Abstract
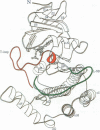
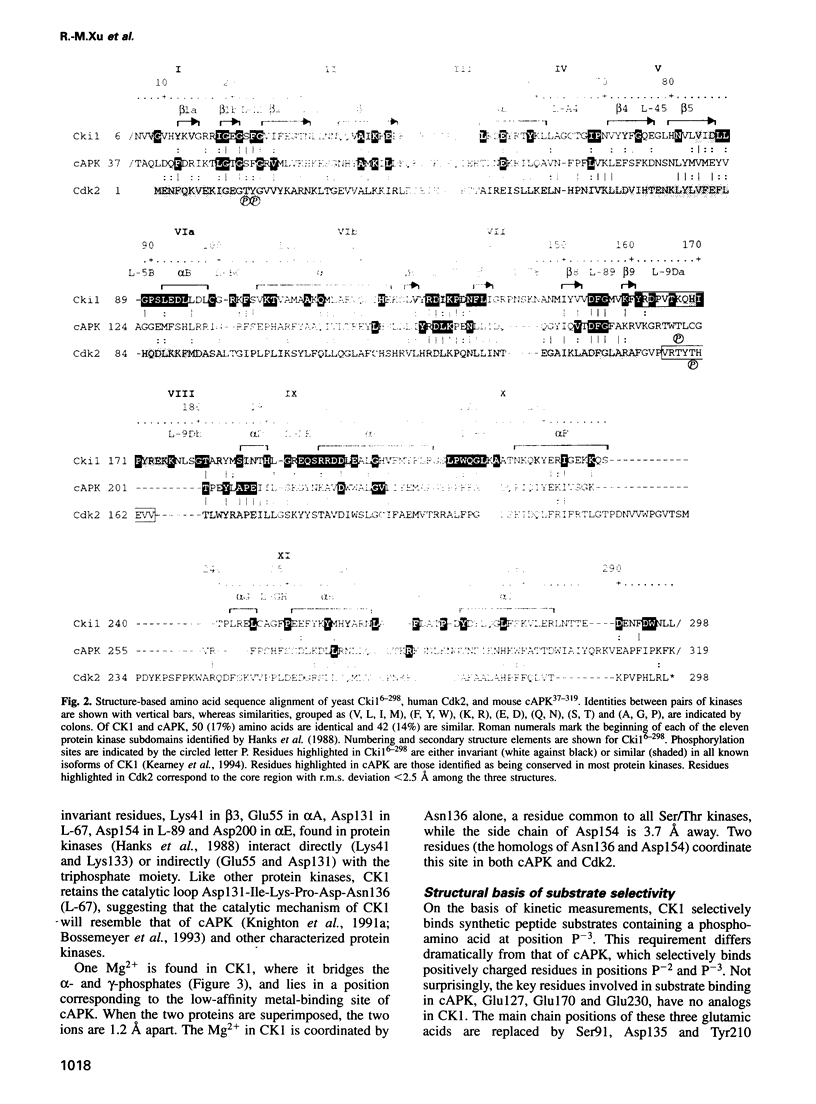
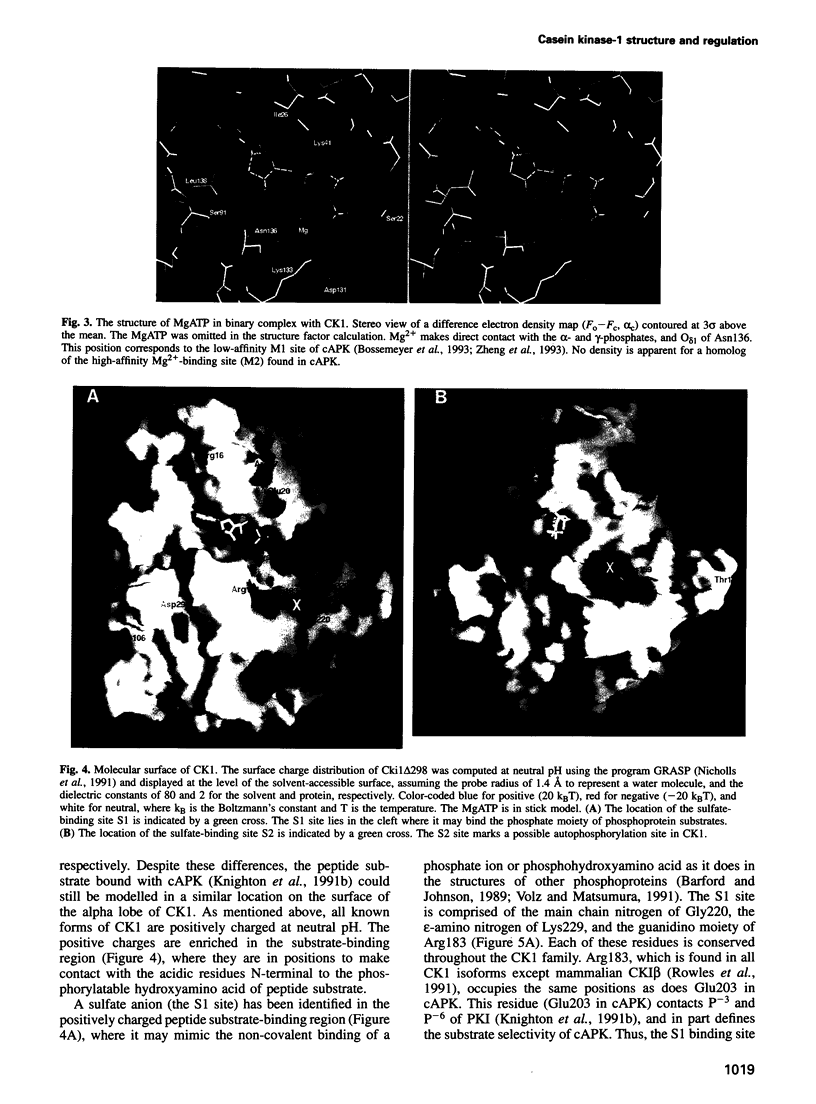
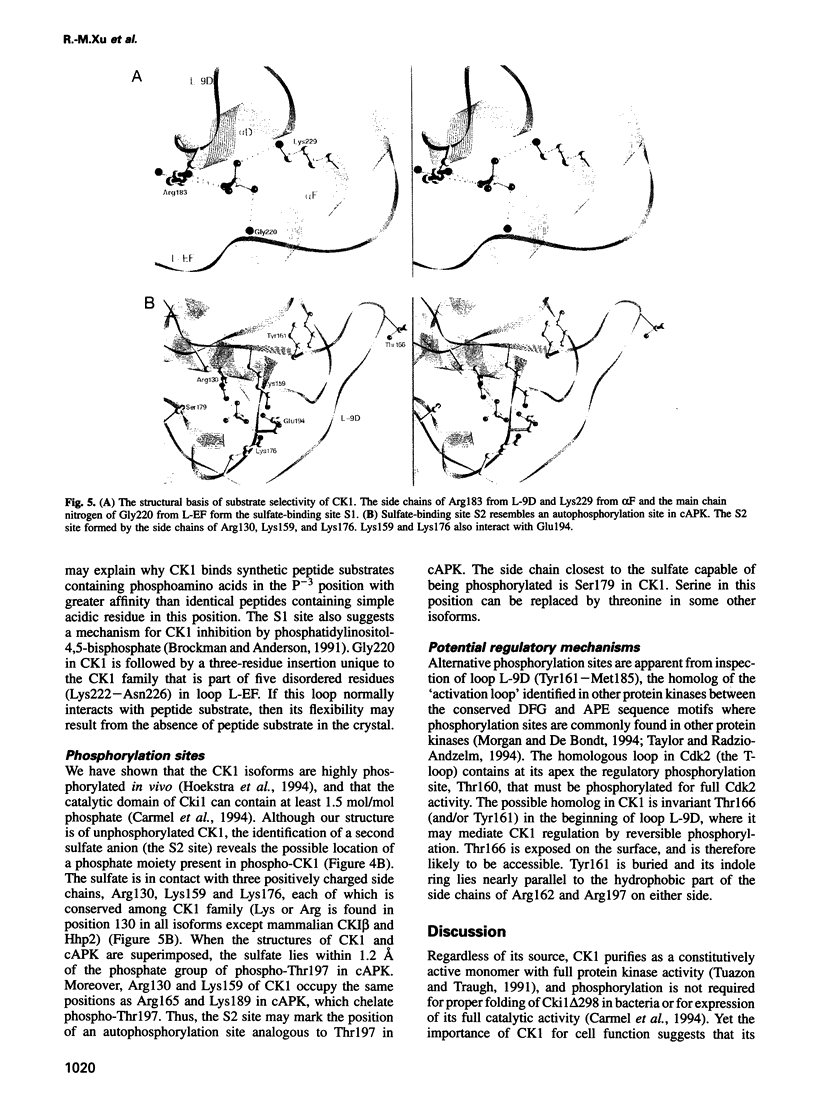
The structure of a truncated variant of casein kinase-1 from Schizosaccharomyces pombe, has been determined in complex with MgATP at 2.0 A resolution. The model resembles the 'closed', ATP-bound conformations of the cyclin-dependent kinase 2 and the cAMP-dependent protein kinase, with clear differences in the structure of surface loops that impart unique features to casein kinase-1. The structure is of unphosphorylated, active conformation of casein kinase-1 and the peptide-binding site is fully accessible to substrate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barford D., Johnson L. N. The allosteric transition of glycogen phosphorylase. Nature. 1989 Aug 24;340(6235):609–616. doi: 10.1038/340609a0. [DOI] [PubMed] [Google Scholar]
- Bossemeyer D., Engh R. A., Kinzel V., Ponstingl H., Huber R. Phosphotransferase and substrate binding mechanism of the cAMP-dependent protein kinase catalytic subunit from porcine heart as deduced from the 2.0 A structure of the complex with Mn2+ adenylyl imidodiphosphate and inhibitor peptide PKI(5-24). EMBO J. 1993 Mar;12(3):849–859. doi: 10.1002/j.1460-2075.1993.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman J. L., Anderson R. A. Casein kinase I is regulated by phosphatidylinositol 4,5-bisphosphate in native membranes. J Biol Chem. 1991 Feb 5;266(4):2508–2512. [PubMed] [Google Scholar]
- Brockman J. L., Gross S. D., Sussman M. R., Anderson R. A. Cell cycle-dependent localization of casein kinase I to mitotic spindles. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9454–9458. doi: 10.1073/pnas.89.20.9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel G., Leichus B., Cheng X., Patterson S. D., Mirza U., Chait B. T., Kuret J. Expression, purification, crystallization, and preliminary x-ray analysis of casein kinase-1 from Schizosaccharomyces pombe. J Biol Chem. 1994 Mar 11;269(10):7304–7309. [PubMed] [Google Scholar]
