Abstract
Objective
Acute lung injury (ALI) is common in the intensive care unit (ICU), typically requiring life support ventilation. Survivors often experience anxiety after hospital discharge. We evaluated general anxiety symptoms 3 months after ALI for: (1) associations with patient characteristics and ICU variables, and (2) cross-sectional associations with physical function and quality of life (QOL).
Methods
General anxiety was assessed as part of a prospective cohort study recruiting patients from 13 ICUs at four hospitals in Baltimore, MD using the Hospital Anxiety and Depression Scale — Anxiety Subscale (HAD-A), with associations evaluated using multivariable linear and logistic regression models.
Results
Of 152 patients, 38% had a positive screening test for general anxiety (HAD-A ≥ 8). Pre-ICU body mass index and psychiatric comorbidity were associated with general anxiety (OR, 95% confidence interval (CI): 1.06 (1.00, 1.13) and 3.59 (1.25, 10.30), respectively). No ICU-related variables were associated with general anxiety. General anxiety was associated with the number of instrumental ADL dependencies (Spearman's rho = 0.22; p = 0.004) and worse overall QOL as measured by EQ-5D visual analog scale (VAS) (rho = −0.34; p < 0.001) and utility score (rho = −0.30; p < 0.001), and by the SF-36 mental health domain (rho = −0.70; p < 0.001) and Mental Component Summary score (rho = −0.73; p < 0.001).
Conclusion
Many patients have substantial general anxiety symptoms 3 months after ALI. General anxiety was associated with patient characteristics and impaired physical function and quality of life. Early identification and treatment of general anxiety may enhance physical and emotional function in patients surviving critical illnesses.
Keywords: Acute lung injury, adult, Anxiety, Critical care, Intensive care units, Physical function, Quality of life, Respiratory distress syndrome, adult
Introduction
Critical illnesses, requiring life support therapies such as mechanical ventilation in the intensive care unit (ICU) setting, can have a significant impact on psychological outcomes [1]. In particular, survivors of critical illnesses are at high risk for experiencing anxiety symptoms [2–4]. Up to 50% of ICU survivors experience clinically important general (or non-specific) anxiety symptoms one year after discharge [1,2], which is much higher than the US general population's 18% prevalence of any type of anxiety disorder [5]. Such anxiety symptoms are associated with somatization, as well as impaired psychological functioning and lower health-related quality of life (HRQOL) [3,6–11].
Within the ICU setting, acute lung injury (ALI), and its more severe subset, acute respiratory distress syndrome (ARDS), is an archetypal critical illness [12]. ALI is characterized by acute onset of severe hypoxemia caused by non-cardiogenic pulmonary edema evidenced by bilateral pulmonary infiltrates on chest x-ray [13,14]. A variety of pulmonary (e.g. pneumonia) and non-pulmonary (e.g. pancreatitis) risk factors are associated with onset of ALI [13,14]. Patients with ALI commonly experience severe dyspnea and require mechanical ventilation in the ICU. In-hospital mortality of ALI may be as high as 45% [15,16].
There have been few prior studies of general anxiety symptoms in survivors of critical illnesses, leaving substantial gaps in the extant literature on risk factors, as well as the association between general anxiety symptoms and HRQOL or physical function. Other studies have identified associations between depressive symptoms and physical functioning in ICU survivors [8,17,18]. We hypothesized a priori that several baseline patient characteristics and ICU variables would be associated with general anxiety symptoms, and that general anxiety symptoms would be associated cross-sectionally with HRQOL and physical function. As such, the objectives of this study were to evaluate survivors 3 months after ALI for general anxiety symptoms and associations with: (1) patient characteristics and ICU variables, and (2) physical function and health-related quality of life (HRQOL).
Methods
Study design and patient sample
This evaluation was conducted as part of amulti-site prospective cohort study. A total of 520 participants were recruited from 13 intensive care units (ICUs) in four hospitals in Baltimore, MD. Participants were consecutive, mechanically-ventilated adults with ALI [19] enrolled between October 2004 and October 2007. ICUs specializing in neurological conditions, and ALI patients with primary neurological disease and/or brain trauma were not eligible for enrollment. In addition, the following were key exclusion criteria: (1) >5 days of mechanical ventilation during hospitalization prior to enrollment; (2) pre-existing ALI for >24 h before transfer to a study ICU; (3) pre-existing illness with a life expectancy <6 months; (4) a limitation in use of life support at time of enrollment; (5) prior lung resection; (6) pre-existing cognitive impairment or communication/language barriers, and (7) no fixed address for follow-up purposes. Informed consent for prospective follow-up was obtained in writing fromthe patients once they regained decision-making capacity or from a substitute decision-maker for patients who remained incapable of making medical decisions. Institutional review board approval was obtained from the Johns Hopkins University and all participating study sites.
Assessment of primary outcome: general anxiety symptoms
The primary outcome for this evaluation was the prevalence of general anxiety symptoms 3 months after ALI onset. Outcome assessment was performed by research assistants who were blinded to the exposure variables. General anxiety symptoms were measured with the 7-item anxiety subscale (range 0 to 21, with higher scores indicating worse symptoms) of the Hospital Anxiety and Depression Scale (HAD-A) [20]. The HAD-A is a validated measure commonly used in a wide variety of medical populations, with a score of ≥8 signifying a positive screening test for general anxiety symptoms (i.e., clinically significant symptoms of anxiety) [20–23].
Assessment of patient characteristics
Patient variables were evaluated based on prior studies of psychological outcomes of ICU patients [3,24,25] and a priori hypotheses for other potentially relevant characteristics. Baseline patient characteristics were collected from the medical record and interview with the patient or proxy, and included demographics, educational achievement, body mass index, and relevant comorbidities. Comorbidities were measured with the Charlson index,which is a standardized method of measuring comorbidity burden that assigns weights to the 19 categories of baseline comorbidity evaluated (e.g. a weight of 1 for diabetes, 2 for moderate to severe renal disease, 3 for moderate to severe liver disease) to derive a total comorbidity score [26]. Specific comorbidities were also examined in this study as follows: cardiovascular disease, chronic pulmonary disease, chronic fatigue, HIV/AIDS, psychiatric history (i.e., any record of psychiatric diagnosis and/or treatment), drug/alcohol abuse (i.e., current or prior illicit drug use or excess alcohol use), and at least moderate pain or discomfort.
