Abstract
Isotopic and molecular analysis on human, fauna and pottery remains can provide valuable new insights into the diets and subsistence practices of prehistoric populations. These are crucial to elucidate the resilience of social-ecological systems to cultural and environmental change. Bulk collagen carbon and nitrogen isotopic analysis of 82 human individuals from mid to late Holocene Brazilian archaeological sites (∼6,700 to ∼1,000 cal BP) reveal an adequate protein incorporation and, on the coast, the continuation in subsistence strategies based on the exploitation of aquatic resources despite the introduction of pottery and domesticated plant foods. These results are supported by carbon isotope analysis of single amino acid extracted from bone collagen. Chemical and isotopic analysis also shows that pottery technology was used to process marine foods and therefore assimilated into the existing subsistence strategy. Our multidisciplinary results demonstrate the resilient character of the coastal economy to cultural change during the late Holocene in southern Brazil.
Introduction
The Brazilian coast encompasses a wide range of tropical and sub-tropical ecosystems that have sustained human populations from the middle Holocene to the present day. The large shell mounds, or sambaquis, are a distinctive feature of this coastline, testament to large-scale exploitation of marine resources, from ∼8,000 to ∼1,000 calibrated years before present (cal BP). In southern Brazil some sambaquis reached more than 35 m high and contained hundreds of burials, post holes and faunal remains testifying the development of a complex social panorama [1]. The exploitation of aquatic (mostly marine) resources was an important subsistence activity at these sites [2] and must have drawn people to the coast. However indirect evidence reveals that the contribution of plants also appears to be important [3]–[8]. Sambaquis containing freshwater and land snail shells are also found along the courses of rivers and their distribution penetrates some distances inland. These “Riverine sambaquis” are the same age or even older than their coastal analogues (∼10,000 to ∼1,000 cal BP) [9] and occasionally finds of marine fauna at these riverine sites suggest some connection to the coast [10], [11], [12].
A dramatic change is seen in the archaeological record at ∼1,500 cal BP with the abrupt cessation of large shell mound formation [13]. At this time it is thought that new populations from the southern highlands (known as the Taquara/Itararé tradition) [14] expanded to the coastal lowlands [15], likely driven by rapid population growth, increasing of social interaction and intensification in food production, involving maize and exploitation of pine forest (Araucaria angustifolia) [16]. The appearance of Taquara/Itararé pottery along the southern coast of Brazil therefore may mark a key turning point in exploitation of rich coastal ecotones, as prehistoric groups gained the knowledge and technology to develop new economic practices. However the extent to which the transmission (or imposition) of this new subsistence system transformed the indigenous coastal economy, and its capacity to adjust, persist and maintain its fundamental properties, is still a matter of debate [17].
While there is some evidence for consumption of new cultigens like maize by Taquara/Itararé groups [7] and increased consumption of terrestrial resources [18], marine fauna continue to be found at high abundance [19]. Similarly, while pottery is often assumed to be associated with the processing of new produced and foraged foods, with parallels in the southern Brazilian Highlands [16], there is no direct evidence of what it was used for. Our understanding of the diet and subsistence economy of the prehistoric inhabitants of coastal Brazil has been largely limited to traditional archaeological information, based on faunal, botanical and artefactual remains. In particular, the contribution of marine and terrestrial foods to the diet of both pre-ceramic and ceramic coastal populations of this region still remains largely unknown [11], [20], [21] and only few studies have considered the use of ceramics during this period [22], [23]. As a result, the impact of new economic and technological strategies on coastal adapted hunter-gatherers societies is not yet understood.
Here we report the results of an integrated study into the dietary variability of coastal and inland sambaqui populations. We analysed the stable carbon and nitrogen isotope composition of human bone collagen, a technique widely used to reconstruct paleodiets, and particularly for distinguishing marine versus terrestrial diets [24], [25]. We determined the stable carbon isotope signature of individual amino acids from bone collagen to identify different macronutrient constituents of diet [26]–[28]. We assessed the potential of bone mineral for isotope analysis in order to provide information on whole diet [24]. Finally we considered additional information regarding the diet of ‘incoming’ ceramic producing groups through the analysis of the organic contents of their pottery [29]–[31]. Hansel et al. [22] and Hansel and Schmitz [23] have already shown the potential for retrieving lipids from pottery in coastal Brazil, but here we report the first compound-specific isotopic analysis of these artefacts.
Archaeological Setting
The archaeological records include four middle and late Holocene sites located in southeast (São Paulo) and southern (Santa Catarina) regions of Brazil (Fig. 1). These consist of one inland riverine site (Moraes) and two coastal sambaquis (Jabuticabeira II, Piaçaguera), along with a recently excavated ceramic coastal site (Galheta IV), dated to the time of the expansion of the highland groups to the coast. The sambaqui sites dated to the preceramic period show a wide range, but well-established chronology (∼6,700 to ∼1,700 cal BP) and offer a unique opportunity to elucidate dietary variability between coastal and inland mound builders. They also offer a valuable isotopic baseline for assessing changes in subsistence strategies associated with the spread of pottery technology (∼1,500 cal BP). Preliminary stable isotope studies have already been carried out on human remains from some of these sites (Moraes, Jabuticabeira II) [11], [21] as well as other coastal sites in southern Brazil [20], [32]. Here we undertake the analysis of 106 human remains with the aim of greatly expanding the dietary isotope record for this region.
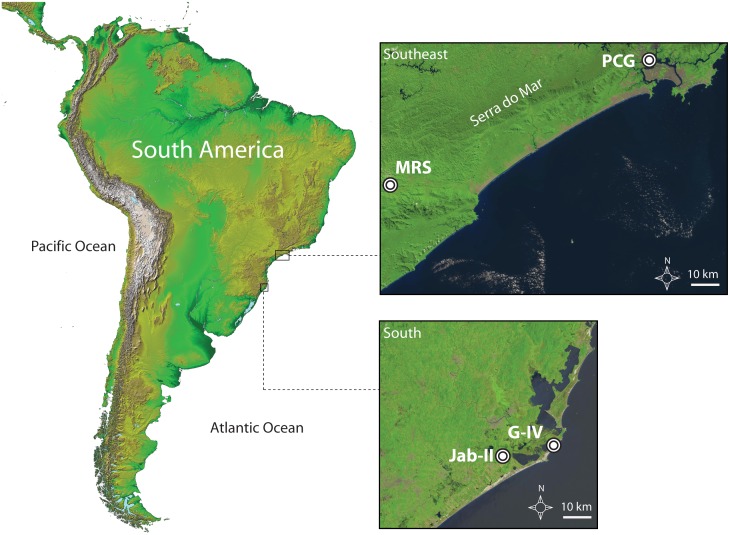
Figure 1. Geographic position of the analysed archaeological records.
Location of the study area showing the archaeological sites from southeast (PCG, MRS) and south (Jab-II, G-IV) regions of Brazil. Satellite imagery from USGS (EarthExplorer) and NASA (Shuttle Radar Topography Mission).
Moraes (MRS) is situated in the Ribera do Iguape Valley, at ∼35 km from the São Paulo coast. The site forms a mound with a conspicuous concentration of land snail shells dated between 6,775–6,499 and 5,289–4,887 cal BP [33]. The mound was used mainly for funerary purposes [34] and 55 human individuals were recorded in the deposit [34], [35], along with a consistent amount of faunal remains mostly represented by terrestrial mammals, followed by amphibians, birds, and only few reptiles and fish bones [10], [11].
Piaçaguera (PCG) is located in the south-east coastal plain of São Paulo state, at ∼12 km from the present day shoreline. PCG similarly provided a stratigraphic succession containing more than 80 human individuals, artefacts and faunal remains dated to 5,894–5,326 and 5,887–5,314 cal BP [35], [36]. Fish and molluscs dominate the assemblage, followed by some crustaceans, a few terrestrial mammals and carbonized seeds [35]–[38].
Jabuticabeira II (Jab-II), at ∼7 km from the present day shoreline at Santa Catarina, is one of the best-studied sambaqui sites of Brazil [21], [39]. The stratigraphic sequence is composed of alternating layers of marine shells and fish bones [21]. Numerous human burials and the absence of compelling domestic activities point to a ceremonial function of the site from 3,137–2,794 to 1,860–1,524 cal BP [13], [40].
Finally, Galheta IV (G-IV) is a funerary site overlooking the Atlantic Sea, at ∼20 km north of Jab-II, in Santa Catarina [41]. The site yielded 8 adult human burials, some of them partially cremated, associated with abundant marine faunal remains (fish, sea mammals, seabirds) and artefacts, including potsherds of Taquara/Itararé tradition. G-IV was 14C dated between 1,304–1,140 and 913–739 cal BP, and it dates to the expansion of inland ceramic producers (southern Jê speakers) to the coast.
Material and Methods
Human and Faunal Remains: Sampling Procedure and Ethical Statement
A total of 106 human individuals (different age and sex) from MRS, PCG, Jab-II and G-IV were sampled for isotopic analysis. In order to build a faunal isotopic reference for the region, 36 animal bone remains (terrestrial mammals, sea mammals, birds and fish) from these four sites were also selected for isotopic analysis (Tab. 1). Human samples were obtained almost entirely from the ribs, whereas fauna samples were from a range of different skeletal elements. All necessary permits were obtained for the described study, which complied with all relevant regulations of the Instituto do Patrimônio Histórico e Artístico Nacional – IPHAN (protocols n° 01506.00407/2012-14, 01506.001516/2006-47 and 01510.000047/2003-37). Archaeological materials used in this study are stored at the University of York (UK), Biology S-Block.