- Cegielska A., Virshup D. M. Control of simian virus 40 DNA replication by the HeLa cell nuclear kinase casein kinase I. Mol Cell Biol. 1993 Feb;13(2):1202–1211. doi: 10.1128/mcb.13.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T., Hagiwara M., Hidaka H. A newly synthesized selective casein kinase I inhibitor, N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase I from bovine testis. J Biol Chem. 1989 Mar 25;264(9):4924–4927. [PubMed] [Google Scholar]
- Cook P. F., Neville M. E., Jr, Vrana K. E., Hartl F. T., Roskoski R., Jr Adenosine cyclic 3',5'-monophosphate dependent protein kinase: kinetic mechanism for the bovine skeletal muscle catalytic subunit. Biochemistry. 1982 Nov 9;21(23):5794–5799. doi: 10.1021/bi00266a011. [DOI] [PubMed] [Google Scholar]
- De Bondt H. L., Rosenblatt J., Jancarik J., Jones H. D., Morgan D. O., Kim S. H. Crystal structure of cyclin-dependent kinase 2. Nature. 1993 Jun 17;363(6430):595–602. doi: 10.1038/363595a0. [DOI] [PubMed] [Google Scholar]
- Dhillon N., Hoekstra M. F. Characterization of two protein kinases from Schizosaccharomyces pombe involved in the regulation of DNA repair. EMBO J. 1994 Jun 15;13(12):2777–2788. doi: 10.1002/j.1460-2075.1994.tb06571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotow H., Graves P. R., Wang A. Q., Fiol C. J., Roeske R. W., Roach P. J. Phosphate groups as substrate determinants for casein kinase I action. J Biol Chem. 1990 Aug 25;265(24):14264–14269. [PubMed] [Google Scholar]
- Flotow H., Roach P. J. Role of acidic residues as substrate determinants for casein kinase I. J Biol Chem. 1991 Feb 25;266(6):3724–3727. [PubMed] [Google Scholar]
- Floyd C. C., Grant P., Gallant P. E., Pant H. C. Principal neurofilament-associated protein kinase in squid axoplasm is related to casein kinase I. J Biol Chem. 1991 Mar 15;266(8):4987–4994. [PubMed] [Google Scholar]
