Abstract
Nasopharyngeal carcinoma (NPC) has a high metastatic character in the clinic, but its mechanism is not clear. As a carcinogen with organ specificity for the nasopharyngeal epithelium, N,N′-Dinitrosopiperazine (DNP) is involved in NPC metastasis. Herein, our data revealed that anterior gradient 2 (AGR2) was overexpressed in human NPC tissues, particularly in cervical lymph node metastatic NPC (LMNPC). High AGR2 expression was associated with NPC metastasis. Importantly, DNP induced AGR2 expression, and increased cell motility and invasion in the NPC cell line 6–10B. However, DNP-mediated cell motility and invasion was dramatically decreased when transfected with siRNA-AGR2. Further, AGR2 directly regulated cathepsin (CTS) B and D by binding them in vitro. These results indicate that DNP induces AGR2 expression, regulates CTSB and CTSD, increases cell motility and invasion, and promotes NPC tumor metastasis. Therefore, DNP-mediated AGR2 expression may be an important factor in prolific NPC metastasis.
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant cancer in southern China [1]. Epidemiological investigations have revealed that the incidence of NPC has remained high in endemic regions, particularly in Southeast Asia, with an incidence of 30–80 per 100,000 people per year in southern China [2]. In spite of significant advances in early diagnosis, surgical intervention, as well as local and systemic adjuvant therapies, the majority of NPC deaths are attributable to tumor invasion and distant metastases that are resistant to available therapies [3]–[5] .
In endemic NPC, >95% are classified as undifferentiated World Health Organization (WHO) type III and is universally associated with the Epstein–Barr virus (EBV) [6], a strong etiological factor interacting with genetic predisposition [7], and dietary intake of preserved foods [8]. Moreover, in studies on Chinese populations in high-incidence regions, the relative risk of NPC is related to the region’s eating habits, particularly with dietary intake of salt-preserved fish [8]–[15] . The process of salt preservation is inefficient and the food can become partially putrefied. Consequently, these foods accumulate significant levels of nitrosamines [16], [17], which are known carcinogens [16], [18], [19]. N,N′-Dinitrosopiperazine (DNP) is one predominant volatile nitrosamine in salted fish [13], [20]. The carcinogenic potential of DNP in salt-preserved fish is supported by experiments in rats, which develop malignant nasal and NPC [21]–[23]. Furthermore, DNP can induce malignant transformations of human embryonic nasopharyngeal epithelial cells [24].
DNP-induced NPC, is organ specific to the nasopharyngeal epithelium, and is involved in nasopharyngeal tumorigenesis, motility and invasion [25], [26], [57]. Our recent work indicates that DNP induces NPC metastasis, following high expression of anterior gradient 2 (AGR2), cathepsin B (CTSB), and cathepsin D (CTSD) [27]. AGR2 is upregulated in multiple cancers, including breast [28], lung adenocarcinoma [29], [30], ovarian [31], and prostate cancers [32]. Its high expression is associated with tumor metastasis and poor prognosis [33], while silencing AGR2 inhibits cell growth and cell cycle progression, and induces cell death [34]. The mechanism linking AGR2 to tumor invasion and metastasis is involved in several molecular alterations such as CTSB and CTSD [35]. CTSB and CTSD degrade extracellular matrix proteins, and recently they have been implicated in cancer invasion and metastasis. As a lysosomal cysteine endoproteinase, CTSB can degrade laminin, fibronectin, collagen, and other extracellular matrix components, and promote the formation of tumor blood vessels; therefore, it is believed to be one of the key enzymes in invasion and metastasis of malignant tumors. It is upregulated in glioma [36], laryngeal cancer [37], cervical cancer [38], [39], bladder cancer [40], gastrointestinal tract cancer [41]–[43], oral carcinoma [44], and breast cancer [45]–[47], and its expression level is correlated to metastatic potential. CTSD, a lysosomal aspartate proteolytic enzyme, also plays a role in invasion and metastasis of cancer. It is upregulated in some malignant tumor metastases [48], gastric cancer with lymphatic and/or blood vessel invasion [49], breast cancer [50], [51], colorectal cancer [52], [53], liver cancer [54] and pancreatic cancer [55]. Furthermore, Cheng et al [56] observed significant CTSD upregulation in lymph node metastasis versus primary NPC, which was significantly correlated with advanced clinical stage, recurrence, and lymph node and distant metastasis.
To explore the role and possible mechanism of DNP-induced invasion and metastasis, we first detected the expression of AGR2, CTSB, and CTSD in metastatic NPC tissues. We then investigated the mechanism of AGR2 in DNP-induced NPC invasion and metastasis. We found that DNP induces AGR2 expression. AGR2 then binds to CTSB and CTSD, and promotes NPC cell motility and invasion leading to NPC metastasis.
Materials and Methods
Ethics Statement
This project’s experimental designs and protocol were submitted to the ethical committee at Zhuhai Hospital of Jinan University and Xiangya Hospital of Central South University before performing the study. The ethical committee members reviewed the experimental designs and protocols to determine whether these studies would hurt the security and privacy of the patients enrolled, and gave ethical approval. Additionally, all patients enrolled agreed to participate in the project and gave signed consent.
Cell Lines and Tissues
The human NPC cell line 5–8F and 6–10B (sublines derived from cell line SUNE-1) were purchased from the Cancer Research Center of Sun Yatsen University (Guangzhou, China). The 5–8F cell has a high metastatic ability. The 6–10B cell has a low metastatic ability [26]. The Cell lines were cultured as a monolayer in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 μg/mL penicillin, and 100 IU/mL streptomycin (Invitrogen, Carlsbad, CA), and were maintained in an incubator with a humidified atmosphere of 95% air and 5% CO2 at 37°C. For DNP treatment, DNP crystals were dissolved in dimethyl sulfoxide (DMSO), and appropriate amounts of the DNP stock solution were added to the culture cells to achieve the indicated concentrations. The cells were then incubated for the indicated times. To investigate the dose-course of DNP treatment, 6–10B cells were treated with 0, 2, 6, 8, or 10 μM DNP for 24 h. For time-course assays, the cells were treated with 8 μM DNP for 0, 6, 18, 24, 36, or 48 h.
One hundred and thirty-four tissue specimens, including 60 cases of primary NPC, 48 normal nasopharyngeal epithelial tissues (NNET), and 26 cervical lymph node metastatic NPC (LMNPC), were obtained from the Xiangya Hospital of Central South University from January 2010 to September 2012. Each specimen was fixed with 40 g/L paraformaldehyde solution, followed by dehydration and paraffin embedment. Four-microgram serial sections were utilized for immunohistochemistry. All patients had only one primary tumor and none had received treatment.
Immunohistochemistry
Immunohistochemistry was performed on the formalin-fixed and paraffin-embedded tissue sections using a standard immunohistochemical technique. Four-microgram-thick tissue sections were deparaffinized in xylene, rehydrated in a graded alcohol series, and treated with an antigen retrieval solution (10 mmol/L sodium citrate buffer, pH 6.0). The sections were incubated with rabbit monoclonal anti-AGR2 (Abcam, Cambridge, UK; dilution 1∶50), mouse monoclonal anti-CTSB (Abcam; dilution 1∶50) and mouse monoclonal anti-CTSD (Santa Cruz, dilution 1∶50) antibody overnight at 4°C. Subsequently, the sections were incubated with a biotinylated secondary antibody (Zhongshan, China), followed by incubation with an avidin–biotin complex (Zhongshan, China) according to the manufacturer’s instructions. Finally, tissue sections were incubated with 3′,3′-diaminobenzidine (DAB) (Sigma-Aldrich) and hydrogen peroxide for 2 min, and counterstained with hematoxylin for 30 s. In negative controls, primary antibodies were omitted.