Assessment of in-ICU variables
Critical illness and ICU hospitalization variables were evaluated based on prior studies of psychological outcomes of ICU patients [18,27–32]. These variables included ICU type (surgical vs. non-surgical); severity of illness at ICU admission (Acute Physiology and Chronic Health Evaluation [APACHE II] score) [33]; maximum daily organ failure in the ICU (Sequential Organ Failure Assessment (SOFA) score) [28–30]; proportion of ICU days with sepsis from microbiologically-proven infection [34]; hospital and ICU length of stay; low blood glucose (mean daily minimum glucose value) [31]; and the mean daily dose and duration of use of selected medications (benzodiazepines [in midazolam-equivalents], opiates [in morphine-equivalents] and systemic corticosteroids [in prednisone-equivalents]).
Correlates of general anxiety symptoms
General anxiety symptoms were evaluated for their cross-sectional association with physical function and HRQOL 3 months after ALI onset. Physical function was evaluated by the number of dependencies in activities of daily living, measured by the Katz Index of Independence in Activities of Daily Living (ADLs) (range 0 to 6, with a higher score indicating more ADL dependencies) and instrumental activities of daily living, measured by the Lawton Instrumental Activities of Daily Living scale (IADLs) (range 0 to 8, with a higher score indicating more IADL dependencies), using validated instruments [35,36]. HRQOL was measured using the EQ-5D [37] and Medical Outcomes Study Short Form-36 version 2 (SF-36) [38] instruments. The EQ-5D provided 2 measures of overall HRQOL used in this evaluation: a visual analog scale (VAS) (range: 0 to 100, with a higher score indicating better HRQOL) and a utility score based on patient responses to 5 items each evaluating a different domain of HRQOL (range: −0.11 to 1.0, with a higher score indicating better HRQOL). The SF-36 has 8 separate domains (4 mental and 4 physical) with 2 overall summary measures of mental and physical health known as the Mental and Physical Component Summary measures. From the SF-36, the Mental and Physical Component Summary measures (norm-based scores, with range 0 to 100, mean = 50, standard deviation = 10, and higher scores indicating better HRQOL) were evaluated in addition to the mental health and physical function domains (not norm-based scores, with range 0 to 100, and higher scores indicating better HRQOL).
Statistical methods
Summary statistics were calculated for the 3-month HAD-A score modeled as both a binary variable (i.e., a positive screening test for general anxiety symptoms (i.e., HAD-A ≥ 8)) and as a continuous variable. Participants were classified according to the binary HAD-A threshold, and the patient- and ICU-related variables were summarized using percentages or median (first quartile, third quartile) within each group. The bivariable association between the continuous and binary measures of general anxiety symptoms and the patient and ICU-related variables was estimated using simple linear and logistic regression models. All patient and ICU-related variables with p < 0.10 in the bivariable analyses, plus all comorbidities regardless of bivariable association, were then analyzed in multivariable linear and logistic regression models. Standard model checking procedures were used for multivariable models, including evaluation of residuals (including normal probability plots for linear regression, DFBeta, and DFFit plots) [39]. Scatterplots and loess curves [40]were generated to visualize the cross-sectional association between the 3-month general anxiety symptoms and physical function and quality of life measures, and Spearman rank correlation coefficients were calculated. All analyses were completed on the available data. Exploratory analyses were conducted to compare the patient and ICU-exposures for participants with and without HAD-A data, revealing no statistically significant or clinically relevant differences between the two groups. All p-values were two-sided with statistical significance defined as p < 0.05. All analyses were performed using R statistical software [41].
Results
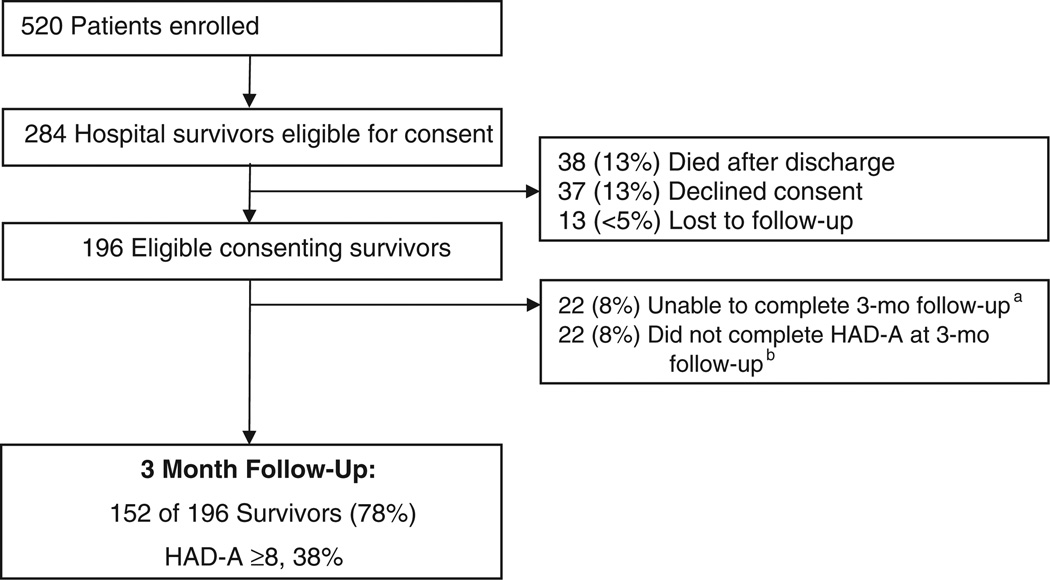
Of the 520 patients with ALI enrolled in the study, 284 (55%) survived to hospital discharge. Among those 284 hospital survivors, prior to 3 month follow-up, an additional 38 (13%) died, 37 (13%) declined consent, and 13 (<5%)were lost to follow-up. Of the eligible consenting survivors, 22 (8%) were unable to complete a 3-month follow-up visit, and 22 (8%) had a visit without completing the HAD-A survey, leaving 152 patients with complete HAD-A data at 3 months (Fig. 1). We compared the patient- and ICU-related variables among the 152 participants that completed the 3-month follow-up and the 44 who were eligible but did not complete either the 3-month follow-up or the HAD-A at their 3-month visit. With the exception of BMI, there were no statistically or clinically significant differences between the patient and ICU exposures in these two groups. Although BMI was statistically significantly different between groups (p < .05), the median BMI in the two groups was not clinically relevant. Of these 152 patients, the median (inter-quartile range (IQR)) age was 49 [40,57] with 59% male and 61% Caucasian (Table 1).