- Goldstein L. S. The kinesin superfamily: tails of functional redundancy. Trends Cell Biol. 1991 Oct;1(4):93–98. doi: 10.1016/0962-8924(91)90036-9. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. F., Liskay R. M., Ou A. C., DeMaggio A. J., Burbee D. G., Heffron F. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science. 1991 Aug 30;253(5023):1031–1034. doi: 10.1126/science.1887218. [DOI] [PubMed] [Google Scholar]
- Issinger O. G. Casein kinases: pleiotropic mediators of cellular regulation. Pharmacol Ther. 1993;59(1):1–30. doi: 10.1016/0163-7258(93)90039-g. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Zheng J., Xuong N., Taylor S. S., Sowadski J. M. Structure of the mammalian catalytic subunit of cAMP-dependent protein kinase and an inhibitor peptide displays an open conformation. Acta Crystallogr D Biol Crystallogr. 1993 Jul 1;49(Pt 4):381–388. doi: 10.1107/S0907444993002306. [DOI] [PubMed] [Google Scholar]
- Kearney P. H., Ebert M., Kuret J. Molecular cloning and sequence analysis of two novel fission yeast casein kinase-1 isoforms. Biochem Biophys Res Commun. 1994 Aug 30;203(1):231–236. doi: 10.1006/bbrc.1994.2172. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Kong C. T., Cook P. F. Isotope partitioning in the adenosine 3',5'-monophosphate dependent protein kinase reaction indicates a steady-state random kinetic mechanism. Biochemistry. 1988 Jun 28;27(13):4795–4799. doi: 10.1021/bi00413a032. [DOI] [PubMed] [Google Scholar]
- Kuret J., Woodgett J. R., Cohen P. Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Identification of the sites phosphorylated by casein kinase-I. Eur J Biochem. 1985 Aug 15;151(1):39–48. doi: 10.1111/j.1432-1033.1985.tb09066.x. [DOI] [PubMed] [Google Scholar]