Table 1. Bone collagen δ13C and δ15N values of faunal remains.
| Site | Taxon | Vernacular name | δ13C% | δ15N% | %C | %N | C:N | Col wt% | Source |
| MRS | Tayassu sp. | Peccary | –21.0 | +8.6 | 27.0 | 9.6 | 3.3 | 0.5 | This study |
| MRS | Tayassu sp. | Peccary | –21.3 | +6.7 | 37.4 | 13.4 | 3.3 | 1.4 | This study |
| MRS | Tayassu sp. | Peccary | –23.5 | +9.2 | 35.6 | 12.7 | 3.3 | 1.5 | This study |
| MRS | Tayassu sp. | Peccary | –22.4 | +8.7 | 40.5 | 14.9 | 3.2 | 1.5 | This study |
| MRS | Mazama sp. | Brocket | –23.0 | +9.6 | 31.6 | 11.2 | 3.3 | 0.6 | This study |
| MRS | Mazama sp. | Brocket | –24.6 | +8.4 | 41.0 | 14.7 | 3.2 | 1.5 | This study |
| MRS | Cuniculus paca | Lowland paca | –20.8 | +7.9 | 42.5 | 14.4 | 3.4 | 1.2 | This study |
| MRS | Cuniculus paca | Lowland paca | –20.6 | +8.7 | 41.7 | 14.8 | 3.3 | 1.0 | This study |
| MRS | Alouatta sp. | Howler monkeys | –21.5 | +7.9 | 26.0 | 9.3 | 3.3 | 3.3 | This study |
| MRS | Alouatta sp. | Howler monkeys | –22.5 | +8.6 | 43.0 | 15.5 | 3.2 | 1.4 | This study |
| MRS | Tayassu sp. | Peccary | –22.3 | +6.5 | - | - | - | - | [11] |
| MRS | Alouatta sp. | Howler monkeys | –21.6 | +6.3 | - | - | - | - | [11] |
| PCG | Selachimorpha | Shark | –11.8 | +15.2 | 29.2 | 9.8 | 3.5 | 3.0 | This study |
| PCG | Euphractus sexcinctus | Six-banded armadillo | –20.3 | +13.3 | 40.5 | 13.7 | 3.4 | 1.9 | This study |
| PCG | Alouatta sp. | Howler monkeys | –22.5 | +6.9 | 56.4 | 19.8 | 3.3 | 2.0 | This study |
| Jab-II | Trichiurus lepturus | Hairtail | –11.1 | +12.8 | 41.1 | 14.8 | 3.2 | 3.5 | This study |
| Jab-II | Lobotes surinamensis | Tripletail | –10.5 | +14.5 | 41.9 | 14.6 | 3.4 | 2.3 | This study |
| Jab-II | Pogonias cromis | Blackdrum | –9.8 | +12.6 | 41.2 | 14.3 | 3.4 | 3.4 | This study |
| Jab-II | Ariidae | Sea catfishes | –9.2 | +15.5 | 42.1 | 15.1 | 3.3 | 2.7 | This study |
| Jab-II | Pomacanthidae | Angelfishes | –10.8 | +13.4 | 41.5 | 14.4 | 3.4 | 1.6 | This study |
| Jab-II | Cetacea | Undeter | –10.8 | +16.1 | 39.5 | 13.8 | 3.3 | 2.2 | This study |
| Jab-II | Aves | unknown bird | –19.5 | +7.2 | 42.3 | 15.0 | 3.3 | 4.8 | This study |
| G-IV | Aves | unknown seabird | –12.5 | +17.4 | 43.3 | 15.7 | 3.2 | 6.6 | This study |
| G-IV | Aves | unknown seabird | –9.8 | +18.9 | 44.3 | 16.0 | 3.2 | 6.2 | This study |
| G-IV | Aves | unknown seabird | –13.6 | +17.9 | 42.7 | 15.5 | 3.2 | 4.6 | This study |
| S Brazil | Arctocephalus tropicalis | Subantarctic fur seal | –11.0 | +16.0 | - | - | - | - | [20] |
| S Brazil | Arctocephalus australis | S American fur seal | –11.4 | +16.4 | - | - | - | - | [20] |
| S Brazil | Eubalaena australis | Southern right whale | –15.2 | +6.9 | - | - | - | - | [20] |
| S Brazil | Spheniscus magellanicus | Magellanic penguin | –11.2 | +14.5 | - | - | - | - | [20] |
| S Brazil | Selachimorpha | Shark | –9.5 | +16.0 | - | - | - | - | [20] |
| S Brazil* | Macrodon ancylodon | King weakfish | –11.8 | +13.0 | 60.0 | 22.4 | 3.1 | 11.0 | This study |
| S Brazil* | Epinephelus marginatus | Dusky grouper | –11.1 | +16.0 | 44.1 | 16.9 | 3.0 | 19.4 | This study |
| S Brazil* | Micropogonias furnieri | whitemouth croaker | –11.3 | +13.9 | 43.5 | 16.4 | 3.1 | 13.5 | This study |
| S Brazil* | Peprilus paru | American harvestfish | –15.0 | +13.0 | 49.9 | 14.7 | 4.0 | 11.1 | This study |
| S Brazil* | Mugilidae | Mullets | –12.5 | +11.5 | 41.5 | 15.5 | 3.1 | 12.8 | This study |
| S Brazil* | Cynoscion acoupa | Acoupa weakfish | –9.0 | +11.3 | 43.3 | 16.4 | 3.1 | 10.7 | This study |
| S Brazil* | Urophycis sp. | Brazilian codling | –10.9 | +14.2 | 41.0 | 15.6 | 3.1 | 13.0 | This study |
| S Brazil* | Coryphaena sp. | Common dolphinfish | –13.3 | +9.2 | 41.9 | 15.8 | 3.1 | 16.1 | This study |
| S Brazil* | Pomatomus saltatrix | Blue fish | –11.9 | +15.3 | 55.7 | 20.8 | 3.1 | 7.8 | This study |
| S Brazil* | Xiphias gladius | Swordfish | –12.1 | +9.9 | 70.9 | 26.2 | 3.2 | 11.5 | This study |
Isotopic analyses were also conducted on modern fish (n = 10) caught using traditional fishing techniques and acquired at the central market of Florianópolis (Santa Catarina, S. Brazil). The use of modern fish specimens was carried out in strict accordance with the recommendations of the Brazilian Institute of Environment and Renewable Natural Resources – IBAMA. No In Vivo experiment was developed, thus ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments) for this study is not applicable. The transport of modern specimens to the University of York was approved under the Convention on International Trade in Endangered Species of Wild Fauna and Flora – CITES-IBAMA (protocol n° 113508). Modern fish collagen δ13C were corrected (+1.14%) for the global decrease of atmospheric δ13C values [42]. We also incorporated δ13C and δ15N values from Plens [11] and De Masi [20], who report collagen isotopic composition of archaeological faunal remains from MRS and other coastal sites of southern Brazil.
Assessing Bone Collagen and Mineral Preservation
Bulk collagen δ13C and δ15N derive primarily from dietary protein, although macronutrients (carbohydrates, lipids) may variably contribute to collagen carbon, particularly for the non-essential amino acids in collagen [43]–[45]. The δ13C of bone apatite instead reflects the total dietary pool of carbon ingested [46]. Therefore the combination of collagen and apatite δ13C has been shown to give information on main energy and protein consumed several years prior to death [47], [48]. In archaeological contexts, however, the burial environment may impact the physical and chemical composition of bones in different ways [49], particularly through loss of collagen and alteration of bio-apatite and the stable isotope signature of this fraction [50].
Assessment of collagen preservation in both human and faunal remains was carried out following the criteria proposed by van Klinken [51]. In addition, Raman spectroscopy studies were performed on randomly selected human bone samples from MRS (n = 9), Jab-II (n = 9), G-IV (n = 4) and PCG (n = 10) to assess diagenetic change to the mineral fraction, with modern lamb bone being used as a control [52]. To optimise the quality of the Raman spectra, the samples of bone were flattened and smoothed by gentle rubbing with fine-grade diamond paper. The Raman spectra were collected from the samples using an HORIBA XploRa instrument at 532 nm laser wavelength and under x100 magnification in confocal mode (NA = 0.9, with 2400 g mm-1 grating). Five spectra were collected from each bone specimen using 1s laser exposure at ∼3.5 mW power at the sample, with each measurement averaged over 40 spectral acquisitions. The spectra were collected over 4 spectral windows to achieve a total spectral range of 200–3200 cm−1. The software package IGOR Pro 6.32 was used to average, baseline correct, and analyse the Raman spectra using Gaussian peak-fitting procedures, with the ν1 carbonate peak (at ∼1070 cm−1) de-convoluted according to published protocols [53].
Collagen Extraction and Isotope Analysis
Collagen preparation follows the protocol described in Craig et al. [54]. Before isotopic analysis, lipids were removed from modern fish bones with dichlormethane:methanol (2∶1, x3). Between 300 and 500 mg of cleaned human and animal bones were used for collagen extraction. Samples were agitated in 8 ml of 0.6 M hydrochloric acid at 4°C to demineralize. Once demineralization had occurred the samples were removed from the acid and washed with ultrapure water three times. The samples were gelatinised in pH 3 hydrochloric acid and maintained for forty-eight hours at 80°C. The gelatinised samples were then ultrafiltered and a >30 kDa fraction was lyophilised. Duplicates (1 mg) were measured using a continuous flow isotope ratio mass spectrometry Thermo Finnigan Delta Plus XL in the Department of Archaeological Sciences of the University of Bradford (UK), to determine the δ13C and δ15N values. The results are reported using the delta scale in % relative to internationally accepted standards, V-PDB and AIR respectively. Analytical error, calculated from repeated measurements of each sample and measurements of the bovine control from multiple extracts, was <0.2% (1σ).
Analysis of Individual Amino Acids in Collagen
Stable carbon isotope analyses were performed on collagen amino acids isolated from randomly selected coastal individuals from Jab-II (n = 10) and G-IV (n = 7). Approximately 1 mg of collagen was hydrolysed under vacuum in amino acid free 6 M hydrochloric acid (1 ml) at 110°C for 24 hours. After hydrolysis the samples were dried in a rotary vacuum concentrator and stored at –20°C until analysis. Prior to isotopic analysis, the samples were redissolved under sonication in MilliQ water with the addition of an internal standard (2-amino-isobutyric acid).
Instrumental analysis was carried out using Thermo Scientific Liquid chromatography isotope ratio mass spectrometry (LC-IRMS) system consisting of an Accela 600 pump connected to a Thermo Scientific LC Isolink and a Delta V Plus Isotope Ratio Mass Spectrometer housed at the La Trobe Institute for Molecular Sciences (LIMS, La Trobe University, Melbourne, Australia). LC-IRMS analysis of single amino acid fractions of collagen hydrolysates were carried out using a three phase LC/IMRS method similar to that described in Smith et al. [55], with the exception that a Primesep A (SCIELC) column (2.1×250 mm, 100 Å, 5 μm) was used. This is a narrower column to that used by Smith et al. [55] and thus lower LC flow rates were used. Conditioning runs were made at 110 μLmin−1 using phases ratios of 85B:15C–95B:5C, analytical runs were made using flow rates of 60–80 μLmin−1 and oxidation reagent flow rates were set at 35 μLmin−1 each. Approximately 10 μg of amino acid hydrolysate (on column) was used for each run. Amino acid peak δ13C values were measured against CO2 gas pulses throughout the run (δ13CVPDB = –2.8%) calibrated against international standard USGS-40 L-Glutamic Acid (δ13CVPDB-LSVEC = –26.4±0.04%). In house standard runs were made during the sample runs to monitor measurement quality.