Fig. 1.
Flow diagram of study participants. Abbreviations: HAD-A, Hospital Anxiety and Depression Scale — Anxiety Subscale. a Patients were unable to complete a 3-month follow-up visit for the following reasons: unable to locate participant (n = 14), participant declined visit (n = 4), cognitively or physically incapable (n = 2), and other reasons (n = 2). b Some patients had a follow-up visit, but did not have complete HAD-A data for the following reasons: physically incapable (n = 9), cognitively incapable (n = 5) and other reasons (n = 8).
Table 1.
Patient characteristics, by HAD-A score at 3 months after acute lung injury
| Variable | All patientsa n = 152 |
HAD-A scorea | |
|---|---|---|---|
| Positive (HAD-A ≥ 8) n = 58 |
Negative (HAD-A < 8) n = 94 |
||
| Patient variables | |||
| Age | 49 (40, 57) | 51 (43, 57) | 46 (38, 58) |
| Male | 89 (59%) | 35 (60%) | 54 (57%) |
| Caucasian | 92 (61%) | 37 (64%) | 55 (59%) |
| Surgical ICU | 20 (13%) | 10 (17%) | 10 (11%) |
| BMI, kg/m2 | 28 (23, 33) | 29 (25, 34) | 27 (23, 32) |
| More than high school education | 57 (39%) | 17 (32%) | 40 (44%) |
| Employed | 60 (39%) | 20 (35%) | 40 (43%) |
| Charlson comorbidity index | 1 (0, 3) | 2 (1,5) | 1 (0, 2) |
| Specific comorbidities: | |||
| Cardiovascular disease | 26 (17%) | 9 (16%) | 17 (18%) |
| Chronic pulmonary disease | 65 (43%) | 32 (55%) | 33 (35%) |
| Chronic fatigue | 52 (34%) | 19 (33%) | 33 (36%) |
| HIV/AIDS | 24 (16%) | 13 (22%) | 11 (12%) |
| Psychiatric history | 38 (25%) | 25 (43%) | 13 (14%) |
| Drug/Alcohol abuse | 68 (45%) | 33 (57%) | 35 (37%) |
| Pain | 103 (82%) | 42 (93%) | 61 (76%) |
| ICU-related variables | |||
| APACHE II severity of illness | 23 (19, 28) | 24 (19, 28) | 23 (19, 28) |
| Maximum daily SOFA organ failure score | 9 (7, 11) | 9 (7, 11) | 9 (7, 11) |
| Proportion of ICU days with sepsis | 75 (6, 96) | 78 (33, 96) | 68 (0, 96) |
| Length of hospital stay, days | 26 (16, 36) | 29 (17, 38) | 25 (16, 34) |
| Length of ICU stay, days | 15 (9, 23) | 16 (10, 25) | 14 (9, 21) |
| Mean daily minimum glucose < 100 mg/dL | 59 (39%) | 20 (35%) | 39 (42%) |
| Mean daily benzodiazepine dose (midazolam-equivalent), mg | 24 (3, 71) | 23 (3, 71) | 24 (3, 73) |
| Total days of benzodiazepine in ICU | 9 (4, 13) | 8 (4, 13) | 9 (4, 12) |
| Mean daily corticosteroid dose (prednisone-equivalent), mg | 5 (0, 31) | 6 (0, 20) | 4 (0, 33) |
| Total days of steroids in ICU | 1 (0, 7) | 1 (0, 8) | 1 (0, 7) |
| Mean daily opiate dose (morphine-equivalent), mg | 104 (44, 204) | 105 (36, 186) | 103 (50, 209) |
| Total days of opiate in ICU | 10 (6, 15) | 10 (6, 17) | 10 (6, 14) |
HAD-A, Hospital Anxiety and Depression Scale — Anxiety Subscale; ICU, intensive care unit; BMI, body mass index; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment.
Data presented as number (proportion) or median (inter-quartile range). Proportions may not add to 100% due to rounding.
At 3-month follow-up, 38% (58/152) of patients had a positive screening test for general anxiety symptoms (HAD-A ≥ 8) with a median (IQR) HAD-A score of 6 (3, 9). In simple logistic regression analyses of all patient- and ICU-related variables with a positive screening test for general anxiety symptoms (HAD-A ≥ 8), the following were associated at p < 0.10: body mass index, and both medical (e.g. Charlson index, chronic pulmonary disease, HIV/AIDS, pain) and psychiatric comorbidities (e.g. psychiatric history, drug/alcohol abuse) (Table 2). Associations of patient- and ICU-related variables with general anxiety symptoms were similar when general anxiety symptoms were modeled as a continuous variable in simple linear regression models (Table 3).
Table 2.
