Abstract
Background and aim
Spontaneous rupture of hepatocellular carcinoma (HCC) carries a high mortality. The use of radiofrequency ablation (RFA) in recent years has enriched the armamentarium for hemostasis of spontaneously ruptured HCCs but its results have not been documented. This study investigated the prognosis and outcome of spontaneous rupture of HCC as well as the results of using RFA for hemostasis.
Patients and method
From January 1991 to December 2010, 5283 patients were diagnosed with HCC at our hospital, and 189 of them had spontaneous rupture of HCCs. They were grouped under two periods: period 1, 1991–2000, n = 70; period 2, 2001–2010, n = 119. RFA was available in period 2 only.
Results
Hepatitis B virus infection was predominant in both periods. Surgical hemostasis was mainly achieved by hepatic artery ligation in period 1 and by RFA in period 2. The 30-day hospital mortality after surgical treatment was 55.6% (n = 18) in period 1 and 19.2% (n = 26) in period 2 (p = 0.012). Multivariate analysis identified 4 independent factors for better overall survival, namely, hemostasis by transarterial chemoembolization (hazard ratio 0.516, 95% confidence interval 0.354–0.751), hemostasis by RFA (hazard ratio 0.431, 95% confidence interval 0.236–0.790), having surgery as a subsequent treatment (hazard ratio 0.305, 95% confidence interval 0.186–0.498), and a serum total bilirubin level <19 umol/L (hazard ratio 1.596, 95% confidence interval 1.137–2.241).
Conclusion
The use of RFA for hemostasis during laparotomy greatly reduced the hospital mortality rate when compared with conventional hepatic artery ligation.
Introduction
Spontaneous rupture is one of the presentations of hepatocellular carcinoma (HCC). It can have catastrophic consequences. The incidence of spontaneous rupture of HCCs ranges from 3–14.5% in regions where HCC is prevalent [1]–[4]. Transarterial chemoembolization (TACE) is a well-established treatment option [5], [6]. Emergency hepatic artery ligation, plication, packing, hepatectomy or radiofrequency ablation (RFA) is required if TACE fails to achieve hemostasis [3], [4]. Unfortunately, many patients having surgical intervention end up in liver failure or even death. This study tried to find out whether using RFA for hemostasis during laparotomy would improve patients' hospital survival.
Patients and Methods
From January 1991 to December 2010, 5283 patients were diagnosed with HCC and 189 of them had spontaneous rupture of HCCs and were admitted to our hospital. All the data used in this study were recorded by a single research assistant. The 189 patients were grouped under two periods: period 1, 1991–2000, n = 70; period 2, 2001–2010, n = 119.
Ethics statement
The study complies with the laws of Hong Kong and follows the principles laid down in the Declaration of Helsinki. Oral consent by the patients was obtained after clear and complete explanation and was recorded in the patients' medical records. Approval in the form of written consent was not needed as it was waived by the institutional review board of The University of Hong Kong, which approved this retrospective study.
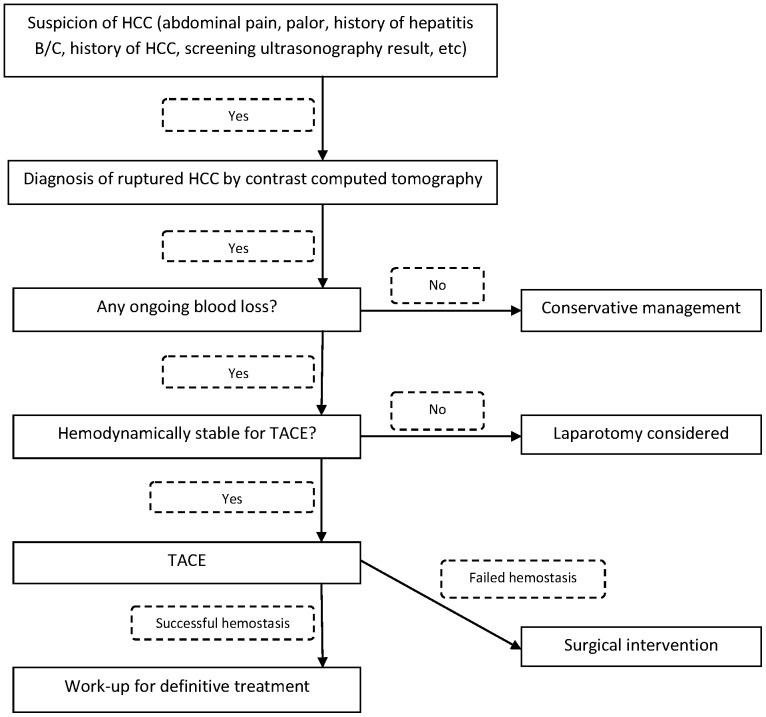
Treatment algorithm
Diagnosis of spontaneous rupture of HCCs was based on clinical pictures acquired by physical examination and screening ultrasonography on presentation. Shock index was used to describe the hemodynamic status of a patient. Shock index was calculated as the division of heart rate reading by systolic blood pressure reading. The shock index of a healthy adult should be in the range between 0.5 and 0.7. A patient was classified as in shock when his/her shock index was >0.7 [7].
