Abstract
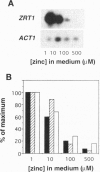
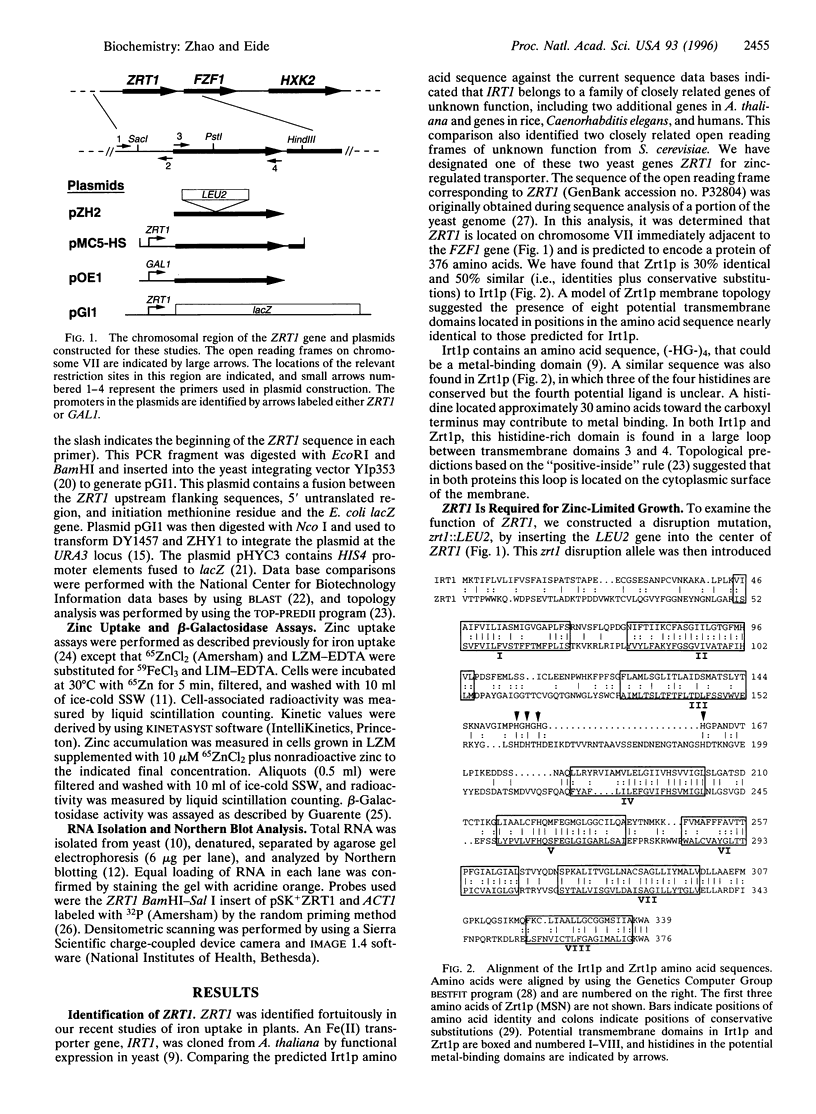
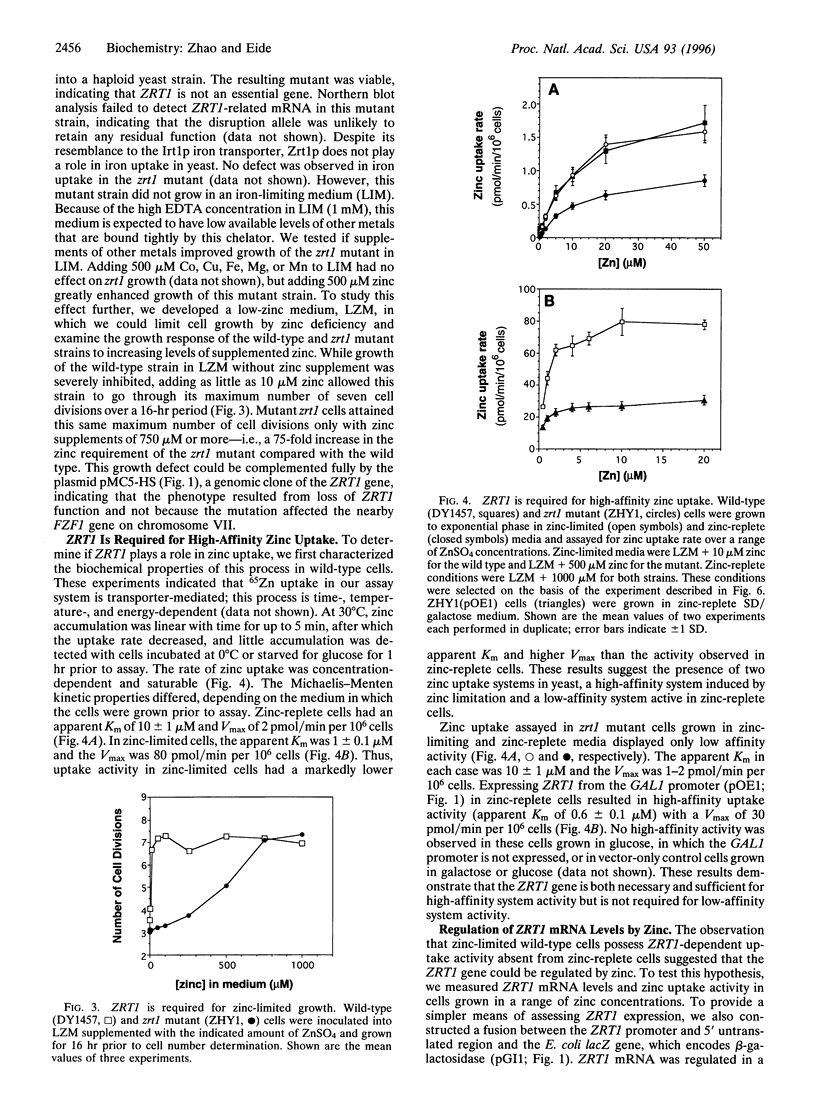
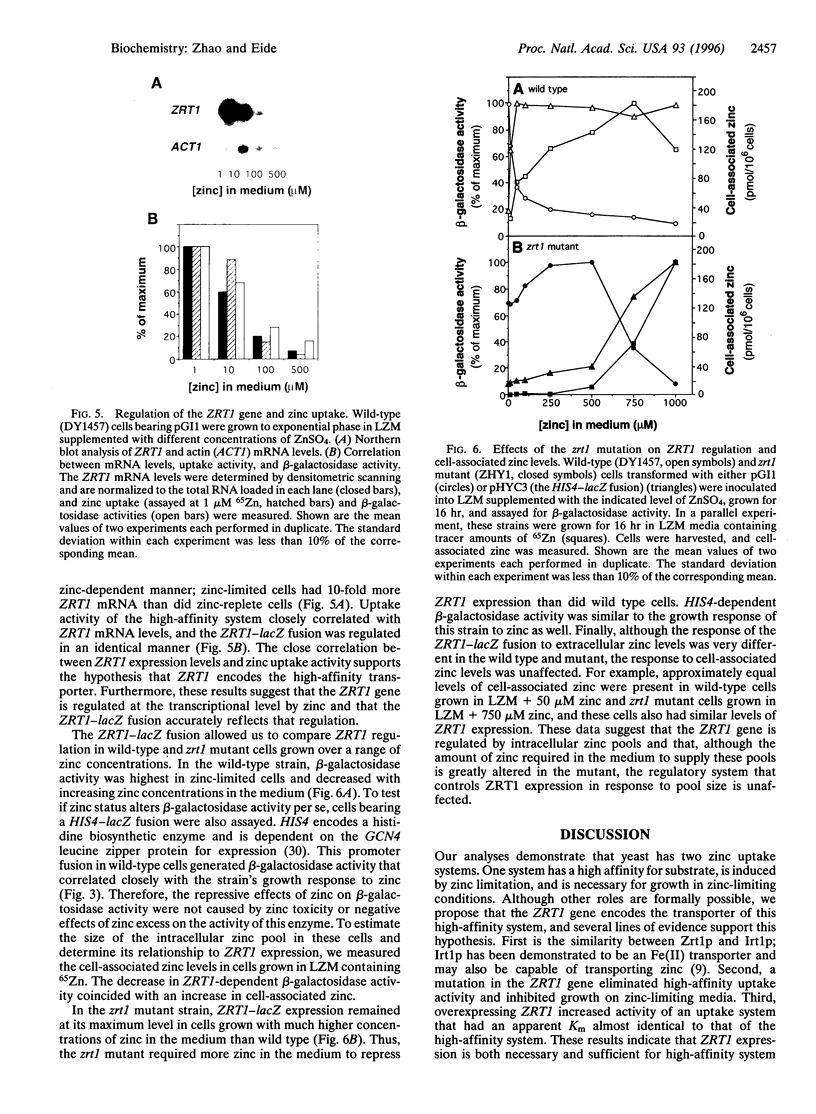
The yeast Saccharomyces cerevisiae has two separate systems for zinc uptake. One system has high affinity for substrate and is induced in zinc-deficient cells. The second system has lower affinity and is not highly regulated by zinc status. The ZRT1 gene encodes the transporter for the high-affinity system, called Zrt1p. The predicted amino acid sequence of Zrt1p is similar to that of Irt1p, a probable Fe(II) transporter from Arabidopsis thaliana. Like Irt1p, Zrt1p contains eight potential transmembrane domains and a possible metal-binding domain. Consistent with the proposed role of ZRT1 in zinc uptake, overexpressing this gene increased high-affinity uptake activity, whereas disrupting it eliminated that activity and resulted in poor growth of the mutant in zinc-limited media. Furthermore, ZRT1 mRNA levels and uptake activity were closely correlated, as was zinc-limited induction of a ZRT1-lacZ fusion. These results suggest that ZRT1 is regulated at the transcriptional level by the intracellular concentration of zinc. ZRT1 is an additional member of a growing family of metal transport proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Breitwieser W., Price C., Schuster T. Identification of a gene encoding a novel zinc finger protein in Saccharomyces cerevisiae. Yeast. 1993 May;9(5):551–556. doi: 10.1002/yea.320090512. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Claros M. G., von Heijne G. TopPred II: an improved software for membrane protein structure predictions. Comput Appl Biosci. 