Associations with positive anxiety screening test at 3 months after acute lung injury
| Variable | Odds ratio (95% CI) for positive anxiety screening test (HAD-A ≥ 8) | |||
|---|---|---|---|---|
| Bivariable | p valuea | Multivariable | p valuea | |
| Patient variables | ||||
| Age | 1.01 (0.99, 1.04) | 0.249 | ||
| Male | 1.13 (0.57, 2.22) | 0.725 | ||
| Caucasian | 1.25 (0.63, 2.49) | 0.518 | ||
| Surgical ICU | 1.75 (0.67, 4.59) | 0.246 | ||
| BMI, kg/m2 | 1.06 (1.01, 1.11) | 0.018 | 1.06 (1.00, 1.13) | 0.052 |
| More than high school education | 0.59 (0.28, 1.21) | 0.139 | ||
| Employed | 0.71 (0.36, 1.42) | 0.324 | ||
| Charlson comorbidity index | 1.15 (1.00, 1.32) | 0.040 | 1.28 (1.00, 1.64) | 0.048 |
| Specific comorbidities | ||||
| Cardiovascular disease | 0.83 (0.34, 2.05) | 0.683 | 0.26 (0.06, 1.10) | 0.061 |
| Chronic pulmonary disease | 2.28 (1.15, 4.50) | 0.016 | 3.41 (1.22, 9.57) | 0.017 |
| Chronic fatigue | 0.89 (0.44, 1.82) | 0.752 | 0.50 (0.18, 1.38) | 0.171 |
| HIV/AIDS | 2.18 (0.89, 5.36) | 0.083 | 1.43 (0.30, 6.75) | 0.643 |
| Psychiatric history | 4.72 (2.12, 10.49) | <0.001 | 3.59 (1.25, 10.30) | 0.015 |
| Drug/Alcohol abuse | 2.23 (1.13, 4.40) | 0.019 | 1.87 (0.71, 4.92) | 0.198 |
| Pain | 4.36 (1.18, 16.09) | 0.024 | 4.81 (0.89, 26.06) | 0.063 |
| ICU-related variables | ||||
| APACHE II severity of illness | 1.01 (0.97, 1.05) | 0.670 | ||
| Maximum daily SOFA organ failure score | 1.01 (0.91, 1.12) | 0.825 | ||
| Maximum daily SOFA > 10 | 0.98 (0.47, 2.03) | 0.950 | ||
| % of ICU days with sepsis per 10% increase | 1.05 (0.97, 1.14) | 0.240 | ||
| Length of ICU stay, days | 1.01 (0.99, 1.03) | 0.333 | ||
| Length of hospital stay, days | 1.00 (0.99, 1.02) | 0.590 | ||
| Mean daily minimum glucose, mg/dL | 1.00 (0.98, 1.02) | 0.768 | ||
| Mean daily minimum glucose < 100 mg/dL | 0.74 (0.37, 1.48) | 0.390 | ||
| Mean daily midazolam equivalent dose per 25 mg | 1.01 (0.92, 1.10) | 0.885 | ||
| Total days of benzodiazepine in ICU | 1.01 (0.97, 1.05) | 0.638 | ||
| Total dose of midazolam equivalent per 75 mg | 1.00 (0.99, 1.01) | 0.827 | ||
| Mean daily midazolam equivalent dose > 100 mg | 1.06 (0.45, 2.50) | 0.892 | ||
| Mean daily prednisone equivalent dose per 40 mg | 0.83 (0.63, 1.09) | 0.177 | ||
| Total days of steroids in ICU | 0.99 (0.95, 1.03) | 0.659 | ||
| Total dose of prednisone equivalent per 40 mg | 0.99 (0.97, 1.01) | 0.232 | ||
| Mean daily morphine equivalent dose per 10 mg | 0.99 (0.98, 1.01) | 0.507 | ||
| Mean daily morphine equivalent dose > 100 mg | 0.98 (0.50, 1.92) | 0.961 | ||
CI, confidence interval; HAD-A, Hospital Anxiety and Depression Scale — Anxiety Subscale; ICU, intensive care unit; BMI, body mass index; Max., maximum.
p values calculated using logistic regression. All variables from Table 1 were presented herein and those with a p value ≤ 0.10 in simple logistic regression analysis were analyzed in the multivariable logistic regression model.
Table 3.
Associations with anxiety symptoms at 3 months after acute lung injury
| Variable | Increase in mean HAD-A score (95% CI) | |||
|---|---|---|---|---|
| Bivariable | p valuea | Multivariable | p valuea | |
| Patient variables | ||||
| Age | 0.01 (−0.04, 0.06) | 0.790 | ||
| Male | −0.19 (−1.60, 1.23) | 0.792 | ||
| Caucasian | 1.09 (−0.33, 2.50) | 0.127 | ||
| Surgical ICU | 0.20 (−1.86, 2.27) | 0.844 | ||
| BMI, kg/m2 | 0.12 (0.04, 0.21) | 0.006 | 0.15 (0.06, 0.25) | 0.002 |
| More than high school education | −1.08 (−2.54, 0.38) | 0.140 | ||
| Employed | −1.28 (−2.69, 0.13) | 0.072 | −0.64 (−2.14, 0.85) | 0.392 |
| Charlson comorbidity index | 0.33 (0.06, 0.61) | 0.015 | 0.33 (−0.05, 0.70) | 0.082 |
| Specific comorbidities: | ||||
| Cardiovascular disease | −0.22 (−2.08, 1.63) | 0.810 | −2.1 (−4.22, 0.02) | 0.050 |
| Chronic pulmonary disease | 1.71 (0.32, 3.09) | 0.015 | 1.89 (0.31, 3.47) | 0.018 |
| Chronic fatigue | −0.21 (−1.70, 1.27) | 0.774 | −1.23 (−2.81, 0.35) | 0.121 |
| HIV/AIDS | 1.86 (−0.03, 3.75) | 0.050 | 1.33 (−1.15, 3.81) | 0.285 |
| Psychiatric history | 3.42 (1.91, 4.93) | <0.001 | 2.33 (0.71, 3.95) | 0.005 |
| Drug/Alcohol abuse | 1.54 (0.16, 2.92) | 0.027 | 1.01 (−0.52, 2.53) | 0.189 |
| Pain | 2.90 (0.93, 4.88) | 0.004 | 1.65 (−0.25, 3.54) | 0.085 |
| ICU-related variables | ||||
| APACHE II severity of illness | 0.04 (−0.04, 0.13) | 0.317 | ||
| Maximum daily SOFA organ failure score | −0.01 (−0.22, 0.20) | 0.915 | ||
| Maximum daily SOFA > 10 | 0.44 (−1.09, 1.97) | 0.565 | ||
| % of ICU days with sepsis, per 10% increase | 0.13 (−0.04, 0.30) | 0.137 | ||
| Length of ICU stay, days | 0.05 (0.00, 0.09) | 0.041 | 0.02 (−0.03, 0.07) | 0.470 |
| Length of hospital stay, days | 0.02 (−0.01, 0.06) | 0.210 | ||
| Mean daily minimum glucose, mg/dL | 0.01 (−0.03, 0.06) | 0.480 | ||
| Mean daily minimum glucose <100 mg/dL | −0.73 (−2.15, 0.70) | 0.310 | ||
| Mean daily midazolam equivalent dose, per 25 mg | 0.11 (−0.08, 0.30) | 0.235 | ||
| Total days of benzodiazepine in ICU | 0.05 (−0.02, 0.13) | 0.175 | ||
| Total dose of midazolam equivalent, per 75 mg | 0.01 (−0.01, 0.03) | 0.225 | ||
| Mean daily midazolam equivalent dose > 100 mg | 0.95 (−0.84, 2.74) | 0.290 | ||
| Mean daily prednisone equivalent dose, per 40 mg | −0.29 (−0.64, 0.05) | 0.094 | −0.22 (−0.57, 0.12) | 0.202 |
| Total days of corticosteroids in ICU | −0.03 (−0.11, 0.05) | 0.524 | ||
| Total dose of prednisone equivalent, per 40 mg | −0.02 (−0.04, 0.01) | 0.122 | ||
| Maximum daily prednisone equivalent dose > 70 mg | −0.93 (−2.47, 0.61) | 0.230 | ||
| Mean daily corticosteroid dose ≥ 40 mg | −1.40 (−3.14, 0.34) | 0.109 | ||
| Mean daily morphine equivalent dose > 100 mg | −0.15 (−1.55, 1.25) | 0.831 | ||
CI, confidence interval; HAD-A, Hospital Anxiety and Depression Scale — Anxiety Subscale; ICU, intensive care unit; BMI, body mass index.