Organic Residues Analysis
Molecular and isotopic analysis of organic residues absorbed into porous vessels or preserved in surface deposits offer valuable information concerning pottery use [29], [30], [54], [56]–[58]. Lipids were extracted (and methylated in one-step) from 14 potsherds according to protocols reported in Craig et al. [31], [59]. Briefly, after cleaning the surface, methanol (4 ml) was added to powdered ceramic samples (70 to 240 mg) and the mixture was sonicated for 15 min and then acidified with concentrated sulphuric acid (800 ml). The acidified suspension was heated in sealed tubes for 4 h at 70°C and then cooled, and lipids were extracted with n-hexane (2 ml×3). The extract was dried under a gentle flux of nitrogen and internal standard (n-hexatriacontane) was added before the direct analysis by gas chromatography/mass spectrometry (GCMS) at the University of York (UK).
Stable isotopic analysis of n-hexadecanoic (C16∶0) and n-octadecanoic (C18∶0) acids from 11 extracted lipid samples were performed using a gas chromatograph (GC) coupled to a combustion isotope ratio mass spectrometry (GC-C-IRMS) at the University of Liverpool (UK) following the protocol reported in Craig et al. [59]. Instrument precision on repeated measurements was 0.2% (s.e.m.).
Charred residues of food were preserved in the internal part of 6 ceramic potsherds. Samples (3–7 mg) were removed and subsamples (1 mg) selected for carbon and nitrogen isotopic analysis at the University of Bradford by using the same IRMS procedure as for bone collagen [56].
Statistical Analysis
The proportional contribution of different food sources to human diet (based on stable isotope values) was estimated using a Bayesian mixing model in SIAR V4 (Stable Isotope Analysis in R) [60]. The bulk δ13C and δ15N values of faunal remains (Tab. 1) was used in the model calculation after adding trophic enrichments of 1% and 4% for carbon and nitrogen respectively [61]. Bulk δ13C and δ15N were also analysed using the parametric One-Way Anova test in the software PAST 2.13 [62], after checking for normal distribution (Shapiro-Wilk) and using a statistical significance probability threshold of α = 0.05. Available radiocarbon ages were calibrated with OxCal 4.2, using the Southern Hemisphere curve SHCal04 [63], [64].
Results
Bone Apatite and Collagen Preservation
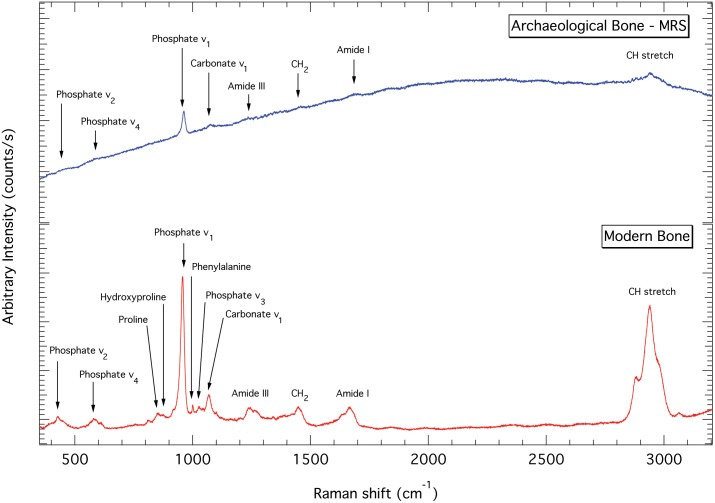
Raman analysis revealed substantial alteration to the mineral phase of all of the archaeological samples that were analysed (Tab. S1, Fig. 2). Substitution of non-biogenic carbonate for biogenic phosphate, or reduction in biogenic phosphate, is indicated by the increased carbonate ν1 (C) to phosphate ν1 (P) intensity ratios in archaeological bones compared to the modern control [65], [66]. An increase in crystallinity due to mineral alteration in the archaeological specimens is expected [49], [67] and confirmed in our Raman data by a decrease in the full width at half maximum (FWHM) of the ν1 P band (at ∼957 cm−1) relative to the modern bone sample [65], [66], [68]. Increasing crystallinity in diagenetically altered bone has been linked to the loss of collagen [49], which is further corroborated in our data by reduced CH/P ratios compared to the modern bone sample. Here, CH refers to the collagen band (i.e. CH stretching) at ∼2933 cm−1. The CH/P trend is also in agreement with the Raman spectroscopy results of Edwards et al. [69], which demonstrated reduced intensity in the collagen modes in human bones collected at JAB-II. Finally, the large fluorescent background in the archaeological samples (Fig. 2) is attributed to spectral emission from luminescent ions incorporated into the bone lattice due to diagenetic alteration [65].
Figure 2. Bone diagenesis.
Examples of Raman spectra of an archaeological bone spectrum (MRS) and a modern lamb bone without baseline correction showing the Raman band assignments of the key peaks. All peaks are identified as per the literature [68] and [112], with results being comparable to those described in Edwards et al. [69].
Despite widespread alteration to the bone mineral phase, most of human burials (82 out of 106) matched the criteria for adequate collagen preservation [51]. Collagen yield and C:N ratios individuals from MRS (n = 15), PCG (n = 11), Jab-II (n = 47) and G-IV (n = 6) range from 0.9 to 7.7 wt% and from 3.2 to 3.5 respectively (Tab. SI2). Three individuals from PCG and one from G-IV contain <1% collagen but still had acceptable C:N ratios and their δ13C and δ15N values are coherent with the other humans. Similarly, most of the animal samples from the study sites (25 out of 36) had adequate (>1%) collagen yields (Tab. 1). Of these, one peccary (Tayassu sp.) and one brocket (Mazama sp.) from MRS show collagen yield of 0.5 and 0.6 wt% respectively, but again the C:N matches the criteria for unaltered collagen. In summary, whilst the bone mineral fraction is unlikely to preserve a vital isotopic signal, the collagen yield and C:N composition suggests acceptable collagen preservation for the majority of the samples [51], [70].
Bulk Collagen Stable Isotope Analysis
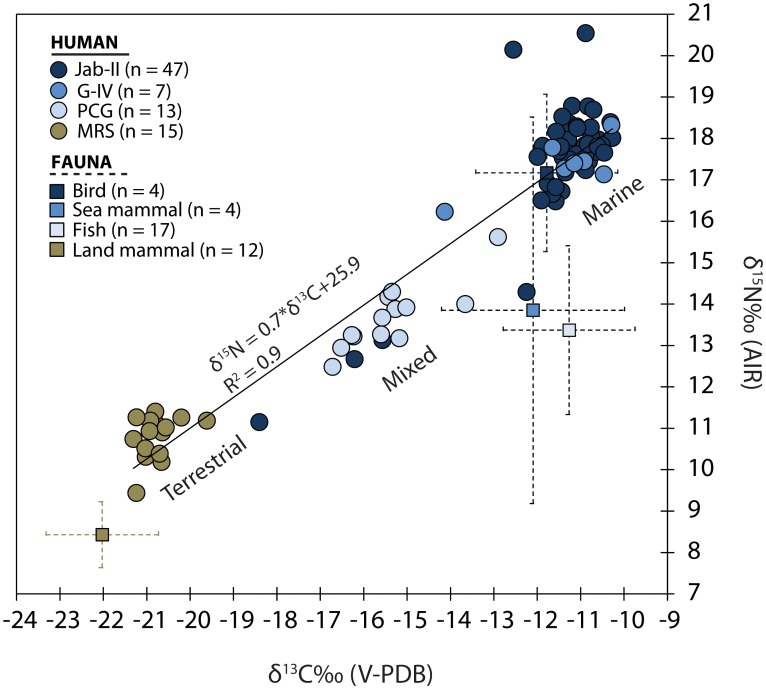
Terrestrial fauna comprehensively show average δ13C and δ15N of −22.0±1.2% and +8.4±1.7% respectively (Tab. 1). Some variability is observed in carbon isotopes at MRS and may be a result of hunting in different environments. The aquatic fauna, modern and archaeological fish, exhibits average δ13C and δ15N of −11.1±1.5% and +13.5±1.8% respectively. Sea mammals show average δ13C and δ15N values of −12.3±1.9% and +12.9±4.5%. Seabirds show average δ13C of −13.3±3.7% and the highest average δ15N value among faunal remains, +15.2±4.8%, pointing to the consumption of higher trophic levels marine resources.
Human δ13C and δ15N values show strong positive linear correlations (r = 0.95; R2 = 0.92; p<0.001; Tab. S2) and fall between the end-points derived from correcting the observed marine and C3 terrestrial fauna for isotopic fractionation (Fig. 3). Therefore the human isotope values can be largely explained by direct routing of both carbon and nitrogen from dietary protein to collagen, which implies that the diets contained sufficiently high protein [71]. The δ13C and δ15N values differ significantly among sites (p<0.001; Tab. 2). Lower δ13C and δ15N values were observed in inland individuals from MRS, as opposed to higher values of coastal pre-ceramic and ceramic individuals from Jab-II and G-IV, in agreement with preliminary isotopic studies [11], [21]. Individuals from PCG, instead, exhibit δ13C and δ15N values consistent with a mixed marine/C3 terrestrial diet.
Figure 3. Bulk collagen δ13C and δ15N values.
Distribution of human and faunal values from Jabuticabeira II (Jab-II), Galheta IV (G-IV), Piaçaguera (PCG) and Moraes (MRS). Fish values also include modern specimens.