Evaluation of Staining
Sections were blindly evaluated by two investigators in an effort to provide a consensus on staining patterns under light microscopy (Olympus). AGR2, CTSB, and CTSD staining were assessed according to the methods described by Cheng et al. [56] with minor modifications. Each case was rated according to a score derived from a scale based on intensity of staining added to a scale based on area of staining. At least 10 high-power fields were chosen randomly, and >1000 cells were counted for each section. The depth of staining was graded on the following scale: 0, no cell coloration; 1+, light yellow; 2+, brown; 3+, tan. The area of staining was evaluated as follows: 0, no staining of cells in any microscopic fields; 1+, <30% of tissue stained positive; 2+, between 30% and 60% stained positive; 3+, >60% stained positive. The sum (extension+intensity) was used as the total score, where 0–1 indicates a negative score (−), ≥2 a positive score (+). Statistical analysis was performed using SPSS (version 18.0). A difference of P<0.05 was considered statistically significant.
Immunofluorescence Analysis
The 6–10B cells treated with DNP were fixed with 2.0% formaldehyde in PBS for 30 min, washed with PBS three times, and then treated with PBS containing 0.2% Triton X-100 for 10 min. After being washed with PBS three times, the cells were incubated with 0.5% bovine serum albumin in PBS. The cells were incubated with AGR2 mouse antibody or cathepsin rabbit antibody, and respectively incubated with the anti-mouse antibody conjugated with FITC or anti-rabbit-IgG antibody conjugated with Texas Red after being washed. Cells were again washed using PBS, mounted onto coverslips, and examined under a Zeiss Axiophot microscope (Carl Zeiss, Oberkochen, Germany). Cells incubated with a non-specific IgG served as the blank control. Cells stained with DAPI served as the cell control.
siRNA-AGR2 and Vectors
The siRNA-AGR2 was synthesized by Guangzhou RiboBio Limited Company (Guangzhou Guangdong China), and the sequence was as follows: 5′-CUGAUUAGGUUAUGGUUUATT-3′. When 30% confluence was reached, 6–10B cells were transfected with siRNA-AGR2 according to the manufacturer’s instructions. In parallel, cells transfected with nonspecific siRNA were used as a control. Control nonspecific siRNA was also purchased from Guangzhou RiboBio Limited Company of China. The pU6pro vector was used to construct pU6pro-si-mock (si-mock) and pU6prosi-AGR2 (si-AGR2) following the manufacture’s protocol. An AGR2 DNA fragment was generated by polymerase chain reaction (PCR) and cloned into the BamHI/XhoI site of the pcDNA3.1 vector to generate pcDNA3.1-AGR2 plasmids.
Gene Transfection and Stable Transfection of Cell
5–8F cells were transfected with U6pro-si-mock (si-mock) and pU6prosi-AGR2 using Lipofectamine 2000 reagent (Invitrogen), following the manufacturer’s suggested protocol. The stably transfected cell lines, 5–8F-si-mock, and 5–8F-si-AGR2 were obtained by selection for G418 resistance, and further confirmed by assessing AGR2 expression. 5–8F-si-AGR2 was further transfected with either pcDNA3.1 or pcDNA3.1-AGR2.
Cell Invasion and Motility Assay
Cell invasion and motility assays were assayed according to the methods described previously with minor modifications [26]. For the invasion assay, untreated 6–10B cells, and 6–10B cells transfected with siRNA-AGR2 or nonspecific siRNA were treated with 8 μM DNP for 24 h. After DNP treatment, cells were removed by trypsinization, and their invasiveness was tested using the Boyden chamber invasion assay in vitro. Matrigel (25 mg/50 mL, Collaborative Biomedical Products, Bedford, MA) was applied to 8-mm pore size polycarbonate membrane filters. The treated cells were seeded into the upper part of the Boyden chamber (Neuro Probe, Cabin John, MD) at a density of 1.5×104 cells/well in 50 μL of serum-free medium, and then incubated for 12 h at 37°C. The bottom chamber also contained standard medium with 20% FBS. The cells that invaded the membrane were fixed with methanol and stained with hematoxylin and eosin. Invaded cell numbers were counted under a light microscope. The motility assay was carried out as described for the invasion assay, but without Matrigel.
Co-immunoprecipitation of AGR2 and CTSB or CTSD
For immunoprecipitation, 6.0×106 6–10B cells treated with DNP were lysed in 0.5 mL cell lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 5 mM beta-glycerophosphate, 1 mM Na3VO4, 1 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 1 μM aprotinin) for 10 min at 4°C. After brief sonication, the cells were centrifuged at 12,000×g for 15 min at 4°C. One hundred micrograms supernatant was incubated overnight with 2 μg anti-AGR2 body (Abcam, rabbit monoclonal) and protein-G beads. The immunoprecipitates were collected and washed three times with RIPA buffer (50 mM Tris, pH7.5, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 Mm Na3VO4, 1 mM NaF, and protease inhibitor), and finally subjected to western blot analysis using anti-CTSB (Abcam, mouse monoclonal), or anti-CTSD (Santa Cruz, mouse monoclonal) antibody.
Western Blotting
Western blotting analysis was performed as previously described [27]. Cell lysates and conditioned media were prepared, and protein concentrations were measured using Bio-Rad reagent (Bio-Rad). Cell lysates were denatured with an equal amount of 2×sample loading buffer by heating at 100°C for 10 min, and were then separated on a 10% polyacrylamide gel and transferred onto a nitrocellulose membrane (Bio-rad). The membranes were subsequently incubated with 5% non-fat milk in Tris-buffered saline containing 0.05% Tween-20 (TBST) for 1 h to block non-specific binding, and then incubated overnight with antibody against AGR2, CTSB, or CTSD (Cell Signaling Technologies). After being washed three times for 5 min each, the membranes were incubated with the secondary antibody for 1 h at room temperature. Finally, target proteins were detected by ECL (Pierce, Rockford, USA). Western blotting with anti-GAPDH antibody (Millipore) was used as an internal control to verify basal level expression and equal protein loading. The abundance ratio to GAPDH was measured.