p values calculated using linear regression. All variables from Table 1 were presented herein and those with a p value ≤ 0.10 in simple linear regression analysis were analyzed in the multivariable linear regression model.
Multivariable logistic regression models demonstrated that the following variables were independently associated (odds ratio (OR) (95% confidence interval (CI))) with a positive screening test for general anxiety symptoms (HAD-A ≥ 8) (Table 2): pre-ICU body mass index (1.06 (1.00, 1.13) per unit increase in BMI), Charlson index (1.28 (1.00, 1.64)), cardiovascular disease (0.26 (0.06, 1.10)), chronic pulmonary disease (3.41 (1.22, 9.57)), psychiatric comorbidity (3.59 (1.25, 10.30)), and pain (4.81 (0.89, 26.06)). Several of these variables also were associated with general anxiety symptoms measured as a continuous variable in multivariable linear regression models (Table 3) including: higher pre-ICU body mass index (0.15 (95% CI: 0.06, 0.25) increase in the HAD-A score for each 1 unit increase in body mass index); Charlson index (0.33 (−0.05, 0.70)); cardiovascular disease (−2.1 (−4.22, 0.02)); chronic pulmonary disease (1.89 (0.31, 3.47)); psychiatric history (2.33 (0.71, 3.95)); and pain (1.65 (−0.25, 3.54)). None of the other patient- and ICU-related variables was associated with a positive screening test for general anxiety symptoms (HAD-A ≥ 8) or the continuous HAD-A score in these multivariable regression analyses.
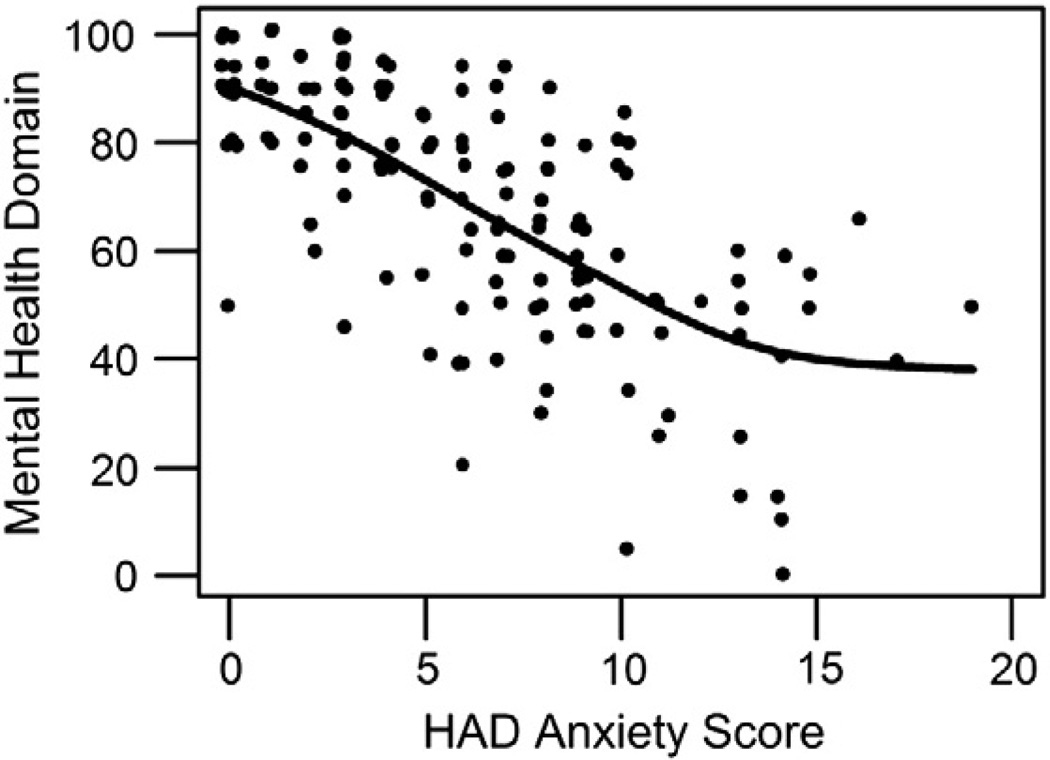
Greater general anxiety symptoms were significantly associated with worse overall HRQOL, as measured by the EQ-5D VAS (Spearman's rho = −0.34; p < 0.001) and utility score (rho = −0.30; p < 0.001), and by the SF-36 mental health domain (rho = −0.70; p < 0.001; Fig. 2) and Mental Component Summary score (rho = −0.73; p < 0.001). Associations were weaker, but statistically significant, for physical aspects of HRQOL as measured by the SF-36 physical function domain (rho = −0.26; p < 0.001) and Physical Component Summary score (rho = −0.18; p = 0.009). Greater general anxiety symptoms were cross-sectionally associated with impaired physical function, as measured by the number of IADL dependencies (rho = 0.22; p = 0.004). There was not a statistically significant association between general anxiety symptoms and number of ADL dependencies (r = 0.11; p = 0.124).
Fig. 2.
SF-36 mental health domain and anxiety symptoms at 3 months after acute lung injury. Mental health domain, SF36 Short-Form Health Survey Mental Health Domain; HAD anxiety score, Hospital Anxiety and Depression Scale – Anxiety Subscale.