- Meggio F., Perich J. W., Reynolds E. C., Pinna L. A. A synthetic beta-casein phosphopeptide and analogues as model substrates for casein kinase-1, a ubiquitous, phosphate directed protein kinase. FEBS Lett. 1991 Jun 3;283(2):303–306. doi: 10.1016/0014-5793(91)80614-9. [DOI] [PubMed] [Google Scholar]
- Milne D. M., Palmer R. H., Campbell D. G., Meek D. W. Phosphorylation of the p53 tumour-suppressor protein at three N-terminal sites by a novel casein kinase I-like enzyme. Oncogene. 1992 Jul;7(7):1361–1369. [PubMed] [Google Scholar]
- Morgan D. O., De Bondt H. L. Protein kinase regulation: insights from crystal structure analysis. Curr Opin Cell Biol. 1994 Apr;6(2):239–246. doi: 10.1016/0955-0674(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Nicholls A., Sharp K. A., Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11(4):281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- RAMACHANDRAN G. N., RAMAKRISHNAN C., SASISEKHARAN V. Stereochemistry of polypeptide chain configurations. J Mol Biol. 1963 Jul;7:95–99. doi: 10.1016/s0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- Roach P. J. Control of glycogen synthase by hierarchal protein phosphorylation. FASEB J. 1990 Sep;4(12):2961–2968. [PubMed] [Google Scholar]
- Robinson R. G., Preston D. F., Spicer J. A., Baxter K. G. Radionuclide therapy of intractable bone pain: emphasis on strontium-89. Semin Nucl Med. 1992 Jan;22(1):28–32. doi: 10.1016/s0001-2998(05)80154-0. [DOI] [PubMed] [Google Scholar]
- Roof D. M., Meluh P. B., Rose M. D. Kinesin-related proteins required for assembly of the mitotic spindle. J Cell Biol. 1992 Jul;118(1):95–108. doi: 10.1083/jcb.118.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M. J., Harper J. W., Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993 Aug;12(8):3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. S., Radzio-Andzelm E. Three protein kinase structures define a common motif. Structure. 