1994 Dec;10(6):685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- Conklin D. S., McMaster J. A., Culbertson M. R., Kung C. COT1, a gene involved in cobalt accumulation in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Sep;12(9):3678–3688. doi: 10.1128/mcb.12.9.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix D. R., Bridgham J. T., Broderius M. A., Byersdorfer C. A., Eide D. J. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J Biol Chem. 1994 Oct 21;269(42):26092–26099. [PubMed] [Google Scholar]
- Eide D., Davis-Kaplan S., Jordan I., Sipe D., Kaplan J. Regulation of iron uptake in Saccharomyces cerevisiae. The ferrireductase and Fe(II) transporter are regulated independently. J Biol Chem. 1992 Oct 15;267(29):20774–20781. [PubMed] [Google Scholar]
- Eide D., Guarente L. Increased dosage of a transcriptional activator gene enhances iron-limited growth of Saccharomyces cerevisiae. J Gen Microbiol. 1992 Feb;138(2):347–354. doi: 10.1099/00221287-138-2-347. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G. F., Rothstein A. The transport of Zn2+, Co2+ and Ni2+ into yeast cells. Biochim Biophys Acta. 1968 Nov 5;163(3):325–330. doi: 10.1016/0005-2736(68)90117-x. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., Lucchini G., Fink G. R. A synthetic HIS4 regulatory element confers general amino acid control on the cytochrome c gene (CYC1) of yeast. Proc Natl Acad Sci U S A. 1985 Jan;82(2):498–502. doi: 10.1073/pnas.82.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamizono A., Nishizawa M., Teranishi Y., Murata K., Kimura A. Identification of a gene conferring resistance to zinc and cadmium ions in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1989 Oct;219(1-2):161–167. doi: 10.1007/BF00261172. [DOI] [PubMed] [Google Scholar]