Table 2. Average bone collagen δ13C and δ15N values of humans, including isotopic variability (Δδ%) and the number of individual analysed.
| Site | δ13C% | Δδ13C% | δ15N% | Δδ15N% | N |
| MRS | –20.8±0.4 | 1.7 | +10.8±0.5 | 2.0 | 15 |
| PCG | –15.4±1.0 | 3.8 | +13.7±0.8 | 3.1 | 13 |
| Jab-II | –11.5±1.5 | 8.1 | +17.4±1.6 | 9.4 | 47 |
| G-IV | –11.4±1.2 | 3.8 | +17.4±0.6 | 2.1 | 7 |
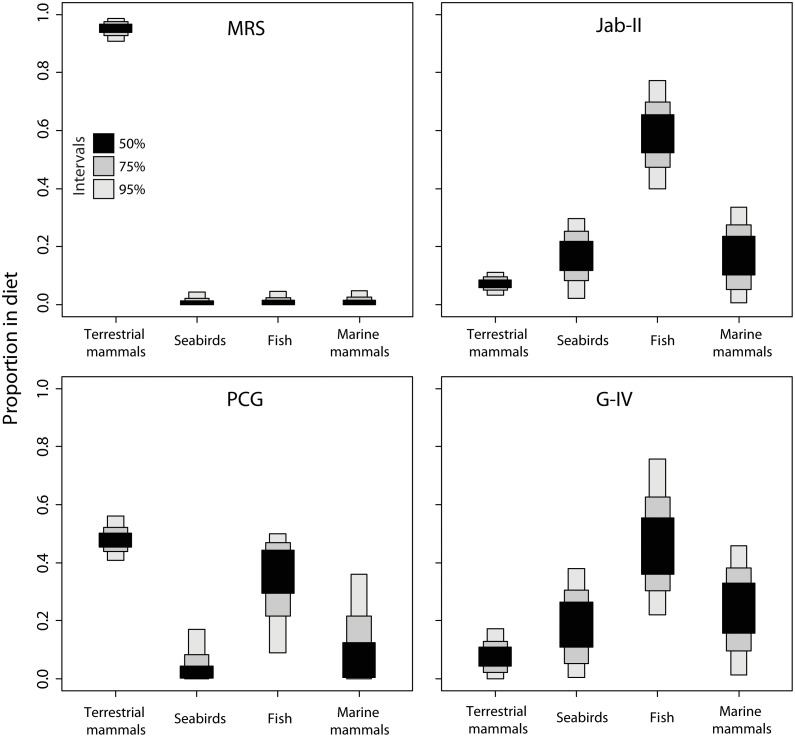
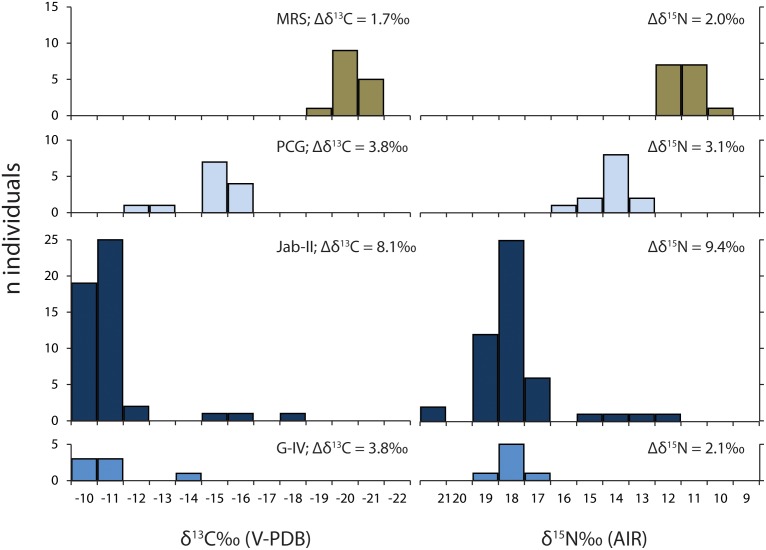
The proportional contribution of different marine and C3 terrestrial animal resources to each individual’s diet can be crudely estimated through linear interpolation between the marine and terrestrial end-members. We also used a Bayesian model which generates possible dietary solutions from multiple dietary source categories. This model predicts that inland peoples acquired >90% of their protein from C3 terrestrial resources, whereas people on the coast were assimilating protein mainly from fish, up to 80%, along with some contribution from seabirds and sea mammals (Fig. 4). A large isotopic variability, however, was detected in coastal groups, in particular at Jab-II (Fig. 5) but it seems not to be related to sex and age (Tab. S3).
Figure 4. Bayesian-derived proportion of protein sources for archaeological site according to 95%, 75% and 50% of the dataset.
Overlap of land mammal and fish in PCG show that human diet was based on marine as well as terrestrial items. This is not observed at MRS, Jab-II and G-IV.
Figure 5. Bulk collagen δ13C and δ15N variability (Δδ) in inland and coastal populations.
Note the large isotopic variability in humans from Jab-II.
It is important to note that these estimations refer to the protein contribution to total dietary protein (by dry weight) and not the contribution to total diet. In this case, the bulk isotopic analyses are highly insensitive to the other dietary components, such as carbohydrate and lipid, which must have been consumed to some extent in order to avoid protein poisoning [72].
Carbon Stable Isotope Analysis of Single Amino Acids
Stable carbon isotope values were obtained from 15 amino acids corresponding to 97.5% of the carbon atoms in collagen (Tab. S4). Mass balance calculations were used to estimate the δ13C of whole collagen from the measured individual amino acid δ13C values. These estimated values were strongly correlated with the observed bulk δ13C values (R2 = 0.98) and the offset between the estimated and observed measurements was <1% in all cases. The δ13C of individual amino acids were strongly and positively correlated with both the δ13C (R2 = >0.8) and δ15N (R2 = >0.7) values of whole collagen for both marine and C3 terrestrial consumers, confirming that both dispensable and non-dispensable amino acids were largely derived from a dietary protein source.
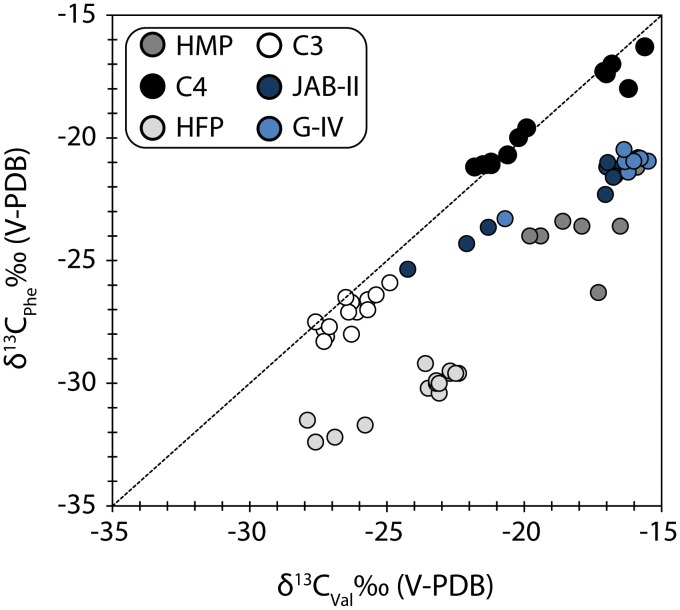
Honch et al. [28] devised a method of interpreting dietary intake of individuals using a plot of δ13C values of phenylalanine (Phe) and valine (Val) of bone collagen hydrolysates. Following this method, the δ13C values again confirm the two dietary groups; those with mixed marine/C3 terrestrial resource diets (Jab-II, burials 17C, 24A, 102 and G-IV, burial 7) and all other samples as high marine protein (HMP) consumers (Fig. 6).
Figure 6. A biplot of phenylalanine and valine δ13C values.
The biplot distinguishes two dietary groups at Jab-II and G-IV; those with mixed marine/C3 terrestrial animal diets and those consuming high marine protein (HMP). Data from Honch et al. [28] are reported for comparison and include C4, C3 and high marine (HMP) and freshwater protein (HFP) consumers.
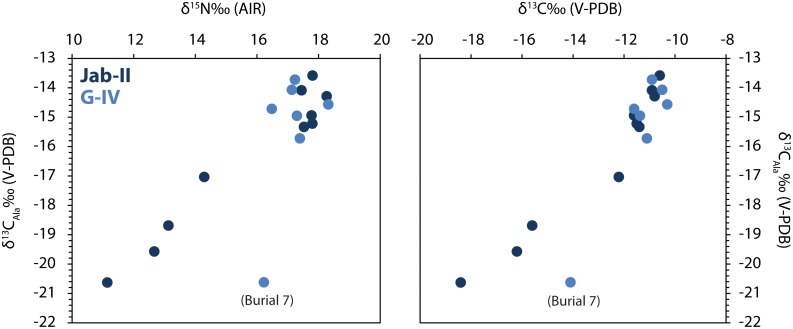
There are some interesting observations concerning the amino acid δ13C values of burial 7 at G-IV. In general the values for both dispensable and non-dispensable amino acids (Tab. S4) for this individual are intermediate between the mixed marine/C3 terrestrial consumers and the HMP consumers, indicating a mixed diet. Unexpectedly however, this individual has a lower δ13C value for alanine (Ala) compared to the others (Fig. 7). In the context of the other amino acid values, the low alanine value is difficult to explain. If the alanine were derived from a protein source with low δ13C values (e.g. C3 terrestrial or freshwater fish) we would expect other amino acids to be 13C depleted (especially Val and Phe; Fig. 6), which is not the case. Ala is a dispensable amino acid and can be directly routed from diet but is also readily synthesized from precursors originating in the first steps of glycolysis [73]. The latter are predominantly derived from dietary carbohydrates. Recently Choy et al. [73] noted that alanine values (in red blood cells and hair keratin of modern individuals) were strongly related to carbohydrate intake and not other dietary sources (i.e. meat, fish, marine mammals and corn products) whilst the other dispensable amino acid δ13C values (Pro, Glx, Asx, Ser and Gly) were not related to carbohydrate intake. We can interpret the δ13C alanine data from the individual in burial 7 at G-IV as an individual who had a long term diet that contained a higher amount of 13C depleted carbohydrate, e.g. from C3 plants, compared to the others. Apart from this exception, the carbon isotope analysis of individual amino acids confirms the bulk collagen analysis and suggests that the majority of individuals consumed adequate protein to supply the nearly all the carbon in collagen. Whether alanine δ13C values can be used as a carbohydrate marker in palaeodietary contexts warrants further testing.
Figure 7. Bulk collagen δ13C and δ15N against alanine δ13C values for individuals from Jab-II and G-IV.
Notice the strong positive correlation of alanine δ13C values with bulk collagen δ13C and δ15N. Exceptionally alanine is depleted in 13C in one individual from G-IV (burial 7) likely implying a larger contribution of C3 plant carbohydrate in the diet.