Contrast computed tomography of the abdomen was performed for patients without renal impairment. Patients with any one of the following three conditions were managed conservatively: (a) major portal vein tumor thrombosis, (b) disseminated extrahepatic disease, (c) Karnosfsky performance status <40%. Other patients were treated according to our standard treatment protocol (Figure 1).
Figure 1. Treatment algorithm for spontaneous rupture of HCCs.
TACE
TACE was conducted by experienced interventional radiologists. Selective cannulation of the feeding artery of the tumor was attempted. Embolization of the feeding artery was performed by injection of pellets (1×2 mm) mixed with 40 mg of gentamycin and contrast. Depending on the size and number of tumor, 4–20 mL of gelfoam emulsion was injected into the catheter placed in the artery supplying the tumor or, for bilobar disease, into the hepatic artery proper beyond the gastroduodenal artery. Gelfoam injection was stopped when the blood flow in the artery supplying the tumor slowed down and occlusion occurred. Relative contraindications to TACE included main portal vein thrombosis, arteriovenous shunting and Child-Pugh C cirrhosis [8].
Operative treatment
All operations were conducted by experienced hepatobiliary surgeons. A bilateral subcostal incision with an upward midline incision was performed in most of the patients. Abdominal packing was performed. Depending on the size of the ruptured tumor and the surgeon's clinical judgement [4], [9], [10], hemostasis was attempted by (a) direct suture of the bleeding site, (b) selective hepatic artery ligation, (c) ligation of the common trunk of the hepatic artery, (d) hepatectomy, or (e) RFA (available in period 2 only). If hepatectomy was required, hepatic parenchymal transection was conducted with an ultrasonic dissector. The Pringle maneuver might be applied during the procedure. All patients who received operative treatment were managed in the intensive care unit.
RFA
RFA was available in period 2 only. It was performed by hepatobiliary surgeons experienced in using the RFA device. Direct puncturing of the bleeding site was performed in most of the cases. A single needle or clustered needles (maximum array diameter 4.0 cm) were used according to the size of the tumor. For most bleeding tumors, a cluster probe was used. RFA was administered using the Olympus Cool-tip system, which consists of a generator that supplies up to 200 W of power until satisfactory hemostasis is achieved [11], [12].
Statistical analysis
The baseline characteristics of patients were expressed as medians and ranges. The Mann-Whitney U test was used to compare continuous variables, and Pearson's chi-squared test was used to compare discrete variables. Hospital mortality was defined as death occurring after admission to and before discharge from hospital. Survival curves were computed with the Kaplan-Meier method and compared between groups by the log-rank test. The logistic regression analysis was performed to define factors that affected hospital mortality. The Cox proportional hazards model was used to define factors that determined the 30-day mortality rate. Statistical significance was denoted by a p value <0.05. All statistical calculations used the computer software SPSS/PC+ (SPSS, Chicago, IL, USA).
Results
The rate of spontaneous rupture of HCC in period 1 was 3.7% (70/1892) and 3.5% (119/3391) in period 2. Demographics, liver function and clinical presentations of the 189 patients are shown in Table 1. In period 1, 33 (47.1%) patients received TACE as the first treatment, 15 (21.4%) patients received surgery as the first treatment, and 22 (31.4%) patients received conservative management. Hemostasis by TACE failed in 3 patients and they subsequently received surgery. In period 2, 58 (48.7%) patients received TACE as the first treatment, 20 (16.8%) patients received surgery as the first treatment, and 41 (34.5%) patients received conservative management. Hemostasis by TACE failed in 6 patients and they subsequently received surgery.