Results
Expression of AGR2, CTSB, and CTSD in NNET, NPC, and Metastatic NPC
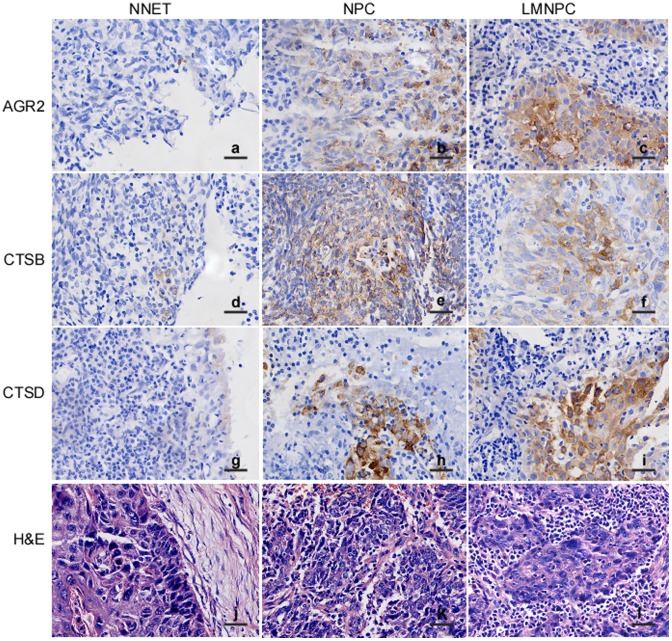
AGR2, CTSB, and CTSD expression was detected using immunohistochemistry in formalin-fixed and paraffin-embedded archival clinical tissues, including 72 cases of NNET, 60 cases of NPC, and 78 cases of cervical LMNPC. The immunohistochemistry results revealed positive AGR2 (Fig. 1A–b, c), CTSB (Fig. 1a–e, f), and CTSD (Fig. 1a–h, i) signals showing brown-yellow granules in the cytoplasm, in the cytoplasm and membrane, and in the cytoplasm and membrane, respectively. Furthermore, as shown in Table 1, the percentage of positive staining in NNET tissues was 20.8%, 25.0%, and 30.5%; NPC tissues: 35.0%, 53.3%, and 36.7%; and LMNPC tissues: 53.8%, 48.7% and 58.9% for AGR2, CTSB, and CTSD respectively. AGR2, CTSB, and CTSD were significantly upregulated in LMNPC compared to NNET (P<0.01), and AGR2 and CTSD were also upregulated in LMNPC when compared to primary NPC (P<0.05), whereas no change of CTSB between LMNPC and primary NPC (P>0.05) was observed. All the results indicate that dysregulation of AGR2, CTSB, and CTSD might be related to development and metastasis of NPC.
Figure 1. Immunohistochemical staining of AGR2, CTSB, and CTSD expressions in normal nasopharyngeal epithelial tissues (NNET), nasopharyngeal carcinoma (NPC), and cervical lymph node metastatic NPC (LMNPC) tissues.
The formalin-fixed and paraffin-embedded NNET, NPC, and LMNPC tissue sections were stained using a standard immunohistochemical technique. a, AGR2 expression in NNET; b, AGR2 expression in NPC; c AGR2 expression in LMNPC; d, CTSB expression in NNET; e, CTSB expression in NPC; f, CTSB expression in LMNPC; g, CTSD expression in NNET; h, CTSD expression in NPC; i, CTSD expression in LMNPC; j, NNET hematoxylin and eosin; k, NPC hematoxylin and eosin; l, LMNPC hematoxylin and eosin. Arrows, positive cells. Original magnification, ×400. Scale bar, 5 μm.
Table 1. Differential AGR2, CTSB, and CTSD expression among NNET, NPC, and LMNPC tissues.
| AGR2 | CTSB | CTSD | |||||||||||
| n | − | + | % | P | − | + | % | P | − | + | % | P | |
| NNET | 72 | 57 | 15 | 20.8 | 0.080* | 54 | 18 | 25.0 | 0.001* | 50 | 22 | 30.5 | 0.466* |
| NPC | 60 | 39 | 21 | 35.0 | 0.038** | 28 | 32 | 53.3 | 0.611** | 38 | 22 | 36.7 | 0.011** |
| LMNPC | 78 | 36 | 42 | 53.8 | 0.000*** | 40 | 38 | 48.7 | 0.004*** | 32 | 46 | 58.9 | 0.001*** |
Note: *, NNET versus NPC, **, NPC versus LMNPC, ***, LMNPC versus NNET.
Expression of AGR2 is Associated with CTSB and CTSD in NPC Tissues
AGR2, CTSB, and CTSD are highly expressed in metastatic NPC, which implies that AGR2 is associated with CTSB or CTSD. The correlation of AGR2 expression with CTSB or CTSD was analyzed in NPC tissues. As shown in Table 2, AGR2 protein expression was positively correlated with CTSB (r = 0.145, P<0.05) and CTSD (r = 0.163, P<0.05). These data show that AGR2 and CTSB or CTSD are co-expressed in NPC tissues.
Table 2. Correlation of AGR2 with CTSB and CTSD expression in NPC and LMNPC tissues.
| AGR2 | CTSB | CTSD | ||||||
| − | + | r | P | − | + | r | P | |
| − | 60 | 40 | 0.145 | 0.035 | 66 | 39 | 0.163 | 0.018 |
| + | 50 | 60 | 49 | 56 | ||||
Note: r represents correlation coefficient.
DNP Induces the Expression of AGR2, CTSB and CTSD
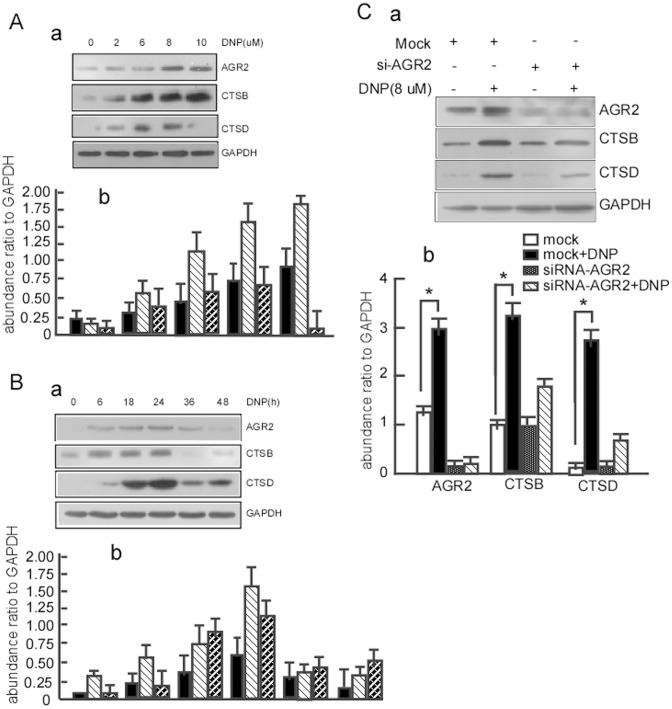
AGR2, CTSB, and CTSD are highly expressed in metastatic NPC. A specific carcinogen for NPC, DNP is involved in NPC metastasis. The next step is to determine whether DNP contributes to high AGR2, CTSB, and CTSD expression. Our previous work showed significant changes in AGR2 levels at 24 hours after DNP treatment [27]. To confirm whether DNP can induce AGR2, CTSB, and CTSD expression, 6–10B cells were treated with 0–10 μM DNP for 24 h for the dose-course assay or with 8 μM DNP for 0–48 h for the time-course assay. AGR2, CTSB, and CTSD expression were then detected using western blotting. DNP induced AGR2 (Fig. 2A, lane 2, 3, 4, 5 vs 1 in 1st panel a, and lane 4, 7, 10, 13 vs 1 in panel b), CTSB (Fig. 2A, lane 2, 3, 4, 5 vs. 1 in 2nd panel a, and lane 5, 8, 11, 14 vs. 2 in panel b) and CTSD (Fig. 2A, lane 2, 3, 4 vs. 1 in 3rd panel a, and lane 6, 9, 12 vs. 3 in panel b) expression in a time-dependent manner, and induced AGR2 (Fig. 2B, lane 2, 3, 4 vs. 1 in 1st panel a, and lane 4, 7, 10 vs. 1 in panel b), CTSB (Fig. 2B, lane 2, 3, 4 vs. 1 in 2nd panel a, and lane 5, 8, 11 vs. 2 in panel b) and CTSD (Fig. 2B, lane 2, 3, 4 vs. 1 in 3rd panel a, and lane 6, 9, 12 vs. 3 in panel b) in a dose-dependent manner. These findings indicate that high AGR2, CTSB, and CTSD expression levels are induced by DNP.