Organic Residue Analysis from Pottery at G-IV
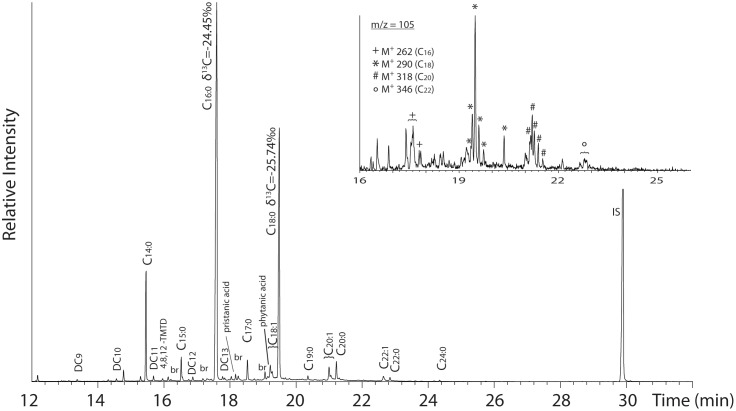
Molecular and isotopic compositions of adsorbed organic residues indicate that pottery vessels were used for the processing of marine products, along with plants and other animal resources. Overall the lipid preservation was poor, and the majority of samples contained only low levels (<0.5 μg mg−1) of palmitic and stearic acids. Nevertheless one sample (G18E) has a lipid profile consisting of medium- and long-chain saturated (C14–C24) and monounsaturated (C18∶1–C22∶1) fatty acids, isoprenoid fatty acids (4,8,12-trimethyltridecanoic acid and phytanic acid) and long-chain (C18–C22) ω-(o-alkylphenyl) fatty acids (Fig. 8, Tab. S5). Such a profile is characteristic of degraded aquatic oils, as established on contemporaneous pottery from this region [22]. A similar lipid distribution was found in G16P but with lesser preservation of ω-(o-alkylphenyl) fatty acids, preventing a clear confirmation of aquatic oils. This sample and G24P contain a series of triterpenes (m/z 189, 218) revealing the presence of plant resins which were also found in Taquara/Itararé pottery assemblages and were interpreted as a waterproofing coating [23]. Finally, one sample (G22P) had low quantities of very long chain fatty acids (up to C28∶0), traces of long chain dicarboxylic fatty acids (C22–C24) and isomers of the C18 ω-(o-alkylphenyl) fatty acid only. The latter compound is formed by heat alteration of polyunsaturated C18 fatty acids, which is consistent with a plant contribution in the profile. It also contains an unusually high concentration of C12∶0 which in this context could be derived from palm kernel oil [74], [75], although the difference in the fatty acid distribution as the various biomarkers present suggest a complex mixture of lipids from different origins.
Figure 8. Partial total ion current chromatograms showing the methylated lipids extracted from a ceramic sherd (G18E).
Cn :x - fatty acids with carbon length n and number of unsaturations x, DCn - α,ω-dicarboxylic acids with carbon length n, br - branched chain acids, phytanic acid, pristanic acid, 4,8,12-TMTD - 4,8,12-tryimethyltridecanoic acid), IS - internal standard (n-hexatriacontane). m/z 105 ion chromatogram showing the presence of ω(ο-alkylphenyl)alkanoic acids with 16 (+), 18(*), 20(#) and 22 (ο) carbon atoms.
The δ13C values (both C16∶0 and C18∶0≥–25%) of medium chain-length n-hexadecanoic (C16∶0) and n-octadecanoic (C18∶0) acids from 7 out of 11 pottery samples are within the range of marine oils reported in previous studies (Tab. S5). Lower δ13C values, in contrast, match those observed in modern pottery vessels used to process freshwater and non-ruminant animal fats and oils [30], [31]. There was no evidence for the processing of ruminants in pottery [59] despite the presence of cervids (Mazama sp., Ozotoceros bezoarticus) in the faunal assemblages [41]. Biomarkers associated with maize processing, e.g. n-dotriacontanol, were absent in all the vessels studied although there are doubts whether these would accumulate or preserve in sufficient quantities to allow identification [76].
Charred surface residues show δ13C and δ15N values ranging from −22.6% to −25.8% and from +6.7% to +12.7% respectively (Tab. S6). Samples enriched in 13C and depleted in 15N may tentatively indicate some contribution of C4 plants [77]. However the correlation between C4 plants (e.g. maize) and δ13C and δ15N values of charred deposit are not straightforward [78], [79], and our δ13C and δ15N results are also consistent with those observed in coastal areas of Northern Europe resulting from the processing of aquatic resources [30]. In spite of the complexity underlying food crust isotopic signatures, compound specific isotopic data from the same potsherds reinforces the interpretation that marine foods principally contributed to the isotopic signal of these charred deposits.
Discussion
Marine and C3 terrestrial animals were the main sources of protein for coastal and inland sambaqui builders of S. Brazil between ∼6,700 and ∼1,700 cal BP. The isotopic gradient from the inland to the coast suggests the existence of confined catchment areas and/or selective targeting of specific resources [80]–[82], which is a common feature amongst sambaqui builders [11], [13], [20], [21] and other coastal populations in South America [81]. There is no isotopic evidence for the contribution of freshwater resources, which is consistent with the very low frequency of these remains in both mainland and coastal sites [11], [21]. Interestingly, some individuals at Jab-II have an unusually high intake of C3 terrestrial proteins, denoting some degree of population variability on the coast. Although the lack of significant isotopic differences between sexes and age is consistent with pervasive food sharing among these populations, the intra-population δ13C and δ15N variability at Jab-II may point to the presence of non-local individuals, as observed in other preceramic coastal populations [18], perhaps assimilated into the group through post-marital residential practices [83]; however isotopic variability may also be associated to food restrictions among members of the community [84].
Our results also revealed that the peoples at G-IV relied substantially on marine resources, to the same extent as the pre-ceramic coastal adapted populations. The isotopic results of diets at coastal sites are broadly supported by the rich archaeozoological evidence dominated by marine resources [21], [41], [85]. Collagen from individuals at Jab-II and G-IV are amongst the most enriched in 13C and 15N in the eastern coast of South America between ∼8,000 and ∼1,000 cal BP [20], [21], [81], [86]–[97].
The carbon isotope signature of individual amino acids indicate a minor contribution of plants to the diet of some individuals, including those that post-date the adoption of pottery on the coast. The occurrence of mortars and plant macro-remains at several, but not all, sites [1], [4], [5], [98], along with variable degree of caries, starch grains in dental calculus [5], [6] and dental wear [99] also indicate that plants made a contribution to the diets of some coastal groups at this time [1], [3], [100]. Furthermore preliminary studies have also successfully extracted phytoliths and starch grains from charred deposits of pottery from G-IV, and one individual provided amino acid (alanine) δ13C values suggestive of a larger intake of C3 carbohydrates. However, the bulk collagen isotope analysis indicates that plants were unlikely to be major dietary staples for these coastal groups, rather the diet was protein-rich and oriented toward marine resources.
The continuity in coastal exploitation is further supported by molecular and isotopic results from organic residues preserved in pottery. These data attest to the use of pottery for the processing of animal products, including marine organisms. Hansen and Schmitz [23] achieved similar results from coeval sites in southern Brazil, revealing that pottery was commonly used for the manipulation of marine resources. Combined results therefore indicate that the adoption of pottery in coastal areas is not directly connected with the imposition of food production and did not affect the proportional contribution of marine resources to the diet of coastal populations. Small ceramic vessels had most likely a ritual and symbolic utility [16], [41]. Therefore, the molecular and isotopic analyses provide new direct evidence of the importance of marine resources in symbolic spheres. This is an interesting finding as it may have been assumed that novel or exotic cultivated plants would have had a more ritual and symbolic role in cuisine and therefore would have been more visible in the pottery contents.
Conclusion
Maritime adaptations sustained South American pre-Columbian populations since the late Pleistocene [101] and stable isotope studies reveal the crucial role of aquatic resources to several Holocene coastal groups (e.g. [86]–[92]), even during the intensification of food production (e.g. [82], [102]–[108]). However these direct lines of evidence are strongly biased towards archaeological records along the western and south-eastern coast of South America. Here we have extended the information to the subtropical Atlantic rainforest coast of Brazil. The isotope results show that it is highly unlikely that these coastal populations relied on plant carbohydrate as a major dietary source. Instead, we demonstrate the strong dependence of marine animal resources, despite the decline of monumental shell mound building and the arrival of a new subsistence strategy at ∼1,500 cal BP, involving domesticated plants and pottery technology, from inland areas. Therefore our results imply that the productive maritime economy was highly resilient to social and cultural change. It remains to be assessed if ceramic producing populations on the coast were directly descended from indigenous coastal foragers or immigrants from the highlands who, having reached the coast, oriented their economy toward aquatic resources. However the resilient character of this subsistence system is further expressed by its flexibility. Rather than transforming the coastal economy, as observed in the Atlantic coasts of Europe [109], [110], the adoption of pottery was incorporated into marine focused subsistence strategies [30]. These results emphasize how the archaeological record offers a unique and exceptional opportunity to illuminate the longstanding trajectory of New World maritime adaptations, which still today play a pivotal role to coastal populations in Latin America [111].
Supporting Information
Carbonate ν1 (C) to phosphate ν1 (P) intensity ratios, full width at half maximum (FWHM) of the phosphate ν1 (P) band and organic (C-H stretch) to phosphate ν1 (P) intensity ratios determined for each averaged spectrum as a function of the sample type. Samples are ordered from youngest to oldest with data acquired using the same Raman confocal settings across all samples. Modern lamb bone is justified as an appropriate control due to the similarities in sheep and human bone as per the RS study of Rehman et al. [52].
(DOCX)
Bone collagen δ13C and δ15N values of humans. Also show the age class or the relative age (young, adult) and the sex (F: female, M: male).
(DOCX)
One-way ANOVA showing a general lack of significant isotopic differences between sexes and age at MRS, Jab-II and PCG. Data from G-IV was not sufficient to be tested statistically. Because of the limited information about the age, individuals from MRS and PCG were sorted out in two categories: <36 and >36 years old. At Jab-II, individuals belonging to the age class 11–20 years old show higher δ15N values (mean 19.1±1.0%, n = 3) than individuals belonging to the age class 36–50 yrs (mean 17.3±0.9%, n = 13).
(DOCX)
Collagen amino acid δ13C values for humans from Jab-II (n = 10) and G-IV (n = 7).