Table 1. Demographics, liver function and clinical presentations of patients.
| 1991–2000 (n = 70) | 2001–2010 (n = 119) | P | |
| Median age (years) | 59.5 (13–94) | 58 (34–92) | 0.557 |
| Male: Female | 57∶13 | 88∶31 | 0.240 |
| Presence of comorbidity | 23 (32.9%) | 48 (40.3%) | 0.305 |
| Child-Pugh class | 0.997 | ||
| A | 30 (46.2%) | 55 (46.2%) | |
| B | 26 (40.0%) | 48 (40.3%) | |
| C | 9 (13.8%) | 16 (13.4%) | |
| Median Model for End-stage Liver Disease score | 12.75 (6–27) | 10.94 (6–37) | 0.522 |
| Median albumin (g/L) | 32 (13–52) | 34 (12–46) | 0.332 |
| Median total bilirubin (umol/L) | 21 (6–750) | 18 (4–180) | 0.272 |
| Median international normalized ratio | 1.2 (0.9–3.2) | 1.15 (0.9–4.1) | 0.514 |
| Median aspartate transaminase (U/L) | 103 (23–1710) | 93 (21–1003) | 0.946 |
| Median alanine transaminase (U/L) | 54.5 (13–652) | 50 (5–679) | 0.221 |
| Median creatinine (umol/L) | 94 (46–379) | 93 (8–296) | 0.328 |
| Median α-fetoprotein (ng/mL) | 2055.5 (2–1720600) | 636.5 (2–1451000) | 0.236 |
| Median tumor size (cm) | 10 (2–23) | 8.5 (2–30) | 0.619 |
| Hepatitis B virus infection | 42 (60.0%) | 86 (72.9%) | 0.001 |
| Peritoneal irritation or severe abdominal pain | 49 (70.0%) | 93 (78.2%) | 0.211 |
| Shock | 33 (47.1%) | 51 (42.9%) | 0.674 |
| Drop in hemoglobin >2 g/dL or hemoglobin <10 g/dL on initial blood test | 46 (65.7%) | 81 (68.1%) | 0.739 |
In period 1, the rate of successful hemostasis was 78.8% (26 patients) for TACE, 77.8% (14 patients) for surgery, and 63.6% (14 patients) for conservative management. In period 2, the rate of successful hemostasis was 75.9% (44 patients) for TACE, 92.3% (24 patients) for surgery, and 58.5% (24 patients) for conservative management. In period 2, surgical hemostasis was mainly achieved by RFA. Operative and postoperative details and mortality rates in the two periods are shown in Table 2.
Table 2. Operative and postoperative details and mortality rates.
| 1991–2000 (n = 18) | 2001–2010 (n = 26) | P | |
| Laparotomy without TACE | 15 (83.3%) | 20 (76.9%) | 0.890 |
| Common hepatic artery ligation | 1 (5.6%) | 0 | 0.852 |
| Left or right hepatic artery ligation | 12 (66.7%) | 3 (11.5%) | 0.0001 |
| Direct suture | 3 (16.7%) | 1 (3.8%) | 0.357 |
| Tumor resection | 2 (11.1%) | 2 (7.7%) | 1 |
| Coagulation by argon beam | 6 (33.3%) | 12 (46.2%) | 0.395 |
| Hemostasis by RFA | 0 | 19 (73.1%) | <0.0001 |
| Median blood loss (L) | 3 (0.8–13) | 2.2 (0.2–7.8) | 0.355 |
| Median operation time (min) | 115 (75–225) | 140 (90–255) | 0.102 |
| Median intensive care unit stay (d) | 2 (0–16) | 2 (0–25) | 0.761 |
| Median hospital stay (d) | 13 (1–34) | 14.5 (0.46–52) | 0.872 |
| Complication (Clavien-Dindo grade) | |||
| 3a | 2 (11.1%) | 3 (11.5%) | 1 |
| 3b | 0 | 1 (3.8%) | 1 |
| 4a | 1 (5.6%) | 1 (3.8%) | 1 |
| 5 | 10 (55.6%) | 6 (23.1%) | 0.028 |
| Mortality for hepatic artery ligation | 8/13 (61.5%) | 2/3 (66.7%) | 1 |
| Hospital mortality for ruptured HCC | 10 (55.6%) | 6 (23.1%) | 0.028 |
| 30-day mortality for ruptured HCC | 10 (55.6%) | 5 (19.2%) | 0.012 |
In period 2, 19 patients received RFA for hemostasis (Table 3). They had a median tumor size of 8.25 cm (range 3–19 cm). Eight (42.1%) patients had 1 tumor, 4 (21.1%) patients had 2 tumors, and 7 (36.8%) patients had >2 tumors. Single-needle probe was applied on 2 (10.5%) occasions and cluster-needle probe was applied on 17 (89.5%) occasions. The median ablation time was 22 min (range 12–72 min). Only 1 patient, who had a 10-cm tumor, received the Pringle maneuver during ablation, and the total inflow occlusion time was 60 min. Six (31.6%) patients who received RFA for hemostasis were found to have complete tumor ablation on computed tomography one month after the ablation.