Figure 2. DNP induces expression of AGR2 and cathepsins B and D.
A, 6–10B cells were treated with 8 μM DNP for the indicated time for a time-course. B, 6–10B cells were treated with the indicated concentration of DNP for a dose-course. AGR2, CTSB, and CTSD expression in the DNP-treated cells was detected using western blotting. GAPDH served as the loading control. C, 6–10B cells were treated with 8 μM DNP for 24 h. AGR2 was immunoprecipitated with anti-AGR2 antibody. CTSB and CTSD in the immunoprecipitations were then detected with western blotting. *indicates P<0.05. One representative experiment is presented.
The above AGR2 expression was associated with CTSB or CTSD in metastatic NPC tissues. Dumartin et al. reported that AGR2 regulates CTSB and CTSD to promote cell dissemination in cancer metastasis [35]. To further clarify whether DNP-mediated AGR2 regulates CTSB or CTSD expression, the untreated 6–10B cells and the 6–10B cells transfected with siRNA-AGR2 or with nonspecific siRNA, were treated with 8 μM DNP for 24 h, and then AGR2, CTSB, and CTSD expression was measured. First, AGR2 knockdown efficiency was examined using western blotting. AGR2 expression was significantly attenuated in AGR2 knockdown cells compared to the cells transfected with nonspecific siRNA (Fig. 2C-a, lane 3 vs. 1 in 1st panel; Fig. 2C-b, lane 3 vs. 1 panel). In AGR2 knockdown cells, CTSB (Fig. 2C-a, lane 4 vs. 3 in 2nd panel; Fig. 2C-b lane 8 vs. 7 panel) and CTSD (Fig. 2C-a, lane 4 vs. 3 in 3rd panel; Fig. 2C-b lane 12 vs. 11 panel) levels did not change after DNP treatment. However, when AGR2 normally expressed, AGR2 (Fig. 2C-a, lane 2 vs. 1 in 1st panel; Fig. 2C-b lane 2 vs. 1 panel), CTSB (Fig. 2C-a, lane 2 vs 1 in 2nd panel; Fig. 2C-b lane 6 vs. 5 panel), and CTSD (Fig. 2C-a, lane 4 vs. 3 in 3rd panel; Fig. 2C-b lane 10 vs. 9 panel) expression all increased after DNP treatment. The results show that DNP induces CTSB and CTSD expression through AGR2.
DNP Increases the Interaction of AGR2 with CTSB and CTSD
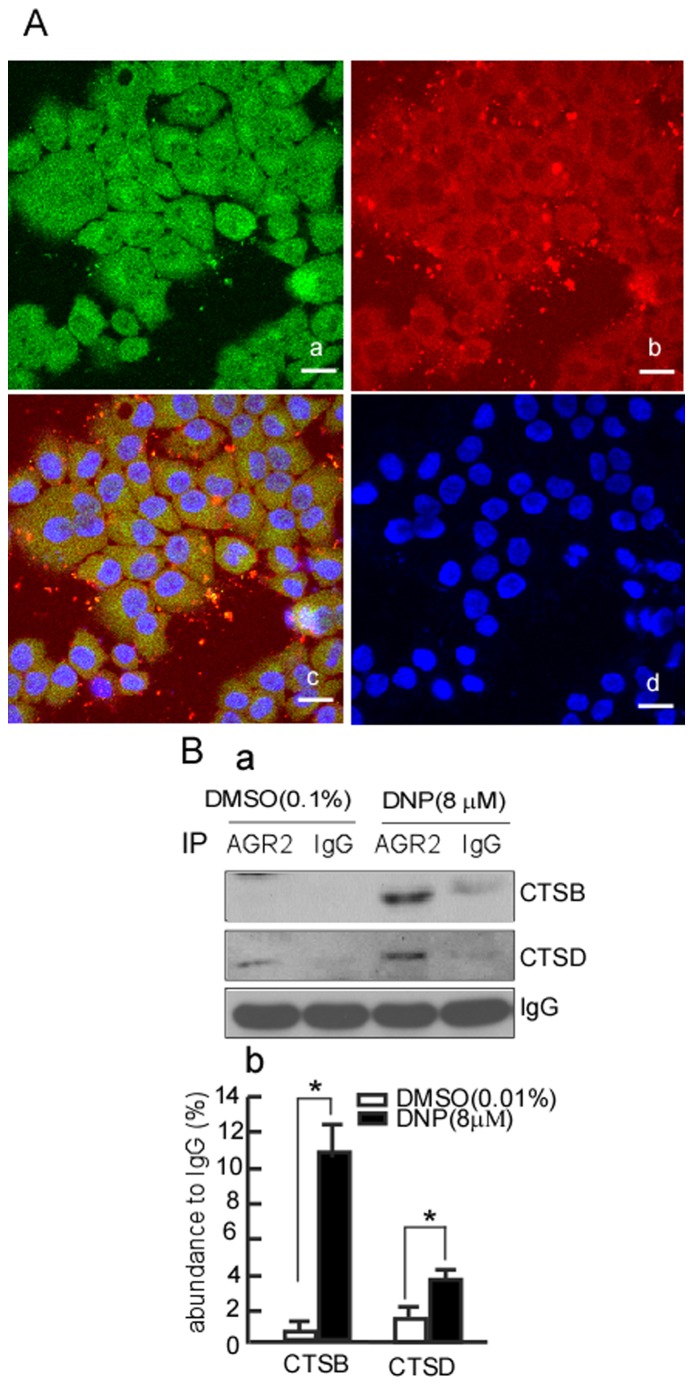
To determine the possibility of AGR2 binding to CTS, we detected the colocalization of AGR2 and CTS using the immunofluorescence assay. The results showed that AGR2 and CTS colocated in the cytoplasm (Fig. 3A–c). To further elucidate whether AGR2 binds to CTSB or CTSD using the immunoprecipitation assay, after DNP treatment, AGR2 was immunoprecipitated by AGR2 antibody, and then CTSB and CTSD were detected in the immunoprecipitates. The results showed that CTSB dramatically increased in the immunoprecipitates of the DNP-treated cells compared to the DNP-untreated group (Fig. 3B-a, lane 3 vs. 1 in 1st panel; Fig. 3B-b lane 1 vs 2, p<0.05). CTSD also increased in the DNP-treated group (Fig. 3B-a, lane 3 vs. 1 in 2nd panel; Fig. 3B-b lane 3 vs. 4, p<0.05). These results indicate that DNP induced binding of AGR2 with CTSD and CTSB.