(DOCX)
Ceramic sherds selected for lipid analysis by GCMS and GC-c-IRMS. FA (Cx:y) - fatty acids with carbon length x and number of unsaturations y, br -branched chain acids, phy- phytanic acid, TMTD - 4,8,12-trimethyltridecanoic acid. APFA (Cn) - ω-(o-alkylphenyl) alkanoic acids with carbon length n. tr - trace. DCx - α,ω-dicarboxylic acids with carbon length x. P - interior, E - exterior. Aquatic oils are interpreted from the presence of isomers of APFA (C20 or C22) and at least one isoprenoid fatty acids (pri, phy or TMTD). Resins are interpreted from the presence of triterpenes. Plant oils are interpreted from the presence of long chain fatty acids, dicarboxylique acids and the presence of isomers of C18 APFA. A high abundance of C12∶0 could be consistent with Palm Kernel. Aquatic (marine) fats are defined on the isotopic characteristics of the C16 and C18 saturated fatty acids.
(DOCX)
Bulk isotope characteristics of charred deposits from the interior of potsherds from G-IV.
(DOCX)
Acknowledgments
The authors are very grateful to Hayley Saul, Beatrice DeMarchi, Harry Robson, Terry O’Connor, Christina Cartaciano, Dave Coulthard (University of York, UK), Anu Thompson (University of Liverpool, UK), Ximena Villagran (Universität Tübingen, Germany), Cecilia Carlucci Petronilho, Celia Boyadjian, Luis Pezo Lanfranco, Tiago Attorre (Universidade de São Paulo, Brazil), Daniela Klokler (Universidade Federal de Sergipe, Brazil) for their helpful assistance in the realization of this paper. We are also grateful to IPHAN, ICMBio, IBAMA and to the anonymous reviewers. The contents of this research paper reflect only the authors’ views and not the views of the European Commission.
Funding Statement
This research was funded by the EU Marie-Curie Action: “Intra-European fellowships for career development” (Coastal resources and South American hunter-gatherers: biochemical perspectives from Brazilian sambaquis - COREBRAS, Ref. 273734). FAPESP: projeto Sambaquis e Paisagem (Ref. 11038-0), Brazil, Australian Research Council Future Fellowship (FT0992258), Australia. Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil and La Trobe University, Australia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. DeBlasis PAD, Fish SK, Gaspar MD, Fish PR (1998) Some references for the discussion of complexity among the Sambaqui moundbuilders from the southern shores of Brasil. Rev Arqueol Amer 15: 75–105. [Google Scholar]
- 2. Figuti L (1993) O homem pré-histórico, o molusco e o sambaqui. Revista do Museu de Arqueologia e Etnologia USP 3: 67–80. [Google Scholar]
- 3.Neves WA, Wesolowski V (2002) Economy, nutrition and disease in prehistoric coastal Brazil: a case study from the state of Santa Catarina. In: Steckel RH, Rose JC editors. The Backbone of History: Health and Nutrition in the Western Hemisphere. Cambridge University Press, 376–405.
- 4. Scheel-Ybert R, Eggers S, Wesolowski V, Petronilho CC, Boyadjian CH, et al. (2003) Novas perspectivas na reconstituição do modo de vida dos sambaquieiros: uma abordagem multidisciplinar. Revista Arqueologia 16: 109–137. [Google Scholar]
- 5. Scheel-Ybert R, Eggers S, Wesolowski V, Petronilho CC, Boyadjian CH, et al. (2009) Subsistence and lifeway of coastal Brazilian moundbuilders. In: Treballs d’Etnoarqueologia Capparelli A, Chevalier A, Piqué R, editors. La alimentación en la América precolombina y colonial: una aproximación interdisciplinaria. 7: 37–53. [Google Scholar]
- 6. Boyadjian CHC, Eggers S, Reinhard K (2007) Dental wash: a problematic method for extracting microfossils from teeth. Journal of Archaeological Science 34: 1622–1628. [Google Scholar]
- 7. Wesolowski V, Mendonça de Souza SMF, Reinhard KJ, Ceccantini G (2010) Evaluating microfossil content of dental calculus from Brazilian sambaquis. Journal of Archaeological Science 37: 1326–1338. [Google Scholar]
- 8.Okumura MMM, Eggers S (2012) Living and eating in coastal Brazil during prehistory. In: Collard D, Morris J, Perego E, editors. Food and Drink in Archaeology 3: Prospect Books. 55–64.
- 9. Figuti L, Plens CR, DeBlasis PAD (2013) Small sambaquis and big chronologies: shellmound building and hunter-gatherers in Neotropical highlands. Radiocarbon 55(2–3): 1215–1221. [Google Scholar]
- 10. Plens CR (2009) O papel dos amontoados de conchas no sambaqui fluvial. Revista de Arqueologia 22(2): 77–93. [Google Scholar]
- 11. Plens CR (2010) Animals for humans in life and death. Museu de Arqueologia e Etnologia 20: 31–52. [Google Scholar]
- 12. Eggers S, Parks M, Grupe G, Reinhard KJ (2011) Paleoamerican diet, migration and morphology in Brazil: archaeological complexity of the earliest Americans. PLoS ONE 6(9): e23962 10.1371/journal.pone.0023962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeBlasis PAD, Kneip A, Scheel-Ybert R, Giannini P, Gaspar M (2007) Sambaquis e paisagem dinâmica natural e arqueologia regional do sul do Brasil. Arqueologia Suramericana/Arqueologia Sul-Americana 3 1: 29–61. [Google Scholar]
- 14. Iriarte J, Behling H (2007) The expansion of Araucaria forest in the southern Brazilian highlands during the last 4000 years and its implications for the development of the Taquara/Itararé Tradition. Environmental archaeology 12(2): 115–127. [Google Scholar]
- 15. Noelli F (2000) A ocupação humana na região sul do Brasil: arqueologia, debates e perspectivas 1972–2000. Revista da USP 44: 218–269. [Google Scholar]
- 16. Iriarte J, Gillam JC, Marozzi O (2008) Monumental burials and memorial feasting: an example from the southern Brazilian highlands. Antiquity 82(318): 947–961. [Google Scholar]
- 17. González EMR (1998) Regional pottery-making groups in southern Brazil. Antiquity 72(277): 616–624. [Google Scholar]
- 18. Bastos MQR, Mendonça de Souza, SMF, Santos RV, Lima BAF, Santos RV, et al. (2011) Human mobility on the Brazilian coast: an analysis of strontium isotopes in archaeological human remains from Forte Marechal Luz sambaqui. Anais da Academia Brasileira de Ciências 83(2): 731–743. [DOI] [PubMed] [Google Scholar]
- 19. Schmitz PI, Rosa AO, Izidro JM, Haubert F, Krever MLB, et al. (1999) Içara: Um jazigo mortuário no litoral de Santa Catarina. Pesquisas, Antropologia 55: 01–164. [Google Scholar]
- 20. De Masi MAN (2001) Pescadores coletores da costa sul do Brasil. Pesquisas 57: 1–136. [Google Scholar]
- 21. Villagran XS, Klokler D, Peixoto S, DeBlasis PAD, Giannini PCF (2011) Building coastal landscapes: zooarchaeology and geoarchaeology of Brazilian shell mounds. Journal of Island & Coastal Archaeology 6: 211–234. [Google Scholar]
- 22. Hansel FA, Copley MS, Madureira LAS, Evershed RP (2004) Thermally produced omega-(o-alkylphenyl)alkanoic acids provide evidence for the processing of marine products in archaeological pottery vessels. Tetrahedron Letters 45: 2999–3002. [Google Scholar]
- 23. Hansel FA, Schmitz PI (2006) Classificação e interpretação dos resíduos orgânicos preservados em fragmentos de cerâmica arqueológica por cromatografia gasosa e cromatografia gasosa-espectrometria de massas. Pesquisas 63: 81–112. [Google Scholar]
- 24. Schwarcz HP, Schoeninger MJ (1991) Stable isotope analyses in human nutritional ecology. Yearbook of Physical Anthropology 34: 283–321. [Google Scholar]
- 25.Schulting RJ (2011) Mesolithic-Neolithic transitions: an isotopic tour through Europe. In: Pinhasi R, Stock J editors. The bioarchaeology of the transition to agriculture. New York: Wiley-Liss. 17–41.
- 26. Fogel M, Tuross N (2003) Extending the limits of paleodietary studies of humans with compound specific carbon isotope analysis of amino acids. Journal of Archaeological Science 30(5): 535–545. [Google Scholar]
- 27. Choy K, Smith CI, Fuller BT, Richards MP (2010) Investigation of amino acid δ13C signatures in bone collagen to reconstruct human palaeodiets using liquid chromatography–isotope ratio mass spectrometry. Geochimica et Cosmochimica Acta 74: 6093–6111. [Google Scholar]
- 28. Honch NV, McCullagh JSO, Hedges REM (2012) Variation of bone collagen amino acid δ13C values in archaeological humans and fauna with different dietary regimes: developing frameworks of dietary discrimination. American Journal of Physical Anthropology 148: 495–511. [DOI] [PubMed] [Google Scholar]
- 29.Evershed RP, Bull ID, Corr LT, Crossman ZM, Vandongen BE, et al. (2007) Compound-specific stable isotope analysis in ecology and paleoecology. In: Michener R, Lajtha K, editors. Stable isotopes in ecology and environmental science. Blackwell Publishing Ltd, Singapore, 480–540.
- 30. Craig OE, Steele VJ, Fischer A, Hartz S, Andersen SH, et al. (2011) Ancient lipids reveal continuity in culinary practices across the transition to agriculture in Northern Europe. Proc. Natl Acad. Sci. USA 108: 17910–17915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Craig OE, Saul H, Lucquin A, Nishida Y, Taché K, et al. (2013) Earliest evidence for the use of pottery. Nature 496: 351–354. [DOI] [PubMed] [Google Scholar]
- 32. De Masi MAN (2009) Aplicações de isótopos estáveis de 18/16O, 13/12C e 15/14N em estudos de sazonalidade, mobilidade e dieta de populações pré-históricas no sul do Brasil. Revista de Arqueologia 22(2): 55–76. [Google Scholar]
- 33.Figuti L, Mendonça CA, Porsani JL, Rocha EB, DeBlasis PAD, et al. (2004) Relatório final FAPESP: Investigação arqueológica e geofísica nos sambaquis fluviais no vale Ribeira de Iguape estado de São Paulo –FAPESP n° 1999/12684–2.
- 34. Eggers S, Petronilho CC, Brandt K, Jericó-Daminello C, Filippini J, Reinhard KJ (2008) How does a riverine setting affect the lifestyle of shellmound builders in Brazil? HOMO–Journal of Comparative Human Biology 59: 405–427. [DOI] [PubMed] [Google Scholar]
- 35.Fischer PF (2012) Os moleques do morro e os moleques da praia: estresse e mortalidade em um sambaqui fluvial (Moraes, vale do Ribeira de Iguape, SP) e em um sambaqui litorâneo (Piaçaguera, Baixada Santista, SP). Unpublished thesis. Universidade de São Paulo.