Table 3. Details of RFA for hemostasis.
| Tumor <5 cm (n = 4) | Tumor 5–10 cm (n = 8) | Tumor >10 cm (n = 7) | P | |
| Median tumor size (cm) | 3.5 (3.0–4.5) | 5.6 (5.0–9.1) | 13.5 (11.1–19.0) | 0.001 |
| Tumor number | 0.253 | |||
| 1 | 3 (75%) | 2 (25.0%) | 3 (42.9%) | |
| 2 | 1 (25%) | 3 (37.5%) | 0 | |
| >2 | 0 | 3 (37.5%) | 4 (57.1%) | |
| Median ablation time (min) | 22 (12–22) | 17 (12–49) | 32 (12–72) | 0.222 |
| No. of patients who had the Pringle maneuver | 0 | 1 (12.5%) | 0 | |
| Time of the Pringle maneuver (min) | - | 60 | - |
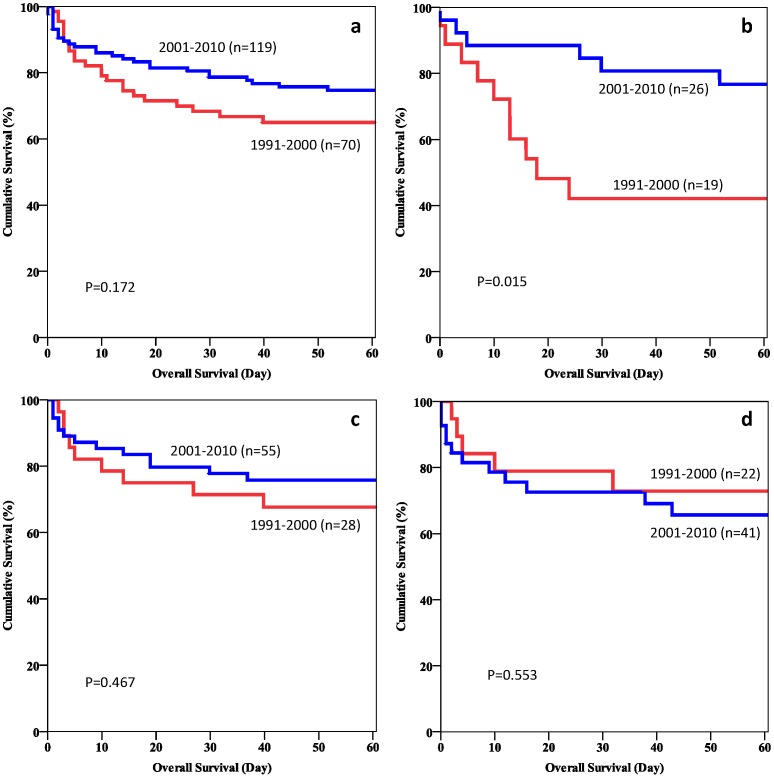
The 30-day hospital mortality rate of patients who had surgery was 55.6% in period 1 and 19.2% in period 2 (p = 0.012). The 30-day hospital mortality rate of patients who had TACE as the first treatment was 11.5% in period 1 and 6.8% in period 2 (p = 0.81). The 30-day hospital mortality rate of patients who had conservative management was 18.2% in period 1 and 24.4% in period 2 (p = 0.805). Figures 2a-2d show the 60-day survival curves of different groups of patients in the two periods.
Figure 2.
a. Overall hospital mortality of patients with spontaneously ruptured HCCs. b. Hospital mortality of patients who had surgery for spontaneously ruptured HCCs. c. Hospital mortality of patients who had TACE as the primary treatment for spontaneously ruptured HCCs. d. Hospital mortality of patients who had conservative management alone for spontaneously ruptured HCCs.
In period 1, 11 patients had tumor resection after spontaneous rupture of tumors. Five of them had minor hepatectomy and 6 had major hepatectomy (resection of >3 liver segments). In period 2, 14 patients had tumor resection after spontaneous rupture of tumors. Four of them had minor hepatectomy and 10 had major hepatectomy. In period 2, 11 patients had RFA and 1 patient had high-intensity focused ultrasound ablation [13], [14] for their HCCs. One patient had liver transplantation at another hospital.
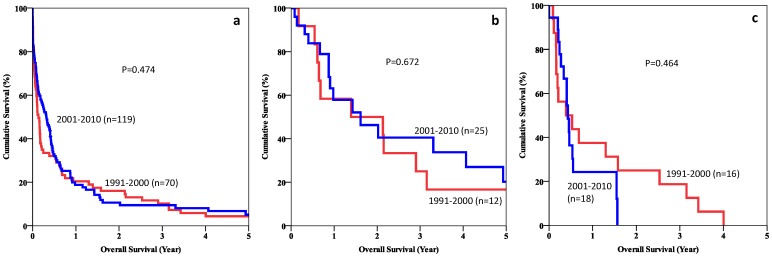
The median survival of patients with spontaneous rupture of HCCs was 1.61 months (range 0–178 months) in period 1 and 3.84 months (range 0–118 months) in period 2 (p = 0.474). Sixteen patients in period 1 and 19 patients in period 2 received TACE as a subsequent treatment. Figures 3a–3c show the 5-year survival curves of different groups of patients in the two periods.