Figure 3. AGR2 interacts with CTSB/CTSD.
A, interaction of endogenous AGR2 and CTS in 6–10B cells. Endogenous AGR2 and CTS expression in 6–10B cells was detected using an immunofluorescence assay. a, AGR2 expression in 6–10B cells; b, CTS expression in 6–10B cells; c, merge of a and b; d, DAPI staining. B, 6–10B cells were respectively transfected with siRNA-AGR2 or si-mock, and then treated with DNP. AGR2 was immunoprecipitated using AGR2 antibody, and CTSB and CTSD were detected in the immunoprecipitates using western blotting. IgG served as the loading control. *indicates P<0.05. One representative experiment is presented.
DNP-mediated Invasion and Motility Through Increased AGR2 Expression
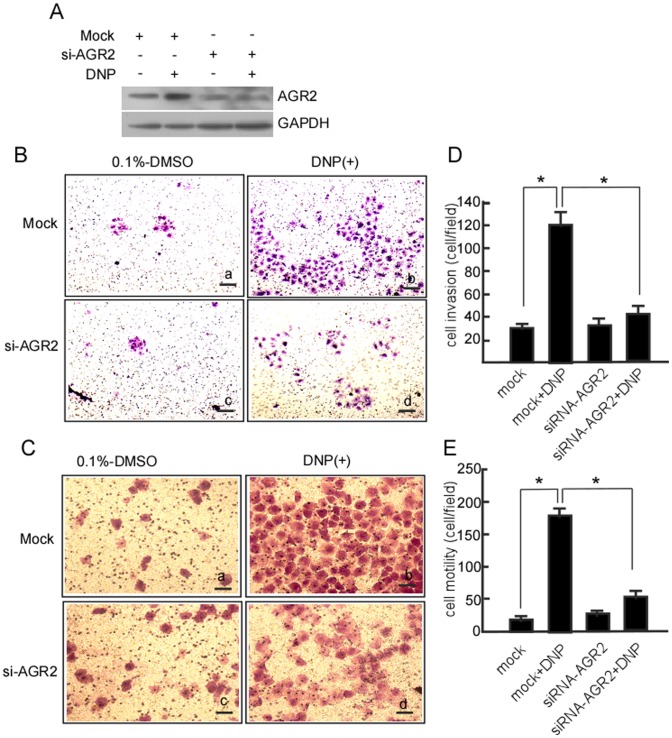
Our previous work has shown that DNP can induce NPC cell motility and invasion [27]. To confirm whether DNP-mediated metastasis occurs through AGR2, 6–10B cells were transfected with siRNA-AGR2 to block AGR2 expression, and then cell motility and invasion were measured. AGR2 was effectively silenced in 6–10B-si-AGR2 cells (Fig. 4A, lane 3 in upper panel). DNP-induced invasion (Fig. 4B–d; Fig. 4D lane 4 vs. 2, P<0.05) and motility (Fig. 4C–d; Fig. 4E, lane 4 vs. 2, P<0.05) were dramatically decreased when AGR2 expression was blocked. However, DNP could effectively induce cell invasion (Fig. 4B-b; Fig. 4D, lane 2 vs. 1, P<0.05) and motility (Fig. 4C-b; Fig. 4E lane 2 vs. 1, P<0.05) when AGR2 was not blocked. Taken together, our data indicate that DNP induces cell metastasis via induction of AGR2.
Figure 4. DNP-mediated invasion and motility through AGR2 in 6–10B cells.
6–10B cells were transiently transfected with siRNA-AGR2 or si-mock, and then treated with DNP. A, AGR2 expression was detected using western blotting. A Matrigel-coated Boyden chamber was used to measure 6–10B cell invasion, and an uncoated Boyden chamber was used to determine cell motility. The cells that invaded the membrane were fixed with methanol and stained with hematoxylin and eosin. Images were taken under a light microscope and random fields of view were counted to determine the number of invading cells. B, cell invasion of the treated cells. a, 6–10B cells transfected with si-mock and treated with 0.1% DMSO; b, 6–10B cells transfected with si-mock and treated with 8 μM DNP; c, the transfect with siRNA-AGR2 plus DMSO treatment; d, the transfect with siRNA-AGR2 and treated with DNP. C, cell motility of the treated cells. a, 6–10B cells transfected with si-mock and treated with DMSO; b, 6–10B cells transfected with si-mock and treated with DNP; c, the transfect with siRNA-AGR2 and treated with DMSO; d, the transfect with siRNA-AGR2 and treated with DNP. D, invasive cells were counted. E, motile cells were counted. The number of traversed cells were counted in three individual experiments and presented as the mean ± SD. *indicates P<0.05.
si-AGR2 Decreased DNP-mediated Invasion and Motility in 5–8F Cells
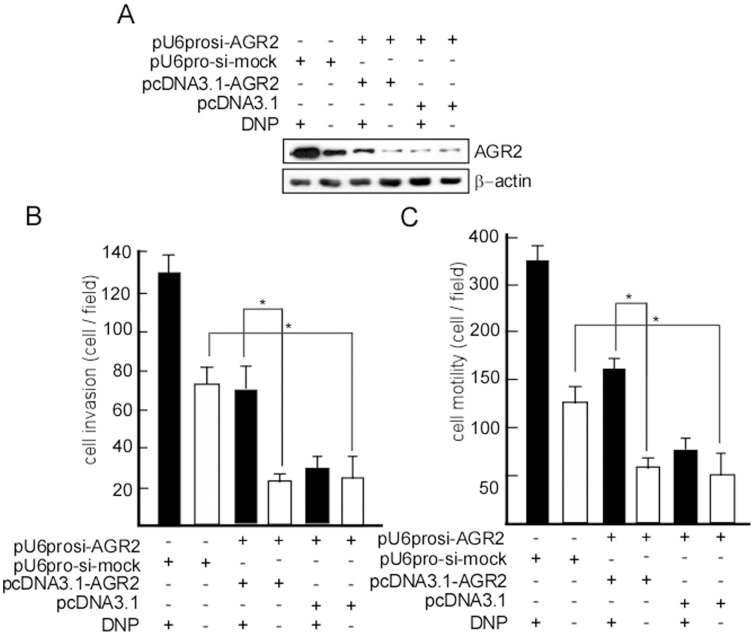
To further confirm that AGR2 is involved in DNP-mediated metastasis, 5–8F, an NPC cell line with high metastatic ability, was transfected with pUprosi-AGR2 (si-AGR2), and 5–8F-si-AGR2 was obtained by selection for G418 resistance. AGR2 expression was blocked in 5–8F-si-AGR2 cells (Fig. 5A, lane 4, 5, 6 in up panel), and motility and invasion of 5–8F-si-AGR2 dramatically decreased (Fig. 5B, lane 6 vs. 2, P<0.05; Fig. 5C, lane 6 vs. 2, P<0.05). DNP could not effectively induce 5–8F-si-AGR2 motility and invasion (Fig. 5B, lane 4 vs. 5; Fig. 5C, lane 4 vs. 5). However, when we introduced AGR2, motility and invasion dramatically increased after DNP treatment (Fig. 5B lane 3 vs. 4, p<0.05; Fig. 5C lane 3 vs. 4, p<0.05).