1994 May 15;2(5):345–355. doi: 10.1016/s0969-2126(00)00036-8. [DOI] [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Umphress J. L., Tuazon P. T., Chen C. J., Traugh J. A. Determinants on simian virus 40 large T antigen are important for recognition and phosphorylation by casein kinase I. Eur J Biochem. 1992 Jan 15;203(1-2):239–243. doi: 10.1111/j.1432-1033.1992.tb19852.x. [DOI] [PubMed] [Google Scholar]
- Vancura A., Sessler A., Leichus B., Kuret J. A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J Biol Chem. 1994 Jul 29;269(30):19271–19278. [PubMed] [Google Scholar]
- Vincent I. J., Davies P. A protein kinase associated with paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2878–2882. doi: 10.1073/pnas.89.7.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz K., Matsumura P. Crystal structure of Escherichia coli CheY refined at 1.7-A resolution. J Biol Chem. 1991 Aug 15;266(23):15511–15519. doi: 10.2210/pdb3chy/pdb. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Wang P. C., Vancura A., Desai A., Carmel G., Kuret J. Cytoplasmic forms of fission yeast casein kinase-1 associate primarily with the particulate fraction of the cell. J Biol Chem. 1994 Apr 22;269(16):12014–12023. [PubMed] [Google Scholar]
- Wang P. C., Vancura A., Mitcheson T. G., Kuret J. Two genes in Saccharomyces cerevisiae encode a membrane-bound form of casein kinase-1. Mol Biol Cell. 1992 Mar;3(3):275–286. doi: 10.1091/mbc.3.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Knighton D. R., ten Eyck L. F., Karlsson R., Xuong N., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MgATP and peptide inhibitor. Biochemistry. 1993 Mar 9;32(9):2154–2161. doi: 10.1021/bi00060a005. [DOI] [PubMed] [Google Scholar]
- de Groot R. P., den Hertog J., Vandenheede J. R., Goris J., Sassone-Corsi P. Multiple and cooperative phosphorylation events regulate the CREM activator function. EMBO J. 1993 Oct;12(10):3903–3911. doi: 10.1002/j.1460-2075.1993.tb06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]