Figure 3.
a. Overall survival of patients with spontaneously ruptured HCCs. b. Overall survival of patients who had hepatectomy or RFA for spontaneously ruptured HCCs. c. Overall survival of patients who had TACE for spontaneously ruptured HCCs.
Thirteen independent factors that might affect the survival outcome were analyzed (Table 4). The 6 independent risk factors for poor survival were (a) tumor size >10 cm, (b) intolerance of TACE for hemostasis, (c) unsuitability for subsequent surgery, (d) poor Child-Pugh grade, (e) serum total bilirubin level >19 umol/L, and (f) international normalized ratio >1.2.
Table 4. Factors that might affect patient survival.
| 1991–2000 | 2001–2010 | 1991–2010 | |||||||
| No. of patients | Median survival (months) | P | No. of patients | Median survival (months) | P | No. of patients | Median survival (months) | P | |
| Tumor size (cm) | 0.003 | 0.001 | <0.0001 | ||||||
| ≤10 | 31 | 4.63 | 62 | 5.06 | 93 | 5.06 | |||
| >10 | 34 | 1.25 | 56 | 1.41 | 90 | 1.35 | |||
| Hemostasis by RFA | - | 0.089 | 0.083 | ||||||
| No | 70 | 1.61 | 100 | 3.16 | 170 | 2.10 | |||
| Yes | 0 | - | 19 | 6.61 | 19 | 6.61 | |||
| Hemostasis by surgery | 0.588 | 0.870 | 0.653 | ||||||
| No | 52 | 1.84 | 106 | 3.68 | 158 | 2.33 | |||
| Yes | 18 | 0.59 | 13 | 3.88 | 31 | 3.29 | |||
| Hemostasis by TACE | 0.003 | 0.133 | 0.001 | ||||||
| No | 44 | 1.08 | 70 | 2.89 | 114 | 1.71 | |||
| Yes | 26 | 6.54 | 49 | 4.86 | 75 | 5.06 | |||
| Subsequent TACE | 0.068 | 0.517 | 0.120 | ||||||
| Yes | 16 | 4.63 | 19 | 5.16 | 35 | 5.16 | |||
| No | 54 | 1.05 | 100 | 3.02 | 154 | 1.61 | |||
| Subsequent surgery | 0.001 | <0.0001 | <0.0001 | ||||||
| Yes | 12 | 16.69 | 16 | 17.12 | 28 | 19.45 | |||
| No | 58 | 1.22 | 103 | 2.56 | 161 | 1.61 | |||
| Shock on presentation | 0.097 | 0.149 | 0.96 | ||||||
| No | 37 | 2.14 | 68 | 3.68 | 105 | 3.02 | |||
| Yes | 33 | 0.59 | 51 | 4.83 | 84 | 1.38 | |||
| Child-Pugh class | <0.0001 | 0.197 | <0.0001 | ||||||
| A | 30 | 8.18 | 55 | 4.86 | 85 | 5.75 | |||
| B | 26 | 0.79 | 48 | 2.76 | 74 | 1.25 | |||
| C | 9 | 0.36 | 16 | 0.62 | 25 | 0.62 | |||
| MELD score | 0.0004 | 0.399 | 0.123 | ||||||
| ≤11 | 28 | 7.76 | 61 | 3.85 | 89 | 4.04 | |||
| >11 | 33 | 1.22 | 57 | 2.76 | 90 | 1.31 | |||
| Total bilirubin (umol/L) | 0.0002 | 0.001 | <0.0001 | ||||||
| ≤19 | 32 | 6.54 | 62 | 5.52 | 94 | 5.75 | |||
| >19 | 36 | 1.05 | 57 | 1.35 | 93 | 1.22 | |||
| Platelet (×109/L) | 0.679 | 0.168 | 0.449 | ||||||
| ≤192.5 | 41 | 1.35 | 53 | 5.06 | 94 | 3.85 | |||
| >192.5 | 27 | 1.94 | 66 | 2.56 | 93 | 2.17 | |||
| International normalized ratio | 0.001 | 0.621 | 0.019 | ||||||
| ≤1.2 | 37 | 2.53 | 77 | 4.47 | 114 | 4.04 | |||
| >1.2 | 25 | 0.89 | 41 | 1.51 | 66 | 1.22 | |||
| Albumin (g/L) | 0.063 | 0.443 | 0.086 | ||||||
| ≤34 | 35 | 1.08 | 67 | 2.89 | 102 | 1.31 | |||
| >34 | 33 | 4.63 | 52 | 4.24 | 85 | 4.47 | |||
MELD score, Model for End-stage Liver Disease score (to the nearest integer of the median).