- 36.Garcia C (1970) Meios de subsistência de populações pré-históricas no litoral do estado de São Paulo. Unpublished thesis. Universidade de São Paulo.
- 37.Garcia C (1972) Estudo comparado das fontes de alimentacao de duas populacoes pre-historicas do litoral paulista. Unpublished PhD thesis. Universidade de São Paulo.
- 38.Uchôa DP (1970) O sítio arqueológico de Piaçagüera: aspectos gerais. Unpublished thesis. Universidade de São Paulo.
- 39. Okumura MMM, Eggers S (2005) The people of Jabuticabeira II: reconstruction of the way of life in a Brazilian shellmound. HOMO - Journal of Comparative Human Biology 55: 263–281. [DOI] [PubMed] [Google Scholar]
- 40.Klokler D (2008) Food for body and soul: mortuary ritual in shell mounds (Laguna - Brazil). Unpublished PhD thesis. The University of Arizona.
- 41.DeBlasis PAD, Farias DS, Kneip A (in press) Velhas tradições e gente nova no pedaço: perspectivas longevas de arquitetura funerária na paisagem do litoral sul catarinense. Revista do Museo de Arqueologia e Etnologia da USP.
- 42. Friedli H, Lötscher H, Oeschger H, Siegenthaler U, Stauffer B (1986) Ice core record of the 13C/12C ratio of atmospheric CO2 in the past two centuries. Nature 234(20): 237–238. [Google Scholar]
- 43.Ambrose SH, Norr L (1993) Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate. In: Lambert JB, Grupe G, editors. Prehistoric Human Bone: Archaeology at the Molecular Level. Springer-Verlag, Berlin, 1–37.
- 44. Jim S, Ambrose SH, Evershed RP (2004) Stable carbon isotopic evidence for differences in the dietary origin of bone cholesterol, collagen and apatite: implications for their use in palaeodietary reconstruction. Geochimica et Cosmochimica Acta 68(1): 61–72. [Google Scholar]
- 45. Jim S, Jones V, Ambrose SH, Evershed RP (2006) Quantifying dietary macronutrient sources of carbon for bone collagen biosynthesis using natural abundance stable carbon isotope analysis. British Journal of Nutrition 95: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 46.Schwarcz HP (2000) Some biochemical aspects of carbon isotopic paleodiet studies. In: Ambrose SH, Katzenberg MA, editors. Biogeochemical approaches to paleodietary analysis. New York: Kluwer Academic/Plenum, 189–209.
- 47. Schoeninger MJ (2009) Stable isotope evidence for the adoption of maize agriculture. Current Anthropology 50(5): 633–640. [DOI] [PubMed] [Google Scholar]
- 48. Fernandes R, Nadeau MJ, Grootes PM (2012) Macronutrient-based model for dietary carbon routing in bone collagen and bioapatite. Archaeol Anthropol Sci 4: 291–301. [Google Scholar]
- 49. Hedges REM (2002) Bone diagenesis: an overview of processes. Archaeometry 44(3): 319–328. [Google Scholar]
- 50. Wright LE, Schwarcz HP (1996) Infrared and isotopic evidence for diagenesis of bone apatite at Dos Pilas, Guatemala: palaeodietary implications. Journal of Archaeological Science 23: 933–944. [Google Scholar]
- 51. van Klinken GJ (1999) Bone collagen quality indicators for palaeodietary and radiocarbon measurements. Journal of Archaeological Science 26: 687–695. [Google Scholar]
- 52. Rehman I, Smith R, Hench LL, Bonfield W (1995) Structural evaluation of human and sheer, bone and comparison with synthetic hydroxyapatite by FT-Raman spectroscopy. Journal of Biomedical Materials Research 29: 1287–1294. [DOI] [PubMed] [Google Scholar]
- 53. Awonusi A, Morris MD, Tecklenburg MMJ (2007) Carbonate assignment and calibration in the Raman Spectrum of Apatite. Calcified Tissue International 81: 46–52. [DOI] [PubMed] [Google Scholar]
- 54. Craig OE, Biazzo M, Colonese AC, Di Giuseppe Z, Martinez-Labarga C, et al. (2010) Stable isotope analysis of Late Upper Palaeolithic human and faunal remains from Grotta del Romito (Cosenza), Italy. Journal of Archaeological Science 37: 2504–2512. [Google Scholar]
- 55. Smith CI, Fuller B T, Choy K, Richards MP (2009) A three-phase liquid chromatographic method for δ13C analysis of amino acids from biological protein hydrolysates using liquid chromatography-isotope ratio mass spectrometry. Anal Biochem 390: 165–172. [DOI] [PubMed] [Google Scholar]
- 56. Evershed RP, Heron C, Goad LJ (1990) Analysis of organic residues of archaeological origin by high-temperature gas chromatography and gas chromatography - mass spectrometry. Analyst 115: 1339–1342. [Google Scholar]
- 57. Craig OE, Forster M, Andersen AH, Koch E, Crombé P, et al. (2007) Molecular and isotopic demonstration of the processing of aquatic products in Northern European prehistoric pottery. Archaeometry 49(1): 135–152. [Google Scholar]
- 58. Reber EA, Dudd SN, van der Merwe NJ, Evershed RP (2003) Direct detection of maize in pottery residues via compound specific stable carbon isotope analysis. Antiquity 78(301): 682–691. [Google Scholar]
- 59. Craig OE, Allen RB, Thompson A, Stevens RE, Steele VJ, Heron C (2012) Distinguishing wild ruminant lipids by gas chromatography/combustion/isotope ratio mass spectrometry. Rapid Communications in Mass Spectrometry 26: 2359–2364. [DOI] [PubMed] [Google Scholar]
- 60. Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5(3): 9672–9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Richards MP, Hedges REM (1999) Stable isotope evidence for similarities in the types of marine foods used by late Mesolithic humans at sites along the Atlantic coast of Europe. Journal of Archaeological Science 26: 717–722. [Google Scholar]
- 62. Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1): 1–9. [Google Scholar]
- 63. McCormac FG, Hogg AG, Blackwell PG, Buck CE, Higham TFG, Reimer PJ (2004) SHCal04 southern Hemisphere calibration, 0–11.0 cal kyr BP. Radiocarbon 46(3): 1087–1092. [Google Scholar]
- 64. Bronk Ramsey C (2009) Bayesian analysis of radiocarbon dates. Radiocarbon 51(1): 337–360. [Google Scholar]
- 65. Thomas DB, Fordyce RE, Frew RD, Gordon KC (2007) A rapid, non-destructive method of detecting diagenetic alteration in fossil bone using Raman spectroscopy. Journal of Raman Spectroscopy 38: 1533–1537. [Google Scholar]
- 66. King CL, Tayles N, Gordon KC (2011) Re-examining the chemical evaluation of diagenesis in human bone apatite. Journal of Archaeological Science 38: 2222–2230. [Google Scholar]
- 67.Nielsen-Marsh C, Gernaey A, Turner-Walker G, Hedges R, Pike A, Collins C (2000) Chemical degradation of bone. In: Cox M, Mays S editors. Human osteology in archaeology and forensic sciences. London: Greenwich Medical Media, 439–453.
- 68. Morris MD, Mandair GS (2011) Raman assessment of bone quality. Clinical Orthopaedics and Related Research 469: 2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Edwards HGM, Farwell DW, de Faria DLA, Monteiro AMF, Afonso MC, et al. (2001) Raman spectroscopy study of 3000-year-old human skeletal remains from a sambaqui, Santa Catarina, Brazil. Journal of Raman Spectroscopy 32: 17–22. [Google Scholar]
- 70. Pestle WJ, Colvard M (2012) Bone collagen preservation in the tropics: a case study from ancient Puerto Rico. Journal of Archaeological Science 39(7): 2079–2090. [Google Scholar]
- 71.Craig OE, Bondioli L, Fattore L, Higham T, Hedges R (2013a) Evaluating marine diets through radiocarbon dating and stable isotope analysis of victims of the AD 79 eruption of Vesuvius. American Journal of Physical Anthropology DOI: 10.1002/ajpa.22352. [DOI] [PubMed]
- 72. Noli D, Avery G (1988) Protein poisoning and coastal subsistence. Journal of Archaeological Science 15: 395–401. [Google Scholar]
- 73. Choy K, Nash SH, Kristal AR, Hopkins S, Boyer BB, O’Brien DM (2013) The carbon isotope ratio of alanine in red blood cells is a new candidate biomarker of sugar-sweetened beverage intake. Journal of Nutrition 143: 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Coimbra MC, Jorge N (2011) Characterization of the Pulp and Kernel Oils from Syagrus oleracea, Syagrus romanzoffiana, and Acrocomia aculeate . Journal of Food Science 76: C1156–C1161. [DOI] [PubMed] [Google Scholar]
- 75. Coimbra MC, Jorge N (2012) Fatty acids and bioactive compounds of the pulps and kernels of Brazilian palm species, guariroba (Syagrus oleraces), jerivá (Syagrus romanzoffiana) and macaúba (Acrocomia aculeata). Journal of the Science of Food and Agriculture 92: 679–684. [DOI] [PubMed] [Google Scholar]
- 76. Reber EA, Evershed RP (2004) How did Mississippians prepare maize? The application of compound-specific carbon isotope analysis to absorbed pottery residues from several Mississippi Valley sites. Archaeometry 46(1): 19–33. [Google Scholar]
- 77. Morton JD, Schwarcz HP (2004) Palaeodietary implications from stable isotopic analysis of residues on prehistoric Ontario ceramics. Journal of Archaeological Science 31: 503–517. [Google Scholar]
- 78. Hart JP, Lovis WA, Schulenberg JK, Urquhart GR (2007) Paleodietary implications from stable carbon isotope analysis of experimental cooking residues. Journal of Archaeological Science 34: 804–813. [Google Scholar]
- 79. Hart JP, Lovis WA, Jeske RJ, Richards JD (2012) The potential of bulk δ13C on encrusted cooking residues as independent evidence for regional maize histories. American Antiquity 77(2): 315–325. [Google Scholar]
- 80.Walker PL, DeNiro MJ (1986) Stable nitrogen and carbon isotope ratios in bone collagen as indices of prehistoric dietary dependence on marine and terrestrial resources in southern California. American Journal of Physical Anthropology 715–761. [DOI] [PubMed]
- 81. Borrero LA, Guichón R, Tykot R, Kelly J, Prieto A, Cárdenas P (2001) Dieta a partir de isótopos estables en restos óseos humanos de Patagonia austral. Estado actual y perspectivas. Anales Instituto de la Patagonia 29: 119–127. [Google Scholar]
- 82. Tomczak PD (2003) Prehistoric diet and socioeconomic relationships within the Osmore Valley of southern Peru. Journal of Anthropological Archaeology 22: 262–278. [Google Scholar]
- 83. Hubbe M, Neves WA, Castro de Oliveira E, Strauss A (2009) Postmarital residence practice in southern Brazilian coastal groups: Continuity and change. Latin American Antiquity 20(2): 267–278. [Google Scholar]
- 84. Begossi A, Hanazaki N, Ramos RM (2004) Food chain and the reasons for fish taboos among amazonian and atlantic forest fishers (Brazil). Ecological Applications 14(5): 1334–1343. [Google Scholar]
- 85. de Castilho PV (2008) Utilization of cetaceans in shell mounds from the southern coast of Brazil. Quaternary International 180: 107–114. [Google Scholar]
- 86.Gómez Otero J, Belardi JB, Tykot R, Grammer S (2000) Dieta y poblaciones humanas en la costa norte del Chubut (Patagonia Argentina). In Desde el paìs de los Gigantes. Perspectivas arqueológicas en Patagonia. Universidad Nacional de la Patagonia Austral: Río Gallegos, 109–122.