Discussion
This multi-site study of 152 survivors of ALI demonstrated that 38% had a positive screening test for general anxiety symptoms at 3-month follow-up. General anxiety symptoms were cross-sectionally associated with worse physical function and HRQOL. Bivariable analyses suggested that several patient variables were associated with increased general anxiety symptoms. Multivariable regression analyses demonstrated significant associations of general anxiety symptoms for higher body mass index, specific medical comorbidities, and psychiatric comorbidity. Of 10 potentially relevant ICU variables evaluated, none was independently associated with general anxiety symptoms.
The 38% prevalence of ALI survivors having a positive screening test for general anxiety symptoms at 3 month follow-up is much higher than the 18% 12-month prevalence of any anxiety disorder in the United States general population [5] and the 21% point prevalence of a HAD-A score ≥8 in a large non-clinical sample of adults [42]. Several other studies also have identified a high prevalence of general anxiety symptoms among survivors of critical illness [3,4,43,44]. Using the Beck Anxiety Inventory (BAI), Mikkelson and colleagues identified a very high prevalence of moderate or severe general anxiety symptoms (62%) among 102 ALI survivors at 12-month follow-up [43]. In a prospective cohort of 62 ALI survivors that also used the BAI, 23% experienced moderate to severe symptoms of general anxiety (BAI ≥ 16) at two years after hospital discharge [3]. Authors of another study of 200 patients recruited from 35 academic, teaching, and community hospitals across the United States, found that approximately half of ALI survivors experienced substantial depression, general anxiety, or insomnia symptoms on a Quality of Well-Being subscale at 6 and 12 months after hospitalization [44]. Hence, the prevalence of general anxiety symptoms in our cohort is comparable with prior research.
Our prospective study revealed that greater general anxiety symptoms following ALI were cross-sectionally associated with impaired physical function and HRQOL. Other studies have revealed similar associations between ALI and HRQOL [4,7,8,11,45], but to our knowledge, none has investigated associations with activities of daily living as done in our study. However, other studies have identified associations between depressive symptoms and physical functioning in ICU survivors [17,18,46].
Several patient risk factors evaluated in this study have not been evaluated in prior studies including: body mass index, specific comorbidities, and pain/discomfort. Other patient risk factors we examined were based on previous studies of psychological outcomes of critically ill patients [3,24,25,47]. Our study was not able to evaluate three patient variables that previously have been associated with symptoms of general anxiety or PTSD: personality traits (e.g. neuroticism), dysfunctional coping strategies, and stressful life events [48–51]. Unlike a prior study of PTSD [52], we did not find that female sex was a risk factor for general anxiety. Our study identified several patient characteristics (body mass index, specific medical comorbidities, and psychiatric history) as independently associated with general anxiety symptoms. Other studies also have identified independent associations between anxiety symptoms and both body mass index [27,31] and psychiatric history [11,24,53] in ALI survivors.
The critical illness and ICU variables we evaluated were based on prior studies of psychological outcomes of ICU patients [18,27–32]. Our study included 10 in-ICU variables which we hypothesized as potentially related to general anxiety symptoms, including severity of illness, the mean daily dose and duration of use of selected medications (benzodiazepines, opioids, and steroids), and hospital and ICU length of stay. Our study did not reveal any significant association between these in-ICU variables and general anxiety symptoms. Conversely, other studies have identified associations between anxiety and benzodiazepine administration, ICU sedation duration, mechanical ventilation duration, dyspnea and ICU length of stay [2,27,54,55].
There may be several reasons our findings differ from prior literature. It is possible that other in-ICU contributors not evaluated in this study could be more closely associated with general anxiety symptoms including dysregulation of the hypothalamic–pituitary–adrenal (HPA) or sympathetic–adrenal–medullary (SAM) axis (i.e., stress response), hypoxia, or perceived level and/or type of social support provided during ICU hospitalization. It is also possible that patient characteristics (e.g. trait anxiety/personality, pre-existing psychopathology, dysfunctional coping strategies) may be more clearly associated with general anxiety symptoms than in-ICU variables. In addition, other ALI studies differed fromours in other important ways. First, the methods for measuring general anxiety symptoms differed. Specifically, other studies used selected questions from a larger general health survey or from a very brief measure (i.e., a visual analog scale) [54,55]. Second, in one study patients were evaluated while still in the ICU [55]. General anxiety symptoms may differ when measured in-hospital versus at 3-months following hospitalization given variability in general anxiety symptoms over time [56] or treatment interventions provided after hospital discharge.
Clinical investigators have recently begun to assess whether early psychological intervention may prevent development of anxiety symptoms, including post-ICU posttraumatic stress disorder symptoms, among survivors of critical illness. A pre–post study of a non-pharmacological psychological intervention in an Italian trauma ICU suggested lower rates of general anxiety symptoms (8.9% vs 17.4%, p = 0.087) among the intervention vs control group at 12-month follow-up [57]. A randomized controlled trial of a music therapy intervention administered during the ICU stay also suggested decreased general anxiety symptoms in the intervention group (p < 0.001) [58].Other studies of music interventions in-ICU indicate that these interventions are anxiolytic [59–62]. Supportive interventions provided in-ICU (e.g. supportive nurse- and physician–patient interactions) [63] and relaxation strategies (e.g. guided imagery) also appear anxiolytic during an ICU stay, but post-hospital follow-up data are lacking from these studies [60,64,65]. Because in-ICU psychological distress may be a risk factor for later general anxiety symptoms, early intervention may be important for improving outcomes after critical illness [66]. Each of these studies suggests that a non-drug intervention in the ICU is feasible and may be effective in reducing general anxiety symptoms in the ICU [58–61].
Our study had several strengths. It is one of only a few studies evaluating general anxiety symptoms following ICU admission in survivors of critical illness [2–4]. We used a well-established measure that has been validated for assessment of general anxiety symptoms in medical patients. In addition, we identified cross-sectional associations between a positive screening test for general anxiety symptoms and important outcomes, including physical function and quality of life.