On multivariate analysis by the Cox regression model, 4 independent factors for improved survival were identified (Table 5). They were (a) hemostasis by TACE, (b) hemostasis by RFA, (c) having surgery as a subsequent treatment, and (d) a serum total bilirubin level <19 umol/L.
Table 5. Factors for improved patient survival.
| P | Hazard ratio | 95% confidence interval | |
| Hemostasis by TACE | 0.0006 | 0.516 | 0.354–0.751 |
| Hemostasis by RFA | 0.0064 | 0.431 | 0.236–0.790 |
| Total bilirubin >19 umol/L | 0.0069 | 1.596 | 1.137–2.241 |
| Surgery as a subsequent treatment | <0.0001 | 0.305 | 0.186–0.498 |
Discussion
To our knowledge, this study has the largest series of patients with cirrhosis and spontaneously ruptured HCCs. Spontaneous rupture is not the most common clinical presentation of HCCs but it can lead to catastrophic consequences. In the study, 3.7% and 3.5% of the HCC patients in period 1 and period 2 respectively had spontaneous rupture of tumors. Compared with the historic figure of 14.5% published by Ong et al. [9], there was a drastic reduction of this clinical manifestation. Early screening of patients with hepatitis B virus infection might have allowed detection of HCC at an early stage. The use of more sophisticated imaging modality like dual-tracer positron emission tomography/computed tomography could have subjected more patients to surgery when the tumors were still small [15], [16].
The management of spontaneously ruptured HCCs remains difficult because patients often present with very poor physical conditions including coagulopathy and hemodynamic instability [17], [18]. A clear and comprehensive treatment protocol for these scenarios is essential. TACE remains as the first-line hemostatic option for patients with a relatively stable hemodynamic condition [19]–[21]. TACE is considered highly effective in hemostasis for ruptured HCCs. In the study, the rates of successful hemostasis in the two periods were comparable. However, the ischemic effect of TACE on cirrhotic livers might have contributed to liver failure, leading to a hospital mortality of 20–30% in the entire period.
In period 1, the hospital mortality after surgery was substantial; more than half of the patients did not survive. On the contrary, there was a significant improvement in hospital mortality after surgery in period 2. The operation mortality reduced to 23%. In period 1, one of the surgical strategies for hemostasis was direct suture of the tumour [4]. This method, however, was far from satisfactory. Since the nature of liver tumors is quite soft, many of the sutures might have cut through the tumors, ending up in ineffective hemostasis. In spontaneous rupture of HCCs, the bleeding mainly comes from the hepatic artery [22]. Selective hepatic artery ligation was applied in period 1. Ligation of the common hepatic artery was performed for patients with ruptured tumors involving both lobes of the liver if selective ligation of the hepatic artery branch had failed to stop the bleeding. Nevertheless, successful cessation of bleeding by hepatic artery ligation could not secure successful survival. More than 60% of the patients ended up in hospital mortality secondary to liver failure. Neither selective ligation of hepatic artery nor ligation of the common hepatic artery was well tolerated in patients with advanced cirrhosis and HCC. In period 2, selective or non-selective hepatic artery ligation also resulted in poor survival outcomes. Three patients who had very unstable hemodynamic condition during operation had ligation of the hepatic artery as a last resort. Two patients developed liver failure as a complication of hepatic artery ligation.
RFA was initially invented as an ablative tool for small HCCs in an elective setting [23]–[25]. It played an important role in period 2 as a treatment for spontaneous rupture of HCCs. RFA can achieve hemostasis and complete ablation at the same time for patients with ruptured HCCs. For lesions <3 cm, a single probe can effectively achieve complete ablation [22], [25], [26]. A cluster probe has an effective ablation diameter of 5 cm and is therefore suitable for large HCCs. The effective ablation effect was demonstrated by the fact that 3 patients with small HCCs had complete ablation of tumors as shown by subsequent contrast computed tomography.
This study has shown that RFA is a safe and simple operative hemostasis method that can produce an excellent survival outcome. According to our treatment algorithm (Figure 1), TACE is the first treatment option since it can provide effective hemostasis with minimal invasiveness whereas RFA is the first-line operative approach when open hemostasis is required. In the study, RFA achieved effective hemostasis in a short period of ablation time. It sped up the whole operation procedure and, most importantly, it improved the overall hospital survival of patients who required open hemostasis.
In general, RFA is safe for and well tolerated by HCC patients. However, in patients with cirrhosis, the complication rate would be slightly higher. As found by one of our previous studies, a continuous ablation time of more than 36 min might induce intolerance in patients with cirrhosis [11]. Therefore, a long ablation time should be avoided.