Figure 5. si-AGR2 decreased DNP-mediated invasion and motility in 5–8F cells.
5–8F cells were transfected with pUprosi-AGR2 (si-AGR2) or pUprosi-mock (si-mock), and 5–8F-si-AGR2 and 5–8F-si-mock were obtained by selection for G418 resistance, and treated with DNP. A, AGR2 expression was detected using western blotting. A Boyden chamber was used to measure the invasion and motility of 5–8F-si-AGR2 and 5–8F-si-mock with or without DNP treatment. The cells that invaded the membrane were fixed with methanol and stained with hematoxylin and eosin. Random fields of view were counted to determine the number of invading cells. B, invasion of the treated cells. C, motility of the treated cells. The number of traversed cells was counted in three individual experiments and presented as the mean ± SD. *indicates P<0.05.
Discussion
In the clinic, NPC has a highly invasive and metastatic character, with approximately 87.9% of patients showing metastasis at initial diagnosis [67]. Well-established risk factors for NPC are genetic susceptibility, infection with the EBV, and regular consumption of salted fish beginning in childhood [2]. As an NPC carcinogen, EBV latent membrane protein-1 and -2 (LMP1 and LMP2) have been confirmed to promote progression and metastasis of NPC, but the positive expression rate of EBV in NPC tissues is only 57% [58], [59]. Presently, we focused on the carcinogen, DNP.
The process of salt preservation is inefficient, allowing fish and other foods to become partially putrefied [16], [60]. Bacteria induce the conversion of nitrates into nitrites, which form important carcinogenic N-nitroso compounds [60]. These foods accumulate significant levels of nitrosamines [16], [17], [19], [61]. When salted foods are consumed, nitrosamines are transformed into DNP in the liver [13], [20], [62], and DNP is a predominant volatile nitrosamine. DNP has been shown to participate in NPC development. In experiments concerning DNP-induced rat NPC, DNP showed organ specificity for the nasopharyngeal epithelium and a high incidence of NPC metastasis [25], [62]. In clinical assays, NPC patients with metastasis have high levels of DNP [26].
In our previous works, to reveal the pathogenesis of DNP, a quantitative proteomic study using stable isotope labeling with amino acids in cell culture (SILAC), coupled with mass spectrometry, was performed on DNP-treated 6–10B cells. Many protein showed significant changes in levels at 24 h after DNP-treatment, including AGR2, CTSB, and CTSD [27]. Recently, much interest has focused on AGR2 for its potential roles in the invasion and metastasis of malignant tumors [63]. AGR2 is frequently overexpressed in many human cancers, including breast carcinoma [28], lung adenocarcinoma [29], [30], ovarian carcinoma [31], and prostate cancers [32]. In the present study we also found that positive expression of AGR2 in NPC tissues was significantly higher than in the normal nasopharyngeal tissues. Further, we analyzed the correlation of AGR2 expression with CTSB or CTSD in NPC tissues. It has been reported that overexpression of AGR2 is associated with poor differentiation, deep invasion, and lymph node metastasis in several types of cancer [32], [34], [64], [65]. Surprisingly, our data demonstrated overexpression of AGR2 was associated with lymph node metastasis in NPC tissues as well. Taken together, the above results suggest that AGR2 acts as a pivotal factor contributing to the progression of NPC, and may be involved in the invasion and metastasis of NPC.
Our previous work has shown that DNP can induce NPC cell motility and invasion [27]. To confirm whether DNP-mediated metastasis occurs through AGR2, AGR2 was silenced in 6–10B cells. Consequently, DNP-mediated motility and invasion of 6–10B cells was decreased. These results were confirmed in 5–8F cells. DNP did not have an effect on 5–8F cell motility and invasion when AGR2 was blocked. On the other hand, AGR2 gene overexpression significantly increased CTSB and CTSD expression [35]. CTSB and CTSD contribute to tumor cell invasion and angiogenesis and are commonly associated with metastasis [38], [46], [48], [52]. Cathepsin is known to remodel the surrounding ECM by proteolysis, allowing tumor cell invasion and metastasis [66]. In the current study, we demonstrated that AGR2 coexpresses with CTSB/CTSD in NPC cells and tissues, and that downregulation of AGR2 by siRNA results in the reduction of the CTSB/CTSD expression in 6–10B cells with DNP treatment. This suggests that activation of CTSB/CTSD is regulated by the AGR2 gene in DNP-mediated metastasis. On the other hand, immunoprecipitation assays have shown that DNP increases the interaction of AGR2 and CTSB. We speculate that a possible mechanism of AGR2-induced NPC metastasis may be the remodeling of the extracellular matrix (ECM) through CTSB and CTSD.
Conclusion
AGR2 is an important factor in the invasion and metastasis of NPC. It may exert control, at least in part, through the regulation of CTSB and CTSD. DNP induces AGR2 expression, and DNP-mediated AGR2 stimulates the production of CTS from endothelial cells, thereby stimulating the remodeling of ECM and accelerating tumor invasion and metastasis. Additionally, DNP-mediated AGR2 and CTSB/CTSD expression involving NPC metastasis provides new avenues for the high incidence of NPC metastasis.
Acknowledgments
We thank the National Natural Science Foundation of China (81372282, 81071718, 30973400 30670990), Program for New Century Excellent Talents in University, NCET (NCET-06-0685), Guangdong Natural Science Foundation (S2013010013360).