Our study also has potential limitations. First, we did not adjust for potentially confounding variables in the correlations described and given our cross-sectional design; we could not explore longitudinal relationships between general anxiety symptoms and physical function or quality of life. Hence, we are unable to determine the mechanisms underlying the relationships described nor the direction of association between general anxiety symptoms and these other outcomes (e.g. impaired physical function leading to general anxiety symptoms or vice versa). Additional research is needed to understand causal associations between general anxiety symptoms and impaired physical function and HRQOL. Second, we are unable to specify any specific psychiatric diagnosis associated with the general anxiety symptoms because a general screening instrument was used rather than a clinical diagnostic interview (e.g. the Structured Clinical Interview for DSM-IV Axis I Disorders). Given the anticipated added burden to participants when they were in the early phases of physically and emotionally recovering from ALI, we believed that a clinical diagnostic interview would not have been feasible and would have resulted in greater loss to follow-up and incomplete data collection. In addition, a clinical diagnostic interview administered in-person by a trained clinician would have been logistically challenging given that 48% of all 3-month follow-up assessments were done outside of the hospital's research clinic (e.g. in patients' homes or long-term care facilities). Third, we also obtained information on prior psychiatric illness and alcohol/drug use/abuse from medical records review rather than gathering this information directly from the patient or proxy. Therefore, we may have underestimated the true impact of these baseline conditions as potential risk factors for post-ALI general anxiety. Fourth, we did not account for possible treatment of anxiety between ALI onset and 3-month follow-up. Hence, our prevalence data may underestimate the true burden of general anxiety symptoms if patients experienced symptoms that resolved with treatment before 3-month follow-up. Lastly, in exploring patient- and ICU-related variables' associations with general anxiety symptoms, we were limited to those included in this prospective cohort study. Additional variables (e.g. personality traits, dysfunctional coping strategies, and stressful life events) should be explored in future studies.
In conclusion, many patients surviving ALI experience substantial general anxiety symptoms at 3-month follow-up. General anxiety symptoms are cross-sectionally associated with impaired physical function and lower quality of life. Although we did find an association between specific patient variables (i.e., body mass index, specific medical comorbidities, and psychiatric history) and general anxiety symptoms, we did not find any association between in-ICU variables and general anxiety symptoms. Early identification of patients with general anxiety symptoms and providing appropriate interventions may be important to enhance ICU survivors' physical and emotional function, as well as quality of life.
Acknowledgments
This research was supported by the National Institutes of Health (Acute Lung Injury SCCOR Grant # P050 HL 73994 and UL1 TR 000424-06), and by the Johns Hopkins Institute for Clinical and Translational Research (ICTR)). The funding body had no role in the study design, manuscript writing or decision to submit the manuscript for publication. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the funding bodies.
Footnotes
Conflict of interest statement
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare that the authors have no competing interests to report.
References
- 1.Bergbom-Engberg I, Haljamae H. Assessment of patients' experience of discomforts during respirator therapy. Crit Care Med. 1989;17:1068–1072. doi: 10.1097/00003246-198910000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70:512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 4.Orme J, Romney JS, Hopkins RO, Pope D, Chan KJ, Thomsen G, et al. Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2003;167:690–694. doi: 10.1164/rccm.200206-542OC. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelluri L, Grenvik A, Silverman M. Intensive care for critically ill elderly: mortality, costs, and quality of life. Review of the literature. Arch Intern Med. 1995;155:1013–1022. [PubMed] [Google Scholar]
- 7.Schelling G, Stoll C, Haller M, Briegel J, Manert W, Hummel T, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med. 1998;26:651–659. doi: 10.1097/00003246-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Weinert CR, Gross CR, Kangas JR, Bury CL, Marinelli WA. Health-related quality of life after acute lung injury. Am J Respir Crit Care Med. 1997;156:1120–1128. doi: 10.1164/ajrccm.156.4.9611047. [DOI] [PubMed] [Google Scholar]
- 9.Dowdy DW, Eid MP, Sedrakyan A, Mendez-Tellez PA, Pronovost PJ, Herridge MS, et al. Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med. 2005;31:611–620. doi: 10.1007/s00134-005-2592-6. [DOI] [PubMed] [Google Scholar]
- 10.Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, et al. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32:1115–1124. doi: 10.1007/s00134-006-0217-3. [DOI] [PubMed] [Google Scholar]
- 11.Kapfhammer HP, Rothenhausler HB, Krauseneck T, Stoll C, Schelling G. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004;161:45–52. doi: 10.1176/appi.ajp.161.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Herridge MS, Angus DC. Acute lung injury — affecting many lives. N Engl J Med. 2005;353:1736–1738. doi: 10.1056/NEJMe058205. [DOI] [PubMed] [Google Scholar]
- 13.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967:319–323. [Google Scholar]
- 14.Fan E, Needham DM, Stewart TE. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA. 2005;294:2889–2896. doi: 10.1001/jama.294.22.2889. [DOI] [PubMed] [Google Scholar]
- 15.Needham DM, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Sevransky JE, Dennison Himmelfarb CR, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 17.Sukantarat K, Greer S, Brett S, Williamson R. Physical and psychological sequelae of critical illness. Br J Health Psychol. 2007;12:65–74. doi: 10.1348/135910706X94096. [DOI] [PubMed] [Google Scholar]
- 18.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, et al. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012;185:517–524. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American–European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 22.Spinhoven PH, Ormel J, Sloekers PPA, Kempen GIJM, Speckens AEM, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27:363–370. doi: 10.1017/s0033291796004382. [DOI] [PubMed] [Google Scholar]
- 23.Sukantarat KT, Williamson RCN, Brett SJ. Psychological assessment of ICU survivors: a comparison between the Hospital Anxiety and Depression Scale and the Depression, Anxiety and Stress Scale. Anaesthesia. 2007;62:239–243. doi: 10.1111/j.1365-2044.2006.04948.x. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins RO, Key CW, Suchyta MR, Weaver LK, Orme JF. Risk factors for depression and anxiety in survivors of acute respiratory distress syndrome. Gen Hosp Psychiatry. 2010;32:147–155. doi: 10.1016/j.genhosppsych.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Rattray JE, Johnston M, Wildsmith JAW. Predictors of emotional outcomes of intensive care. Anaesthesia. 2005;60:1085–1092. doi: 10.1111/j.1365-2044.2005.04336.x. [DOI] [PubMed] [Google Scholar]