Hemostasis of large ruptured HCCs is difficult even for RFA. For large lesions or when hemostasis could not be achieved in an initial 12-min cycle, the Pringle maneuver should be considered as a measure to decrease the blood flow to the lesion. In the study, a patient who had massive bleeding from the tumor underwent the Pringle maneuver for 60 min, and successful hemostasis was achieved. No patient in the study developed liver failure as a consequence of hemostasis by RFA. If RFA fails to stop tumor bleeding, hemostasis has to be performed by other surgical means such as hepatic artery ligation or liver resection, with an increased risk of hospital mortality.
Liver resection can be carried out for peripheral lesions in a very short period of time. With the advancement of laparoscopic surgery, new technology (e.g. LigaSure), new equipment (e.g. the Harmonic scalpel) and new linear staplers with good hemostatic effect are available [27], [28]. Theoretically these devices can speed up the resection of peripherally located lesions. Laparoscopic liver resection can be safely performed even in patients with cirrhosis [29]. Two patients in the study had liver resection for lesions located at the left lateral section with uneventful recovery.
The overall survival of HCC patients depends largely on the stage of disease [17], [30], [31]. Patients in both period 1 and period 2 presented with advanced tumors and cirrhosis. On multivariate analysis, patients with poor liver function had poorer survival. Their poor liver function precluded them from receiving further treatment such as locoregional therapy, targeted therapy, systemic therapy and hepatectomy, which could prolong their survival [32]–[36].
In conclusion, in the management of spontaneous rupture of HCCs, hemostasis by RFA instead of hepatic artery ligation can largely improve patients' hospital survival.
Funding Statement
The authors have no support or funding to report.
References
- 1. Ong GB, Chu EP, Yu FY, Lee TC (1965) Spontaneous rupture of hepatocellular carcinoma. Br J Surg 52: 123–129. [DOI] [PubMed] [Google Scholar]
- 2. Chearanai O, Plengvanit U, Asavanich C, Damrongsak D, Sindhvananda K, et al. (1983) Spontaneous rupture of primary hepatoma: report of 63 cases with particular reference to the pathogenesis and rationale treatment by hepatic artery ligation. Cancer 51: 1532–1536. [DOI] [PubMed] [Google Scholar]
- 3. Cherqui D, Panis Y, Rotman N, Fagniez PL (1993) Emergency liver resection for spontaneous rupture of hepatocellular carcinoma complicating cirrhosis. Br J Surg 80: 747–749. [DOI] [PubMed] [Google Scholar]
- 4. Lai EC, Wu KM, Choi TK, Fan ST, Wong J (1989) Spontaneous ruptured hepatocellular carcinoma. An appraisal of surgical treatment. Ann Surg 210: 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim JY, Lee JS, Oh DH, Yim YH, Lee HK (2012) Transcatheter arterial chemoembolization confers survival benefit in patients with a spontaneously ruptured hepatocellular carcinoma. Eur J Gastroenterol Hepatol 24: 640–645. [DOI] [PubMed] [Google Scholar]
- 6. Sato Y, Fujiwara K, Furui S, Ogata I, Oka Y, et al. (1985) Benefit of transcatheter arterial embolization for ruptured hepatocellular carcinoma complicating liver cirrhosis. Gastroenterology 89: 157–159. [DOI] [PubMed] [Google Scholar]
- 7. Rady MY, Smithline HA, Blake H, Nowak R, Rivers E (1994) A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Ann Emerg Med 24: 685–690. [DOI] [PubMed] [Google Scholar]
- 8. Yau T, Yao TJ, Chan P, Epstein RJ, Ng KK, et al. (2009) The outcomes of elderly patients with hepatocellular carcinoma treated with transarterial chemoembolization. Cancer 115: 5507–5515. [DOI] [PubMed] [Google Scholar]
- 9. Ong GB, Taw JL (1972) Spontaneous rupture of hepatocellular carcinoma. Br Med J 4: 146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu CL, Fan ST, Lo CM, Tso WK, Poon RT, et al. (2001) Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol 19: 3725–3732. [DOI] [PubMed] [Google Scholar]
- 11. Cheung TT, Ng KK, Poon RT, Fan ST (2009) Tolerance of radiofrequency ablation by patients of hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 16: 655–660. [DOI] [PubMed] [Google Scholar]
- 12. Ng KK, Lam CM, Poon RT, Law WL, Seto CL, et al. (2003) Radiofrequency ablation as a salvage procedure for ruptured hepatocellular carcinoma. Hepatogastroenterology 50: 1641–1643. [PubMed] [Google Scholar]