- Liu H., Krizek J., Bretscher A. Construction of a GAL1-regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics. 1992 Nov;132(3):665–673. doi: 10.1093/genetics/132.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini G., Hinnebusch A. G., Chen C., Fink G. R. Positive regulatory interactions of the HIS4 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jul;4(7):1326–1333. doi: 10.1128/mcb.4.7.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. M., Tzagoloff A., Kinney D. M., Lusty C. J. Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene. 1986;45(3):299–310. doi: 10.1016/0378-1119(86)90028-4. [DOI] [PubMed] [Google Scholar]
- Okorokov L. A., Andreeva N. A., Lichko L. P., Valiakhmetov AYa Transmembrane gradient of K+ ions as an energy source in the yeast Saccharomyces carlsbergensis. Biochem Int. 1983 Apr;6(4):463–472. [PubMed] [Google Scholar]
- Palmiter R. D., Findley S. D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995 Feb 15;14(4):639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHSTEIN A., HAYES A., JENNINGS D., HOOPER D. The active transport of Mg++ and Mn++ into the yeast cell. J Gen Physiol. 1958 Jan 20;41(3):585–594. doi: 10.1085/jgp.41.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Klug A. Zinc fingers. Sci Am. 1993 Feb;268(2):56-9, 62-5. doi: 10.1038/scientificamerican0293-56. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Broach J. R. Cloning genes by complementation in yeast. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Boeke J. D. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]