- 26.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 27.Dowdy DW, Bienvenu OJ, Dinglas VD, Mendez-Tellez PA, Sevransky J, Shanholtz C, et al. Are intensive care factors associated with depressive symptoms 6 months after acute lung injury? Crit Care Med. 2009;37:1702–1707. doi: 10.1097/CCM.0b013e31819fea55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Mendonca A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, et al. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 29.Routsi C, Pratikaki M, Sotiropoulou C, Platsouka E, Markaki V, Paniara O, et al. Application of the sequential organ failure assessment (SOFA) score to bacteremic ICU patients. Infection. 2007;35:240–244. doi: 10.1007/s15010-007-6217-6. [DOI] [PubMed] [Google Scholar]
- 30.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 31.Dowdy DW, Dinglas V, Mendez-Tellez PA, Bienvenu OJ, Sevransky J, Dennison CR, et al. Intensive care unit hypoglycemia predicts depression during early recovery from acute lung injury. Crit Care Med. 2008;36:2726–2733. doi: 10.1097/CCM.0b013e31818781f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Needham DM, Dennison CR, Dowdy DW, Mendez-Tellez PA, Ciesla N, Desai SV, et al. Study protocol: the improving care of acute lung injury patients (ICAP) study. Crit Care. 2006;10:R9. doi: 10.1186/cc3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 34.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 35.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 36.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 37.The EQG. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 38.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. Boston: The Health Institute; 1993. [Google Scholar]
- 39.Belsey DA, Kuh E, Welsch RE. Regression diagnostics: identifying influential data and sources of collinearity. New York: Wiley; 1980. [Google Scholar]
- 40.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- 41.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 42.Crawford J, Henry J, Crombie C, Taylor E. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol. 2001;40:429–434. doi: 10.1348/014466501163904. [DOI] [PubMed] [Google Scholar]
- 43.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson T, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1389–1394. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 45.Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354–360. doi: 10.1001/jama.281.4.354. [DOI] [PubMed] [Google Scholar]
- 46.Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics. 2006;47:399–407. doi: 10.1176/appi.psy.47.5.399. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 48.Blazer D, Hughes D, George LK. Stressful life events and the onset of a generalized anxiety syndrome. Am J Psychiatry. 1987;144:1178–1183. doi: 10.1176/ajp.144.9.1178. [DOI] [PubMed] [Google Scholar]
- 49.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30:421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davydow DS, Zatzick DF, Rivara FP, Jurkovich GJ, Wang J, Roy-Byrne PP, et al. Predictors of posttraumatic stress disorder and return to usual major activity in traumatically injured intensive care unit survivors. Gen Hosp Psychiatry. 2009;31:428–435. doi: 10.1016/j.genhosppsych.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faurbach JA, Lawrence JW, Schmidt CW, Munster AM, Costa PT. Personality predictors of injury-related posttraumatic stress disorder. J Nerv Ment Dis. 2000;188:510–517. doi: 10.1097/00005053-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Girard TD, Shintani AK, Jackson JC, Gordon SM, Pun BT, Henderson MS, et al. Risk factors for post-traumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care. 2007;11:R28. doi: 10.1186/cc5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jubran A, Lawm G, Kelly J, Duffner LA, Gungor G, Collins EG, et al. Depressive disorders during weaning from prolonged mechanical ventilation. Intensive Care Med. 2010;36:828–835. doi: 10.1007/s00134-010-1842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson BJ, Weinert CR, Bury CL, Marinelli WA, Gross CR. Intensive care unit drug use and subsequent quality of life in acute lung injury patients. Crit Care Med. 2000;28:3626–3630. doi: 10.1097/00003246-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt M, Demoule A, Polito A, Porchet R, Aboab J, Siami S, et al. Dyspnea in mechanically ventilated critically ill patients. Crit Care Med. 2011;39:2059–2065. doi: 10.1097/CCM.0b013e31821e8779. [DOI] [PubMed] [Google Scholar]
- 56.Chlan L, Savik K. Patterns of anxiety in critically ill patients receiving mechanical ventilatory support. Nurs Res. 2011;60:S50–S57. doi: 10.1097/NNR.0b013e318216009c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peris A, Bonizzoli M, Iozzelli D, Migliaccio ML, Zagli G, Bacchereti A, et al. Early intra-intensive care unit psychological intervention promotes recovery from post traumatic stress disorders, anxiety and depression symptoms in critically ill patients. Crit Care. 2011;15:R41. doi: 10.1186/cc10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chlan L. Effectiveness of a music therapy intervention on relaxation and anxiety for patients receiving ventilatory assistance. Heart Lung. 1998;27:169–176. doi: 10.1016/s0147-9563(98)90004-8. [DOI] [PubMed] [Google Scholar]
- 59.Evans D. The effectiveness of music as an intervention for hospital patients: a systematic review. J Adv Nurs. 2002;37:8–18. doi: 10.1046/j.1365-2648.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 60.Guzzetta CE. Effects of relaxation and music therapy on patients in a coronary care unit with presumptive acute myocardial infarction. Heart Lung. 1989;18:609–616. [PubMed] [Google Scholar]
- 61.Wong HLC, Lopez-Nahas V, Molassiotis A. Effects of music therapy on anxiety in ventilator-dependent patients. Heart Lung. 2001;30:376–387. doi: 10.1067/mhl.2001.118302. [DOI] [PubMed] [Google Scholar]
- 62.Chlan LL, Weinert CR, Heiderscheit A, Tracy MF, Skaar DJ, Guttormson JL, et al. Effects of patient-directed music intervention on anxiety and sedative exposure in critically ill patients receiving mechanical ventilatory support: a randomized clinical trial. JAMA. 2013:E1–E10. doi: 10.1001/jama.2013.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wunderlich RJ, Perry A, Lavin MA, Katz B. Patients' perceptions of uncertainty and stress during weaning from mechanical ventilation. Dimens Crit Care Nurs. 1999;18:8–12. [PubMed] [Google Scholar]
- 64.Deisch P, Soukup SM, Adams P, Wild MC. Guided imagery: replication study using coronary artery bypass graft patients. Nurs Clin North Am. 2000;35:417–425. [PubMed] [Google Scholar]
- 65.Tusek DL, Cwynar R, Cosgrove DM. Effect of guided imagery on length of stay, pain and anxiety in cardiac surgery patients. J Cardiovasc Manag. 1999;10:22–28. [PubMed] [Google Scholar]
- 66.Papathanassoglou EDE. Psychological support and outcomes for ICU patients. Nurs Crit Care. 2010;15:118–128. doi: 10.1111/j.1478-5153.2009.00383.x. [DOI] [PubMed] [Google Scholar]