Funding Statement
This work was supported in part by the National Natural Science Foundation of China (81372282, 81071718, 30973400 30670990), Program for New Century Excellent Talents in University, NCET (NCET-06-0685), Guangdong Natural Science Foundation (S2013010013360). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wei WI, Sham JST (2005) Nasopharyngeal carcinoma. The Lancet 365: 2041–2054. [DOI] [PubMed] [Google Scholar]
- 2. Cao SM, Simons MJ, Qian CN (2011) The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 30: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan AT (2010) Nasopharyngeal carcinoma. Annals of Oncology 21(Suppl): vii308–312. [DOI] [PubMed] [Google Scholar]
- 4. Chua DT, Sham JS, Kwong DL, Wei WI, Au GK, et al. (1998) Locally recurrent nasopharyngeal carcinoma: treatment results for patients with computed tomography assessment. Int J Radiat Oncol Biol Phys 41: 379–386. [DOI] [PubMed] [Google Scholar]
- 5. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, et al. (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. Journal of Clinical Oncology 16: 1310–1317. [DOI] [PubMed] [Google Scholar]
- 6. Liebowitz D (1994) Nasopharyngeal carcinoma: the Epstein-Barr virus association. Semin Oncol 21: 376–381. [PubMed] [Google Scholar]
- 7. Simons MJ Nasopharyngeal carcinoma as a paradigm of cancer genetics (2011) Chin J Cancer. 30: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu MC (1990) Diet and nasopharyngeal carcinoma. FEMS Microbiol Immunol 2: 235–242. [DOI] [PubMed] [Google Scholar]
- 9.Henderson BE, Louie E. (1978) Discussion of risk factors for nasopharyngeal carcinoma. IARC Sci Publ 251–260. [PubMed]
- 10. Yu MC, Ho JH, Lai SH, Henderson BE (1986) Cantonese-style salted fish as a cause of nasopharyngeal carcinoma: report of a case-control study in Hong Kong. Cancer Res 46: 956–961. [PubMed] [Google Scholar]
- 11. Yu MC, Huang TB, Henderson BE (1989) Diet and nasopharyngeal carcinoma: a case-control study in Guangzhou, China. Int J Cancer 43: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong RW, Imrey PB, Lye MS, Armstrong MJ, Yu MC, et al. (1998) Nasopharyngeal carcinoma in Malaysian Chinese: salted fish and other dietary exposures. Int J Cancer 77: 228–235. [DOI] [PubMed] [Google Scholar]
- 13. Yuan JM, Wang XL, Xiang YB, Gao YT, Ross RK, et al. (2000) Preserved foods in relation to risk of nasopharyngeal carcinoma in Shanghai, China. Int J Cancer 85: 358–363. [DOI] [PubMed] [Google Scholar]
- 14. Zou J, Sun Q, Akiba S, Yuan Y, Zha Y, et al. (2000) A case-control study of nasopharyngeal carcinoma in the high background radiation areas of Yangjiang, China. J Radiat Res (Tokyo) 41(Suppl): 53–62. [DOI] [PubMed] [Google Scholar]
- 15. Jia WH, Luo XY, Feng BJ, Ruan HL, Bei JX, et al. (2010) Traditional Cantonese diet and nasopharyngeal carcinoma risk: a large-scale case-control study in Guangdong, China. BMC Cancer 10: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zou XN, Lu SH, Liu B (1994) Volatile N-nitrosamines and their precursors in Chinese salted fish–a possible etological factor for NPC in china. Int J Cancer 59: 155–158. [DOI] [PubMed] [Google Scholar]
- 17.Poirier S, Hubert A, de-Thé G, Ohshima H, Bourgade MC, et al.. (1987) Occurrence of volatile nitrosamines in food samples collected in three high-risk areas for nasopharyngeal carcinoma. IARC Sci Publ 415–419. [PubMed]
- 18. Lijinsky W, Kovatch RM (1993) Carcinogenic effects in rats of nitrosopiperazines administered intravesically: possible implications for the use of piperazine. Cancer Lett 74: 101–103. [DOI] [PubMed] [Google Scholar]
- 19. Jakszyn P, Gonzalez CA (2006) Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence. World J Gastroenterol 12: 4296–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gallicchio L, Matanoski G, Tao XG, Chen L, Lam TK, et al. (2006) Adulthood consumption of preserved and nonpreserved vegetables and the risk of nasopharyngeal carcinoma: a systematic review. Int J Cancer 119: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 21.Huang DP, Ho JH, Saw D, Teoh TB (1978) Carcinoma of the nasal and paranasal regions in rats fed Cantonese salted marine fish. IARC Sci Publ 315–328. [PubMed]
- 22. Yu MC, Nichols PW, Zou XN, Estes J, Henderson BE (1989) Induction of malignant nasal cavity tumours in Wistar rats fed Chinese salted fish. Br J Cancer 60: 198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng X, Luo Y, Christensson B, Drettner B (1994) Induction of nasal and nasopharyngeal tumours in Sprague-Dawley rats fed with Chinese salted fish. Acta Otolaryngol 114: 98–104. [DOI] [PubMed] [Google Scholar]
- 24. Tang F, Jiang H, Duan Z, Chen B, Jing Z, et al. (2001) [Profile of telomerase and telomerase RNA expression in nasopharyngeal carcinogenesis of rats induced by N, N′dinitrosopiperazine (DNP)]. Zhonghua Bing Li Xue Za Zhi 30: 125–128. [PubMed] [Google Scholar]
- 25. Tang FQ, Duan CJ, Huang DM, Wang WW, Xie CL, et al. (2009) HSP70 and mucin 5B: novel protein targets o f N,N′-dinitrosopiperazine -induced nasopharyngeal tumorigenesis. Cancer Sci 100: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang F, Zou F, Peng Z, Huang D, Wu Y, et al. (2011) N,N′-Dinitrosopiperazine-mediated Ezrin Protein Phosphorylation via Activation of Rho Kinase and Protein Kinase C Is Involved in Metastasis of Nasopharyngeal Carcinoma 6–10B Cells. J Biol Chem 286: 36956–36967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Liu N, Huang D, Zhang Z, Peng Z, et al. (2012) Proteomic analysis on N, N′-dinitrosopiperazine-mediated metastasis of nasopharyngeal carcinoma 6–10B cells. BMC Biochem 13: 25. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Salmans ML, Zhao F, Andersen B (2012) The estrogen-regulated anterior gradient 2 (AGR2) protein in breast cancer: a potential drug target and biomarker. Breast Cancer Res 15: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pizzi M, Fassan M, Balistreri M, Galligioni A, Rea F, et al. (2012) Anterior gradient 2 overexpression in lung adenocarcinoma. Appl Immunohistochem Mol Morphol 20: 31–36. [DOI] [PubMed] [Google Scholar]
- 30. Chung K, Nishiyama N, Wanibuchi H, Yamano S, Hanada S, et al. (2012) AGR2 as a potential biomarker of human lung adenocarcinoma. Osaka City Med J 58: 13–24. [PubMed] [Google Scholar]
- 31. Park K, Chung YJ, So H, Kim K, Park J, et al. (2011) AGR2, a mucinous ovarian cancer marker, promotes cell proliferation and migration. Exp Mol Med 43: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bu H, Bormann S, Schäfer G, Horninger W, Massoner P, et al. (2011) The anterior gradient 2 (AGR2) gene is overexpressed in prostate cancer and may be useful as a urine sediment marker for prostate cancer detection. Prostate 71: 575–587. [DOI] [PubMed] [Google Scholar]
