligand binding, and activation of the receptor. J Biol Chem. 1992 Jun 5;267(16):11619–11625. [PubMed] [Google Scholar]