- 13. Cheung TT, Chu FS, Jenkins CR, Tsang DS, Chok KS, et al. (2012) Tolerance of high-intensity focused ultrasound ablation in patients with hepatocellular carcinoma. World J Surg 36: 2420–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheung TT, Fan ST, Chu FS, Jenkins CR, Chok KS, et al. (2013) Survival analysis of high-intensity focused ultrasound ablation in patients with small hepatocellular carcinoma. HPB (Oxford) 15: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheung TT, Chan SC, Ho CL, Chok KS, Chan AC, et al. (2011) Can positron emission tomography with the dual tracers [11 C]acetate and [18 F]fludeoxyglucose predict microvascular invasion in hepatocellular carcinoma? Liver Transpl 17: 1218–1225. [DOI] [PubMed] [Google Scholar]
- 16. Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, et al. (2013) 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeon's perspective. J Nucl Med 54: 192–200. [DOI] [PubMed] [Google Scholar]
- 17. Fan ST (1998) Problems of hepatectomy in cirrhosis. Hepatogastroenterology 45 Suppl 31288–1290. [PubMed] [Google Scholar]
- 18. Silva MA, Muralidharan V, Mirza DF (2004) The management of coagulopathy and blood loss in liver surgery. Semin Hematol 41: 132–139. [DOI] [PubMed] [Google Scholar]
- 19. Kim JY, Lee JS, Oh DH, Yim YH, Lee HK (2012) Transcatheter arterial chemoembolization confers survival benefit in patients with a spontaneously ruptured hepatocellular carcinoma. Eur J Gastroenterol Hepatol 24: 640–645. [DOI] [PubMed] [Google Scholar]
- 20. Toshikuni N, Takuma Y, Morimoto Y, Shimomura H, Yamamoto H (2011) Transarterial embolization for ruptured hepatocellular carcinoma: survival predictors. Hepatogastroenterology 58: 565–569. [PubMed] [Google Scholar]
- 21. Buczkowski AK, Kim PT, Ho SG, Schaeffer DF, Lee SI, et al. (2006) Multidisciplinary management of ruptured hepatocellular carcinoma. J Gastrointest Surg 10: 379–386. [DOI] [PubMed] [Google Scholar]
- 22. Hermann RE, David TE (1973) Spontaneous rupture of the liver caused by hepatomas. Surgery 74: 715–719. [PubMed] [Google Scholar]
- 23. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, et al. (2008) Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 47: 82–89. [DOI] [PubMed] [Google Scholar]
- 24. Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, et al. (2004) Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg 240: 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Curley SA, Izzo F, Ellis LM, Nicolas VJ, Vallone P (2000) Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg 232: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poon RT, Ng KK, Lam CM, Ai V, Yuen J, et al. (2004) Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol 11: 281–289. [DOI] [PubMed] [Google Scholar]
- 27. Chiappa A, Bertani E, Biffi R, Zbar AP, Viale G, et al. (2008) Effectiveness of LigaSure diathermy coagulation in liver surgery. Surg Technol Int 17: 33–38. [PubMed] [Google Scholar]
- 28. Cherqui D, Husson E, Hammoud R, Malassagne B, Stephan F, et al. (2000) Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 232: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, et al. (2013) Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 257: 506–511. [DOI] [PubMed] [Google Scholar]
- 30. Altekruse SF, McGlynn KA, Reichman ME (2009) Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 27: 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chun YH, Kim SU, Park JY, Kim DY, Han KH, et al. (2011) Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer 47: 2568–2575. [DOI] [PubMed] [Google Scholar]
- 32. Fan ST (1999) Surgical therapy of hepatocellular carcinoma in the cirrhotic liver. Swiss Surg 5: 107–110. [DOI] [PubMed] [Google Scholar]
- 33. Yau T, Yao TJ, Chan P, Ng K, Fan ST, et al. (2008) A new prognostic score system in patients with advanced hepatocellular carcinoma not amendable to locoregional therapy: implication for patient selection in systemic therapy trials. Cancer 113: 2742–2751. [DOI] [PubMed] [Google Scholar]
- 34. Yau T, Chan P, Ng KK, Chok SH, Cheung TT, et al. (2009) Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: presence of lung metastasis predicts poor response. Cancer 115: 428–436. [DOI] [PubMed] [Google Scholar]
- 35. Kan RW, Tsang SH, Poon RT, Cheung TT (2012) Update on yttrium-90-based radio-embolization for treatment of hepatocellular carcinoma. ANZ J Surg 82: 505–509. [DOI] [PubMed] [Google Scholar]
- 36. Kaneko S, Furuse J, Kudo M, Ikeda K, Honda M, et al. (2012) Guideline on the use of new anticancer drugs for the treatment of Hepatocellular Carcinoma 2010 update. Hepatol Res 42: 523–542. [DOI] [PubMed] [Google Scholar]