- 87. Guichón RA, Borrero LA, Prieto IA, Cardenas P, Tykot R (2001) Nuevas determinaciones de isótopos estables para Tierra del Fuego. Revista Argentina de Antropología Biológica 3(1): 113–126. [Google Scholar]
- 88. Yesner DR, Torres MJF, Guichon RA, Borrero LA (2003) Stable isotope analysis of human bone and ethnohistoric subsistence patterns in Tierra del Fuego. Journal of Anthropological Archaeology 22: 279–291. [Google Scholar]
- 89. Panarello H, Zangrando F, Tessone A, Kozameh LF, Testa N (2006) Comparative analysis of human diets between the Beagle Channel region and Peninsula Mitre: perspectives from stable isotopes. Magallania 34(2): 37–46. [Google Scholar]
- 90.Favier Dubois C, Borella F, Tykot R (2009) Explorando tendencias en el uso humano del espacio y los recursos en el litoral rionegrino (Argentina) durante el Holoceno medio y tardío. In: Salemme M, Santiago F, Álvarez M, Piana E, Vázquez M, Mansur E, editors. Arqueología de la Patagonia - Una mirada desde el último confín. Editorial Utopías, Ushuaia, 985–998.
- 91. Moreno E, Zangrando AF, Tessone A, Castro A, Panarello H (2011) Isótopos estables, fauna y tecnología en el estudio de los cazadores-recolectores de la costa norte de santa Cruz. Magallania 39(1): 265–276. [Google Scholar]
- 92. Borrero LA, Barberena R, Franco NV, Charlin J, Tykot RH (2009) Isotopes and rocks: geographical organisation of southern Patagonian hunter-gatherers. International Journal of Osteoarchaeology 19: 309–327. [Google Scholar]
- 93. Orquera LA, Piana EL (1996) El sitio Shamakush I. Relaciones de la Sociedad Argentina de Antropología. XXI: 215–265. [Google Scholar]
- 94. Martínez G, Zangrando AF, Prates L (2009) Isotopic ecology and human palaeodiets in the lower basin of the Colorado River, Buenos Aires province, Argentina. International Journal of Osteoarchaeology 19: 281–296. [Google Scholar]
- 95. Politis GG, Scabuzzo C, Tykot R (2009) An approach to Pre-Hispanic diets in the Pampas during the early/middle Holocene. International Journal of Osteoarchaeology 19: 266–280. [Google Scholar]
- 96. Santiago F, Salemme M, Suby J, Guichón R (2011) Restos humanos en el norte de Tierra del Fuego. Aspectos contextuales, dietarios y paleopatológicos. Intersecciones en Antropología 12: 147–162. [Google Scholar]
- 97. Borrero LA, Barberena R (2006) Hunter-gatherer home ranges and marine resources. An archaeological case from southern Patagonia. Current Anthropology 47(5): 855–867. [Google Scholar]
- 98. Scheel-Ybert R, Dias OF (2007) Corondó: palaeoenvironmental reconstruction and palaeoethnobotanical considerations in a probable locus of early plant cultivation (south-eastern Brazil). Environmental Archaeology 12(2): 129–138. [Google Scholar]
- 99. Turner II C, Machado LMC (1983) A new dental wear pattern and evidence for high carbohydrate consumption in a Brazilian archaic skeletal population. American Journal of Physical Anthropology 61: 125–130. [DOI] [PubMed] [Google Scholar]
- 100.Boyadjian CHC (2012) Análise e identificação de microvestígios vegetais de cálculo dentário para a reconstrução de dieta sambaquieira: estudo de caso de Jabuticabeira II, SC. Unpublished thesis, Universidade de São Paulo.
- 101. Sandweiss DH, McInnis H, Burger RL, Cano A, Ojeda B, et al. (1998) Quebrada Jaguay: early South American maritime adaptations. Science 281: 1830–1832. [DOI] [PubMed] [Google Scholar]
- 102.van der Merwe NJ, Lee-Thorp JA, Raymond JS (1993) Light, stable isotopes and the subsistence base of Formative cultures at Valdivia, Ecuador. In: Lambert JB, Grupe G, editors. Prehistoric Human Bone: Archaeology at the Molecular Level. Berlin: Springer-Verlag, 63–97.
- 103. Tykot RH, Staller JE (2002) The importance of early maize agriculture in coastal Ecuador: new data from La Emerenciana. Current Anthropology 43(4): 666–677. [Google Scholar]
- 104.Tykot RH, Burger R, van der Merwe NJ (2006) The importance of maize in initial period and early horizon Peru. In: Staller JE, Tykot RH, Benz BF editors. Histories of maize: multidisciplinary approaches to the prehistory, linguistics, biogeography, domestication, and evolution of maize. Academic Press, Burlington, Massachusetts, 187–197.
- 105. Torres-Rouff C, Pestle WJ, Gallardo F (2012) Eating fish in the driest desert in the world: osteological and biogeochemical analyses of human skeletal remains from the San Salvador cemetery: North Chile. Latin American Antiquity 23(1): 51–69. [Google Scholar]
- 106. Aufderheide AC (1994) Contribution of chemical dietary reconstruction to the assessment of adaptation by ancient highland immigrants (Alto Ramirez) to coastal conditions at Pisagua, North Chile. Journal of Archaeological Science 21: 515–524. [Google Scholar]
- 107. White CD, Nelson AJ, Longstaffe FJ, Grupe G, Jung A (2009) Landscape bioarchaeology at Pacatnamu, Peru: inferring mobility from δ13C and δ15N values of hair. Journal of Archaeological Science 36: 1527–1537. [Google Scholar]
- 108. Knudson KJ, Pestle WJ, Torres-Rouff C, Pimentel G (2010) Assessing the life history of an Andean traveller through biogeochemistry: stable and radiogenic isotope analyses of archaeological human remains from Northern Chile. International Journal of Osteoarchaeology 22(4): 435–451. [Google Scholar]
- 109. Richards MP, Schulting RJ, Hedges REM (2003) Sharp shift in diet at onset of Neolithic. Nature 425: 366. [DOI] [PubMed] [Google Scholar]
- 110. Cramp LJE, Jones J, Sheridan A, Smyth J, Whelton H, et al. (2014) Immediate replacement of fishing with dairying by the earliest farmers of the northeast Atlantic archipelagos. Proc. R. Soc. B 2014 281: 20132372 10.1098/rspb.2013.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Begossi A (2010) Small-scale fisheries in Latin America: Management models and challenges. MAST 9: 5–12. [Google Scholar]
- 112. Movasaghi Z, Rehman S, Rehman IU (2007) Raman spectroscopy of biological tissues, Applied Spectroscopy Reviews. 42: 493–541. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Carbonate ν1 (C) to phosphate ν1 (P) intensity ratios, full width at half maximum (FWHM) of the phosphate ν1 (P) band and organic (C-H stretch) to phosphate ν1 (P) intensity ratios determined for each averaged spectrum as a function of the sample type. Samples are ordered from youngest to oldest with data acquired using the same Raman confocal settings across all samples. Modern lamb bone is justified as an appropriate control due to the similarities in sheep and human bone as per the RS study of Rehman et al. [52].
(DOCX)
Bone collagen δ13C and δ15N values of humans. Also show the age class or the relative age (young, adult) and the sex (F: female, M: male).
(DOCX)
One-way ANOVA showing a general lack of significant isotopic differences between sexes and age at MRS, Jab-II and PCG. Data from G-IV was not sufficient to be tested statistically. Because of the limited information about the age, individuals from MRS and PCG were sorted out in two categories: <36 and >36 years old. At Jab-II, individuals belonging to the age class 11–20 years old show higher δ15N values (mean 19.1±1.0%, n = 3) than individuals belonging to the age class 36–50 yrs (mean 17.3±0.9%, n = 13).
(DOCX)
Collagen amino acid δ13C values for humans from Jab-II (n = 10) and G-IV (n = 7).
(DOCX)
Ceramic sherds selected for lipid analysis by GCMS and GC-c-IRMS. FA (Cx:y) - fatty acids with carbon length x and number of unsaturations y, br -branched chain acids, phy- phytanic acid, TMTD - 4,8,12-trimethyltridecanoic acid. APFA (Cn) - ω-(o-alkylphenyl) alkanoic acids with carbon length n. tr - trace. DCx - α,ω-dicarboxylic acids with carbon length x. P - interior, E - exterior. Aquatic oils are interpreted from the presence of isomers of APFA (C20 or C22) and at least one isoprenoid fatty acids (pri, phy or TMTD). Resins are interpreted from the presence of triterpenes. Plant oils are interpreted from the presence of long chain fatty acids, dicarboxylique acids and the presence of isomers of C18 APFA. A high abundance of C12∶0 could be consistent with Palm Kernel. Aquatic (marine) fats are defined on the isotopic characteristics of the C16 and C18 saturated fatty acids.
(DOCX)
Bulk isotope characteristics of charred deposits from the interior of potsherds from G-IV.
(DOCX)