- 33. Barraclough DL, Platt-Higgins A, de Silva Rudland S, Barraclough R, Winstanley J, et al. (2009) The metastasis-associated anterior gradient 2 protein is correlated with poor survival of breast cancer patients. Am J Pathol 175: 1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vanderlaag KE, Hudak S, Bald L, Fayadat-Dilman L, Sathe M, et al. (2010) Anterior gradient-2 plays a critical role in breast cancer cell growth and survival by modulating cyclin D1, estrogen receptor-alpha and survivin. Breast Cancer Res 12: R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dumartin L, Whiteman HJ, Weeks ME, Hariharan D, Dmitrovic B, et al. (2011) AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. 71: 7091–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rao Malla R, Gopinath S, Alapati K, Gorantla B, Gondi CS, et al. (2013) Knockdown of cathepsin B and uPAR inhibits CD151 and alpha3beta1 integrin-mediated cell adhesion and invasion in glioma. Mol Carcinog 52: 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37. Li C, Chen L, Wang J, Zhang L, Tang P, et al. (2011) Expression and clinical significance of cathepsin B and stefin A in laryngeal cancer. Oncol Rep 26: 869–875. [DOI] [PubMed] [Google Scholar]
- 38. Wu D, Wang H, Li Z, Wang L, Zheng F, et al. (2012) Cathepsin B may be a potential biomarker in cervical cancer. Histol Histopathol 27: 79–87. [DOI] [PubMed] [Google Scholar]
- 39. Wu D, Li ZN, Xu Y, Wang LH, Ding L, et al. (2010) [Clinical significance of cathepsin B expressions in cervical cancer in tissues]. Nan Fang Yi Ke Da Xue Xue Bao 30: 1330–1332. [PubMed] [Google Scholar]
- 40. Lodillinsky C, Rodriguez V, Vauthay L, Sandes E, Casabé A, et al. (2009) Novel invasive orthotopic bladder cancer model with high cathepsin B activity resembling human bladder cancer. J Urol 182: 749–755. [DOI] [PubMed] [Google Scholar]
- 41. Herszényi L, István G, Cardin R, De Paoli M, Plebani M, et al. (2008) Serum cathepsin B and plasma urokinase-type plasminogen activator levels in gastrointestinal tract cancers. Eur J Cancer Prev 17: 438–445. [DOI] [PubMed] [Google Scholar]
- 42. Czyzewska J, Guzińska-Ustymowicz K, Kemona A, Bandurski R (2008) The expression of matrix metalloproteinase 9 and cathepsin B in gastric carcinoma is associated with lymph node metastasis, but not with postoperative survival. Folia Histochem Cytobiol 46: 57–64. [DOI] [PubMed] [Google Scholar]
- 43. Yu B, Li SY, An P, Zuo FY, Cai HY (2004) [Expression of cathepsin B and its clinical importance in colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi 8: 507–509. [PubMed] [Google Scholar]
- 44. Wickramasinghe NS, Nagaraj NS, Vigneswaran N, Zacharias W (2005) Cathepsin B promotes both motility and invasiveness of oral carcinoma cells. Arch Biochem Biophys 436: 187–195. [DOI] [PubMed] [Google Scholar]
- 45. Sevenich L, Werner F, Gajda M, Schurigt U, Sieber C, et al. (2011) Transgenic expression of human cathepsin B promotes progression and metastasis of polyoma-middle-T-induced breast cancer in mice. Oncogene 30: 54–64. [DOI] [PubMed] [Google Scholar]
- 46. Withana NP, Blum G, Sameni M, Slaney C, Anbalagan A, et al. (2012) Cathepsin B inhibition limits bone metastasis in breast cancer. Cancer Res 72: 1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nouh MA, Mohamed MM, El-Shinawi M, Shaalan MA, Cavallo-Medved D, et al. (2011) Cathepsin B: a potential prognostic marker for inflammatory breast cancer. J Transl Med 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Paksoy M, Hardal U, Caglar C (2011) Expression of cathepsin D and E-cadherin in primary laryngeal cancers correlation with neck lymph node involvement. J Cancer Res Clin Oncol 137: 1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. del Casar JM, Corte MD, Alvarez A, García I, Bongera M, et al. (2008) Lymphatic and/or blood vessel invasion in gastric cancer: relationship with clinicopathological parameters, biological factors and prognostic significance. J Cancer Res Clin Oncol 134: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abbott DE, Margaryan NV, Jeruss JS, Khan S, Kaklamani V, et al. (2010) Reevaluating cathepsin D as a biomarker for breast cancer: serum activity levels versus histopathology. Cancer Biol Ther 9: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ohri SS, Vashishta A, Proctor M, Fusek M, Vetvicka V (2008) The propeptide of cathepsin D increases proliferation, invasion and metastasis of breast cancer cells. Int J Oncol 32: 491–498. [PubMed] [Google Scholar]
- 52. Szajda SD, Snarska J, Jankowska A, Roszkowska-Jakimiec W, Puchalski Z, et al. (2008) Cathepsin D and carcino-embryonic antigen in serum, urine and tissues of colon adenocarcinoma patients. Hepatogastroenterology 55: 388–393. [PubMed] [Google Scholar]
- 53. Kaneko I, Tanaka S, Oka S, Yoshida S, Hiyama T, et al. (2007) Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastroenterol 13: 3829–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gan Y, Zhao X, Hu J, Wang ZG, Zhao XT (2008) HCCS1 overexpression induces apoptosis via cathepsin D and intracellular calcium, and HCCS1 disruption in mice causes placental abnormality. Cell Death Differ 15: 1481–1890. [DOI] [PubMed] [Google Scholar]
- 55. Szajda SD, Snarska J, Roszkowska-Jakimiec W, Waszkiewicz N, Siedlecka K, et al. (2006) [Activity of cathepsin D in the blood serum and urine of patients with cancer of the stomach, pancreas and liver]. Pol Arch Med Wewn 116: 1150–1154. [PubMed] [Google Scholar]
- 56. Cheng AL, Huang WG, Chen ZC, Zhang PF, Li MY, et al. (2008) Identificating cathepsin D as a biomarker for differentiation and prognosis of nasopharyngeal carcinoma by laser capture microdissection and proteomic analysis. J Proteome Res 7: 2415–2426. [DOI] [PubMed] [Google Scholar]
- 57. Peng Z, Liu N, Huang D, Duan C, Li Y, et al. (2013) N,N′-dinitrosopiperazine–mediated heat-shock protein 70–2 expression is involved in metastasis of nasopharyngeal carcinoma. Plos One 8: e62908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chew MM, Gan SY, Khoo AS, Tan EL (2010) Interleukins, laminin and Epstein - Barr virus latent membrane protein 1 (EBV LMP1) promote metastatic phenotype in nasopharyngeal carcinoma. BMC Cancer 10: 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH, et al. (2010) Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog 6: e1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bartsch H, Ohshima H, Pignatelli B, Calmels S (1992) Endogenously formed N-nitroso compounds and nitrosating agents in human cancer etiology. Pharmacogenetics 2: 272–277. [DOI] [PubMed] [Google Scholar]
- 61. Preston-Martin S, Correa P (1989) Epidemiological evidence for the role of nitroso compounds in human cancer. Cancer Surv 8: 459–973. [PubMed] [Google Scholar]
- 62.Chen ZC, Pan SC, Yao KT (1991) Chemical transformation of human embryonic nasopharyngeal epithelial cells in vitro. IARC Sci Publ 1991; 434–438. [PubMed]
- 63. Brychtova V, Vojtesek B, Hrstka R (2011) Anterior gradient 2: a novel player in tumor cell biology. Cancer Lett 304: 1–7. [DOI] [PubMed] [Google Scholar]
- 64. Ramachandran V, Arumugam T, Wang H, Logsdon CD (2008) Anterior gradient 2 is expressed and secreted during the development of pancreatic cancer and promotes cancer cell survival. Cancer Res 68: 7811–7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zweitzig DR, Smirnov DA, Connelly MC, Terstappen LW, O’Hara SM, et al. (2007) Physiological stress induces the metastasis marker AGR2 in breast cancer cells. Mol Cell Biochem 306: 255–260. [DOI] [PubMed] [Google Scholar]
- 66. Lively S, Schlichter LC (2013) The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J Neuroinflammation 10: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang X, Li L, Hu C, Zhou Z, Ying H, et al. (2008) Patterns of level II node metastasis in nasopharyngeal carcinoma. Radiother Oncol 89: 28–32. [DOI] [PubMed] [Google Scholar]